Abstract
The objective of this work was to develop new coating materials based on poly(ethyleneoxide) (PEO), which was grafted onto polysilazane (PSZ) by hydrosilylation. Three types of PEO with different molecular weights (350, 750, 2000 g/mol) were studied. The kinetics and yields of this reaction have been surveyed by 1H and 13C NMR spectroscopy. The PEO grafting-density onto PSZ by hydrosilylation increases with a reduction of the S–H/allyl ratio and a decrease of the PEO chain-length. The PEO-graft-PSZ (PSZ-PEO) hybrid coatings, which can be used to prevent the adhesion of marine bacteria on surfaces, were applied by moisture curing at room temperature. The anti-adhesion performance, and thus the anti-fouling activity, of the coatings against three marine bacteria species, Clostridium sp. SR1, Neisseria sp. LC1 and Neisseria sp. SC1, was examined. The anti-fouling activity of the coatings depends on the grafting density and the chain length of PEO. The shortest PEO(350 g/mol)-graft-PSZ with the highest graft density was found to have the best anti-fouling activity. As the density of grafted PEO(750 g/mol) and PEO(2000 g/mol) chains onto the PSZ surface is approximately equal, the relative effectiveness of these two types of PEO is controlled by the length of the PEO chain. The PEO(2000 g/mol)-graft-PSZ coatings are more efficient than the PEO(750 g/mol)-graft-PSZ coatings for the bacterial anti-adhesion.
Introduction
To date, polysilazanes with the general formula –(SiR1R2–NR3)n–, have been mainly used as ceramic precursors Si/C/N for high-temperature applications [1-3]. The Clariant Company has developed formulations based on polysilazanes that could crosslinked by humid air through hydrolysis and condensation reactions [4,5]. This method was used to prepare coatings of thicknesses from 0.3 to 50 micrometers that have an excellent resistance against corrosion and abrasion because of their exceptional adhesion to a variety of substrates (glass, polycarbonate, aluminium, steel, etc.) combined with a high brightness, a non-stick surface, and the resistance to heat, fire and UV irradiation.
Poly(ethylene oxide) or poly(oxyethylene) (PEO) is a non-toxic polymer, which is used as a surface-modification agent, because it is effective in reducing bio-adhesion, i.e., protein adsorption, or the adhesion of bacteria and cells. The environmentally friendly coatings obtained by the grafting of PEO onto PSZ are a promising way to prevent the deposition of marine fouling materials onto the hulls of ships [6]. Several factors have been hypothesized to explain the anti-fouling property of these surfaces. These include the hydrophilicity [7], the mobility, the large exclusion volume in water and the effect of steric repulsion [8-11]. The effect of the molecular weight and the grafting density of PEO on the anti-fouling capacity has been widely studied [12-14].
Several methods to modify surfaces with PEO chains were investigated. These include the covalent grafting [15,16], the chemical adsorption [17], the formation of self-assembled monolayers [18,19], plasma treatment [12,20] or the use of supercritical CO2 [21,22]. Kingshott et al. have demonstrated that the grafting of PEO onto the substrates by covalent bonding is necessary for an anti-fouling activity [23]. Herein, we propose a new synthetic strategy to prepare hybrid materials based on polysilazane (PSZ), which have an enhanced resistance against bacteria adhesion, through the incorporation of allyl PEO monomethyl ether by covalent grafting.
Experimental
Materials. Monomethoxy poly(ethylene oxide) glycol (MPEG) with average molecular weights of 350 g/mol, 750 g/mol and 2000 g/mol and Karstedt’s catalyst were purchased from Sigma-Aldrich. All three types of MPEG were dried under vacuum before usage. The polysilazane precursor, polylmethyhydrosilazane containing triethoxysilanes, was provided by the Clariant Company. The molecular structure of the polysilazane precursor is described on Figure 1. PSZ was used as received without any further purification. Allyl bromide (allyl-Br) purchased from Acros was distilled under a nitrogen atmosphere in the dark and subsequently kept in darkness before usage. Allyl-Br is sensitive to light and may polymerize on exposure to light.
Figure 1: Molecular structure and distribution of the functional groups of the polysilazane precursor provided by the Clariant Company.
Figure 1: Molecular structure and distribution of the functional groups of the polysilazane precursor provide...
NMR. 1H and 13C NMR spectra were obtained on a Bruker FT-NMR 400 MHz spectrometer.
Protocol of the marine bacterial adhesion. Three marine bacteria species, bacillus Clostridium sp. SR1, micrococcus Neisseria sp. LC1 and micrococcus Neisseria sp. SC1, were used for the adhesion studies. The culture media was Vätäänen nine-salt solution (VNSS). The PSZ and PSZ-PEO coatings, which were moisture-cured at ambient conditions, were previously sterilized in methanol, immersed in VNSS that contained the bacteria and were finally incubated at 30 °C for 24 h. Then coatings were rinsed in sterilized phosphate buffered saline to remove the non-adhered bacteria. The adhered bacteria were removed from the surfaces using an ultrasonic cleaner for 5 min with sterilized synthetic sea water. The number of adhered bacteria (CFU/cm2) was determined by using a Colony Counter BZG30.
Synthesis of allyl-PEO. Allyl-terminated monomethyl poly(ethylene oxide) (allyl-PEO) ethers were synthesized via the reaction of MPEG with an excess amount of allyl-Br [24]. The equation of this synthesis reaction is described in Scheme 1.
Scheme 1: Equation of the allyl-PEO synthesis reaction.
Scheme 1: Equation of the allyl-PEO synthesis reaction.
Synthesis of PSZ-PEO. Grafting of allyl-PEO molecules onto the polysilazane (PSZ) chain was performed by using a hydrosilylation reaction between the Si–H group of PSZ and the terminal C=C bond of the allyl-PEO with an excess amount of PSZ in presence of Karstedt’s catalyst (Scheme 2). We define that a, b, c are the different protons and carbon atoms on the allyl branch of the PEO molecule, whereas a′, b′, c′ are the different protons and carbon atoms of the same branch after being grafted onto the PZS chain.
Scheme 2: Principle of hydrosilylation between the Si–H group of PSZ and the C=C bond of the allyl-PEO.
Scheme 2: Principle of hydrosilylation between the Si–H group of PSZ and the C=C bond of the allyl-PEO.
The most favorable conditions for the grafting of allyl-PEO chains on PSZ are the following: a temperature of 80 °C and a molar ratio of Pt/allyl of 3 × 10−3 under argon atmosphere. Different Si–H/allyl molar ratios of 10, 16.5 and 26.5, respectively, were investigated for all three types of allyl-PEO. The grafted products are symbolized as PSZ-PEOX-Y with X being the molecular weight of MPEG and Y being the Si–H/allyl molar ratio.
Results and Discussion
Grafting of allyl-PEO molecules onto the PSZ chain
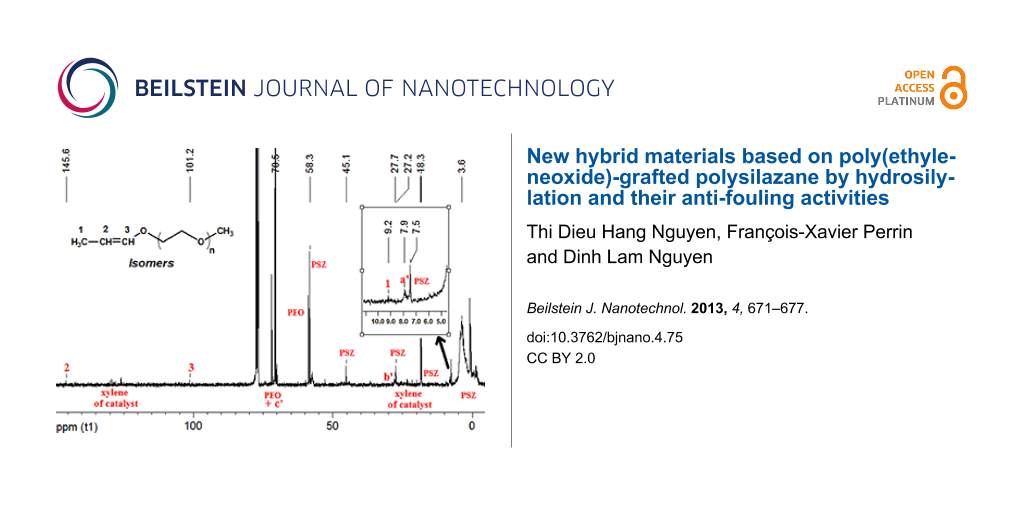
The kinetics and yields of the grafting reactions have been surveyed by 1H and 13C NMR spectroscopy. Figure 2 shows the 1H NMR and 13C NMR spectra of the mixture of the reactants and of the grafting products. In Figure 1 and in Scheme 2, it is found that the structure of the grafted group ≡Si–CH2–CH2–CH2–O– of PSZ-PEO is very close to that of the group ≡Si–CH2–CH2–CH2–N– of the PSZ precursor. In the 1H NMR spectra, the resonance peaks of the protons Ha' and Hb' of PSZ-PEO therefore appear at chemical shifts very similar to those of the protons ≡Si–CH2–CH2– (0.62 ppm) and ≡Si–CH2–CH2– (1.5 ppm) of PSZ, respectively. Likewise, the chemical shift of the proton Hc' of PSZ-PEO is very close to that of protons –O–CH3 and –O–CH2–CH2–O– of PEO, situated at around 3.4 ppm (Figure 2A). The same difficulties in identifying the grafted PEO are encountered during the analysis of the 13C NMR spectra. This is because the 13C NMR chemical shifts of the carbon atoms at positions Ca’ and ≡Si–CH2–CH2–, Cb’ and ≡Si–CH2–CH2–, Cc’ and –O–CH2–CH2–O– of PEO are very close, as shown in Figure 2B. In order to circumvent theses complications, the kinetic investigation of the hydrosilylation reaction was carried out by following the decrease of the intensity of the allyl-proton resonances in the 1H NMR spectra.
![[2190-4286-4-75-2]](/bjnano/content/figures/2190-4286-4-75-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Identification of the grafting of PEO350 molecules onto PSZ chain. (A) Comparison of 1H NMR spectra of initial mixture and grafting product. (B) 13C NMR spectrum of grafting product.
Figure 2: Identification of the grafting of PEO350 molecules onto PSZ chain. (A) Comparison of 1H NMR spectra...
The evolution of the 1H NMR spectra of the reactant mixture over the reaction time is shown in Figure 3. These results verify that the intensities of the 1H MNR peaks attributed to the allyl group (–CH = at 5.87ppm, CH2 = at 5.22ppm and –CH2− at 3.99ppm) [24] decrease continuously. This diminution can be used to confirm the participation of the allyl group in the hydrosilylation during the grafting of allyl-PEO onto the PSZ chain.
![[2190-4286-4-75-3]](/bjnano/content/figures/2190-4286-4-75-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Investigation of grafting allyl-PEO350 onto the PSZ chain by 1H NMR (SiH/allyl = 10).
Figure 3: Investigation of grafting allyl-PEO350 onto the PSZ chain by 1H NMR (SiH/allyl = 10).
After few hours of the grafting reaction, we recognized the appearance of two new 1H NMR peaks at chemical shifts of 5.9 and 6.2 ppm. The intensities of two new peaks increase with reaction time. These two peaks have been verified to match the =CH− protons of the cis- and trans-isomers, respectively, of the propenyl ether group, which results from the isomerization of allyl-PEO [25] (Figure 4). In the 13C NMR spectrum (Figure 2), the position of the propenyl ether group has been recognized in the region of low fields (CH3− at 9.2ppm, −CH= at 145.6ppm and =CH−O at 101.2ppm). The isomerization of the allyl-PEO was therefore considered as a secondary reaction that takes place simultaneously to the hydrosilylation. The optimum conditions for the grafting of PEO were defined to reach a high selectivity toward the hydrosilylation in comparison to the isomerisation.
Figure 4: Chemical structure of cis- and trans-isomers of allyl-PEO.
Figure 4: Chemical structure of cis- and trans-isomers of allyl-PEO.
It was observed that the conversion of allyl-PEO350-10 was 100% after 48 h. In contrast, allyl-PEO750 and allyl-PEO2000 were not converted completely in any ratio of SiH/allyl after 55 h of grafting (Figure 5 and below in Table 1). The amount of non-reacted allyl-PEO2000 can be seen very clearly in the 1H NMR spectra. This means, the longer the molecule the more difficult is its grafting. Therefore, the products of the grafting of allyl-PEO750 and allyl-PEO2000 are mixtures of PSZ-PEO, PSZ surplus, propenyl ether isomers and unreacted allyl-PEO.
![[2190-4286-4-75-5]](/bjnano/content/figures/2190-4286-4-75-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Investigation of grafting allyl-PEO750 (A) and allyl-PEO2000 (B) onto the PSZ chain by 1H NMR (SiH/allyl = 10).
Figure 5: Investigation of grafting allyl-PEO750 (A) and allyl-PEO2000 (B) onto the PSZ chain by 1H NMR (SiH/...
The decrease in the 1H NMR signal of CH2= was also used to evaluate the conversion of allyl-PEO during grafting. The total conversion of allyl-PEO (%CAP,total), the conversion of Allyl-PEO associated with isomerization (%CAP,iso) and the conversion of allyl-PEO associated with hydrosilylation (%CAP,hydro) were calculated according to the following equations:
in which ICH2=(0) is the integral of the CH2= resonance in the 1H-NMR spectrum of the initial mixture and ICH2=(t), ICH,cis(t) and ICH,trans(t) are the integrals of the CH2=, cis-CH=, and trans-CH= resonances at time t, respectively.
The density (δ) of grafted PEO molecules onto the PSZ chain expressed by the amount of PEO attaching to 10 equiv of initial S−H has been calculated according to
The conversions of all three types of allyl-PEO in the different reactions during grafting as well as the density of grafted allyl-PEO molecules onto the PSZ chain are listed in Table 1. It should be noted that the degree of hydrosilylation increases when increasing the Si–H/allyl ratio and decreasing the molecular weight of PEO. Contrary to this, the degree of isomerization reduces significantly when increasing the Si–H/allyl ratio. In particular, when Si–H/allyl = 26.5, the trans-isomer could not be identified for both allyl-PEO750 and allyl-PEO2000 in their 1H NMR spectra.
Table 1: Conversion of allyl-PEO and density of the grafted PEO chains onto PSZ calculated from 1H NMR spectra.
| X = Mallyl-PEO (g/mol) | Y = SiH/allyl | t (h) | %CAP,total | %CAP,hydro | %CAP,iso | δ (mol PEO grafted/10 mol of Si–H) |
|---|---|---|---|---|---|---|
| 350 | 10 | 48 | 100 | 71.78 | 28.22 | 0.72 |
| 16.5 | 40 | 100 | 83.63 | 16.37 | 0.51 | |
| 26.5 | 30 | 100 | 91.84 | 8.16 | 0.35 | |
| 750 | 10 | 55 | 96.72 | 62.84 | 33.88 | 0.63 |
| 16.5 | 55 | 91.60 | 68.02 | 23.59 | 0.41 | |
| 26.5 | 55 | 97.35 | 88.86 | 8.49 | 0.34 | |
| 2000 | 10 | 55 | 80.15 | 59.95 | 20.20 | 0.60 |
| 16.5 | 55 | 72.65 | 61.96 | 10.69 | 0.38 | |
| 26.5 | 55 | 86.61 | 78.07 | 8.54 | 0.29 | |
Table 1 clearly shows that the density of the grafted PEO onto PSZ chain increases when the initial S–H/allyl ratio and the PEO molecular weight decrease. To increase the Si–H/allyl ratio means that the concentration of allyl-PEO molecules will become low. So in this case, the density of grafted PEO was reduced despite increasing the degree of hydrosilylation.
Marine bacterial adhesion on modified surfaces
Figure 6 shows the influence of the length of the grafted PEO and of the Si–H/allyl ratio on the bacterial adhesion in VNSS media. Compared to the unmodified PSZ coating, all PSZ-PEO surfaces exhibit lower levels of bacteria adhesion. The inhibition efficiency varies inversely with the ratio of Si–H/allyl. It is remarkable that the PSZ-PEO350-10 surface shows the best inhibitory properties and leads to a reduction in the amount of adhered bacteria of approximately 70% for all three types of bacteria when compared to the pure PSZ coating. The anti-fouling activities of PSZ-PEO showed an increase in the sequence PEO750 < PEO2000 < PEO350. This result may seem to disagree with several previous studies showing that a PEO2000 chain is most effective against bacterial adhesion [12,26,27]. However, all PSZ-PEO350 coatings have a PEO grafting density that is higher than that of PSZ-PEO750 and PSZ-PEO2000 at the same Si–H/allyl ratio (see Table 1). The higher PEO grafting density of PSZ-PEO350 could explain its higher anti-fouling activity. These results are also in accordance with the works of Jeon et al. [9,10]. Based on a surface–protein interaction model these authors demonstrated that the surface density of the PEO chains have a greater effect than the length of the PEO chains in inhibiting bacterial adhesion. As the grafting density of PEO750 and PEO2000 onto PSZ is approximately equivalent, the effectiveness of these two types of hybrid materials is controlled by the chain length of PEO. Consequently, the PSZ-PEO2000 surfaces are more efficient than the PSZ-PEO750 surfaces in regarding to the anti-fouling activity.
![[2190-4286-4-75-6]](/bjnano/content/figures/2190-4286-4-75-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Marine bacteria adhesion on PSZ-PEO surfaces (mean ± SD, 100% corresponds to 13 × 102 CFU/cm2 for Clostridium sp. SR1 (A); 8 × 103CFU/cm2 for Neisseria sp. LC1 (B) and 11 × 103CFU/cm2 for Neisseria sp. SC1 (C)).
Figure 6: Marine bacteria adhesion on PSZ-PEO surfaces (mean ± SD, 100% corresponds to 13 × 102 CFU/cm2 for C...
Conclusion
The grafting of allyl-PEO molecules onto a PSZ chain in presence of Karstedt’s catalyst has been successfully realized by hydrosilylation. The structure of the grafted products have been identified by 1H NMR and 13C NMR. After a few hours of the grafting reaction, there are the appearances of trans- and cis-isomers of allyl-PEO molecules. Apparently, isomerization occurs simultaneously to the hydrosilylation. The grafting density of PEO onto the PSZ chain increases with a reduction of the S–H/allyl ratio and a decrease of the chain length of PEO. At a molar ratio of Si–H/allyl = 10 for grafting allyl-PEO350 molecules, there is 0.72 chains of allyl-per ten Si–H groups of the PSZ chain. While at a molar ratio of Si–H/allyl = 26.5 for grafting allyl-PEO2000 molecules, there is 0.29 chains of allyl-PEO per ten Si–H groups of the PSZ chain.
The bacterial adhesion inhibition depends on the grafting density and the chain length of PEO. The role of the grafting density is more important than that of the chain length. The PSZ-PEO350 coatings prepared at a ratio SiH/allyl = 10 with the highest grafting density show the best anti-fouling activity for all three studied marine bacteria. While the densities of PEO750 and PEO2000 chains on the PSZ surface are practically equal, the PSZ-PEO2000 coatings are more efficient in anti-fouling than those with PSZ-PEO750. It is indispensable to find the optimal conditions for grafting allyl-PEO2000 on the PSZ chain in order to improve the degree of hydrosilylation and to increase the density of grafted allyl-PEO2000 molecules in PSZ-PEO anti-fouling surfaces.
Acknowledgements
The authors would like to thank the Clariant Company for its kind supply of the polysilazane. The contribution of Dinh Hung Lan Tran, Ngoc Tuan Nguyen, Thi Trang Nguyen, University of Danang - University of Science and Technology, with their experimental work on the marine bacterial adhesion is also very much acknowledged.
References
-
Choong Kwet Yive, N. S.; Corriu, R. J. P.; Leclercq, D.; Mutind, P. H.; Vioux, A. Chem. Mater. 1992, 4, 141–146. doi:10.1021/cm00019a029
Return to citation in text: [1] -
Ya-Li, L.; Kroke, E.; Riedel, R.; Fasel, C.; Gervais, C.; Babonneau, F. Appl. Organomet. Chem. 2001, 15, 820–832. doi:10.1002/aoc.236
Return to citation in text: [1] -
Seitz, J.; Bill, J.; Egger, N.; Aldinger, F. J. Eur. Ceram. Soc. 1996, 16, 885–891. doi:10.1016/0955-2219(96)00007-6
Return to citation in text: [1] -
Lukacs, A., III; Knasiak, G. J. Thermally stable, moisture curable polysilazanes and polysiloxazanes. US Pat. 0083453A1, May 1, 2003.
Return to citation in text: [1] -
Bauer, F.; Decker, U.; Dierdorf, A.; Ernst, H.; Heller, R.; Liebe, H.; Mehnert, R. Prog. Org. Coat. 2005, 53, 183–190. doi:10.1016/j.porgcoat.2005.02.006
Return to citation in text: [1] -
Glinel, K.; Jonas, A. M.; Jouenne, T.; Leprince, J.; Galas, L.; Huck, W. T. S. Bioconjugate Chem. 2009, 20, 71–77. doi:10.1021/bc800280u
Return to citation in text: [1] -
Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. J. Am. Chem. Soc. 2003, 125, 9359–9366. doi:10.1021/ja034820y
Return to citation in text: [1] -
Knoll, D.; Hermans, J. J. Biol. Chem. 1983, 258, 5710–5715.
Return to citation in text: [1] -
Jeon, S. I.; Lee, J. H.; Andrade, J. D.; De Gennes, P. G. J. Colloid Interface Sci. 1991, 142, 149–158. doi:10.1016/0021-9797(91)90043-8
Return to citation in text: [1] [2] -
Jeon, S. I.; Andrade, J. D. J. Colloid Interface Sci. 1991, 142, 159–166. doi:10.1016/0021-9797(91)90044-9
Return to citation in text: [1] [2] -
Knerr, R.; Weiser, B.; Drotleff, S.; Steinem, C.; Gopferich, A. Macromol. Biosci. 2006, 6, 827–838. doi:10.1002/mabi.200600106
Return to citation in text: [1] -
Dong, B.; Manolache, S.; Wong, A. C. L.; Denes, F. S. Polym. Bull. 2011, 66, 517–528. doi:10.1007/s00289-010-0358-y
Return to citation in text: [1] [2] [3] -
Nguyen, T. D. H. Revêtements polysilazane à activités antibactériennes. Ph.D. Thesis, University of the South, Toulon-Var, France, 2011.
Return to citation in text: [1] -
Sofia, S. J.; Premnath, V.; Merrill, E. W. Macromolecules 1998, 31, 5059–5070. doi:10.1021/ma971016l
Return to citation in text: [1] -
Murthy, R.; Cox, C. D.; Hahn, M. S.; Grunlan, M. A. Biomacromolecules 2007, 8, 3244–3252. doi:10.1021/bm700543c
Return to citation in text: [1] -
Chen, H.; Zhang, Z.; Chen, Y.; Brook, M. A.; Sheardown, H. Biomaterials 2005, 26, 2391–2399. doi:10.1016/j.biomaterials.2004.07.068
Return to citation in text: [1] -
Kenausis, G. L.; Voros, J.; Elbert, D. L.; Huang, N.; Hofer, R.; Ruiz-Taylor, L.; Textor, M.; Hubbell, J. A.; Spencer, N. D. J. Phys. Chem. B 2000, 104, 3298–3309. doi:10.1021/jp993359m
Return to citation in text: [1] -
Ostuni, E.; Chapman, R. G.; Liang, M. N.; Meluleni, G.; Pier, G.; Ingber, D. E.; Whitesides, G. M. Langmuir 2001, 17, 6336–6343. doi:10.1021/la010552a
Return to citation in text: [1] -
Huang, N. P.; Michel, R.; Voros, J.; Textor, M.; Hofer, R.; Rossi, A.; Elbert, D. L.; Hubbell, J. A.; Spencer, N. D. Langmuir 2001, 17, 489–498. doi:10.1021/la000736+
Return to citation in text: [1] -
Johnston, E. E.; Bryers, J. D.; Ratner, B. D. Langmuir 2005, 21, 870–881. doi:10.1021/la036274s
Return to citation in text: [1] -
Cooper, A. I.; DeSimone, J. M. Curr. Opin. Solid State Mater. Sci. 1996, 1, 761–766. doi:10.1016/S1359-0286(96)80100-8
Return to citation in text: [1] -
Harrison, K. L.; Johnston, K. P.; Sanchez, I. C. Langmuir 1996, 12, 2637–2644. doi:10.1021/la9510137
Return to citation in text: [1] -
Kingshott, P.; Wei, J.; Bagge-Ravn, D.; Gadegaard, N.; Gram, L. Langmuir 2003, 19, 6912–6921. doi:10.1021/la034032m
Return to citation in text: [1] -
Lestel, L.; Cheradame, H.; Boileau, S. Polymer 1990, 31, 1154–1158. doi:10.1016/0032-3861(90)90266-2
Return to citation in text: [1] [2] -
Chung, D.; Kim, T. G. J. Ind. Eng. Chem. 2007, 13, 571–577.
Return to citation in text: [1] -
Benhabbour, S. R.; Sheardown, H.; Adronov, A. Macromolecules 2008, 41, 4817–4823. doi:10.1021/ma8004586
Return to citation in text: [1] -
Roosjen, A.; Van der Mei, H. C.; Busscher, H. J.; Norde, W. Langmuir 2004, 20, 10949–10955. doi:10.1021/la048469l
Return to citation in text: [1]
| 9. | Jeon, S. I.; Lee, J. H.; Andrade, J. D.; De Gennes, P. G. J. Colloid Interface Sci. 1991, 142, 149–158. doi:10.1016/0021-9797(91)90043-8 |
| 10. | Jeon, S. I.; Andrade, J. D. J. Colloid Interface Sci. 1991, 142, 159–166. doi:10.1016/0021-9797(91)90044-9 |
| 1. | Choong Kwet Yive, N. S.; Corriu, R. J. P.; Leclercq, D.; Mutind, P. H.; Vioux, A. Chem. Mater. 1992, 4, 141–146. doi:10.1021/cm00019a029 |
| 2. | Ya-Li, L.; Kroke, E.; Riedel, R.; Fasel, C.; Gervais, C.; Babonneau, F. Appl. Organomet. Chem. 2001, 15, 820–832. doi:10.1002/aoc.236 |
| 3. | Seitz, J.; Bill, J.; Egger, N.; Aldinger, F. J. Eur. Ceram. Soc. 1996, 16, 885–891. doi:10.1016/0955-2219(96)00007-6 |
| 8. | Knoll, D.; Hermans, J. J. Biol. Chem. 1983, 258, 5710–5715. |
| 9. | Jeon, S. I.; Lee, J. H.; Andrade, J. D.; De Gennes, P. G. J. Colloid Interface Sci. 1991, 142, 149–158. doi:10.1016/0021-9797(91)90043-8 |
| 10. | Jeon, S. I.; Andrade, J. D. J. Colloid Interface Sci. 1991, 142, 159–166. doi:10.1016/0021-9797(91)90044-9 |
| 11. | Knerr, R.; Weiser, B.; Drotleff, S.; Steinem, C.; Gopferich, A. Macromol. Biosci. 2006, 6, 827–838. doi:10.1002/mabi.200600106 |
| 7. | Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. J. Am. Chem. Soc. 2003, 125, 9359–9366. doi:10.1021/ja034820y |
| 12. | Dong, B.; Manolache, S.; Wong, A. C. L.; Denes, F. S. Polym. Bull. 2011, 66, 517–528. doi:10.1007/s00289-010-0358-y |
| 26. | Benhabbour, S. R.; Sheardown, H.; Adronov, A. Macromolecules 2008, 41, 4817–4823. doi:10.1021/ma8004586 |
| 27. | Roosjen, A.; Van der Mei, H. C.; Busscher, H. J.; Norde, W. Langmuir 2004, 20, 10949–10955. doi:10.1021/la048469l |
| 6. | Glinel, K.; Jonas, A. M.; Jouenne, T.; Leprince, J.; Galas, L.; Huck, W. T. S. Bioconjugate Chem. 2009, 20, 71–77. doi:10.1021/bc800280u |
| 24. | Lestel, L.; Cheradame, H.; Boileau, S. Polymer 1990, 31, 1154–1158. doi:10.1016/0032-3861(90)90266-2 |
| 4. | Lukacs, A., III; Knasiak, G. J. Thermally stable, moisture curable polysilazanes and polysiloxazanes. US Pat. 0083453A1, May 1, 2003. |
| 5. | Bauer, F.; Decker, U.; Dierdorf, A.; Ernst, H.; Heller, R.; Liebe, H.; Mehnert, R. Prog. Org. Coat. 2005, 53, 183–190. doi:10.1016/j.porgcoat.2005.02.006 |
| 24. | Lestel, L.; Cheradame, H.; Boileau, S. Polymer 1990, 31, 1154–1158. doi:10.1016/0032-3861(90)90266-2 |
| 18. | Ostuni, E.; Chapman, R. G.; Liang, M. N.; Meluleni, G.; Pier, G.; Ingber, D. E.; Whitesides, G. M. Langmuir 2001, 17, 6336–6343. doi:10.1021/la010552a |
| 19. | Huang, N. P.; Michel, R.; Voros, J.; Textor, M.; Hofer, R.; Rossi, A.; Elbert, D. L.; Hubbell, J. A.; Spencer, N. D. Langmuir 2001, 17, 489–498. doi:10.1021/la000736+ |
| 21. | Cooper, A. I.; DeSimone, J. M. Curr. Opin. Solid State Mater. Sci. 1996, 1, 761–766. doi:10.1016/S1359-0286(96)80100-8 |
| 22. | Harrison, K. L.; Johnston, K. P.; Sanchez, I. C. Langmuir 1996, 12, 2637–2644. doi:10.1021/la9510137 |
| 17. | Kenausis, G. L.; Voros, J.; Elbert, D. L.; Huang, N.; Hofer, R.; Ruiz-Taylor, L.; Textor, M.; Hubbell, J. A.; Spencer, N. D. J. Phys. Chem. B 2000, 104, 3298–3309. doi:10.1021/jp993359m |
| 23. | Kingshott, P.; Wei, J.; Bagge-Ravn, D.; Gadegaard, N.; Gram, L. Langmuir 2003, 19, 6912–6921. doi:10.1021/la034032m |
| 15. | Murthy, R.; Cox, C. D.; Hahn, M. S.; Grunlan, M. A. Biomacromolecules 2007, 8, 3244–3252. doi:10.1021/bm700543c |
| 16. | Chen, H.; Zhang, Z.; Chen, Y.; Brook, M. A.; Sheardown, H. Biomaterials 2005, 26, 2391–2399. doi:10.1016/j.biomaterials.2004.07.068 |
| 12. | Dong, B.; Manolache, S.; Wong, A. C. L.; Denes, F. S. Polym. Bull. 2011, 66, 517–528. doi:10.1007/s00289-010-0358-y |
| 13. | Nguyen, T. D. H. Revêtements polysilazane à activités antibactériennes. Ph.D. Thesis, University of the South, Toulon-Var, France, 2011. |
| 14. | Sofia, S. J.; Premnath, V.; Merrill, E. W. Macromolecules 1998, 31, 5059–5070. doi:10.1021/ma971016l |
| 12. | Dong, B.; Manolache, S.; Wong, A. C. L.; Denes, F. S. Polym. Bull. 2011, 66, 517–528. doi:10.1007/s00289-010-0358-y |
| 20. | Johnston, E. E.; Bryers, J. D.; Ratner, B. D. Langmuir 2005, 21, 870–881. doi:10.1021/la036274s |
© 2013 Nguyen et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Nanotechnology terms and conditions: (http://www.beilstein-journals.org/bjnano)