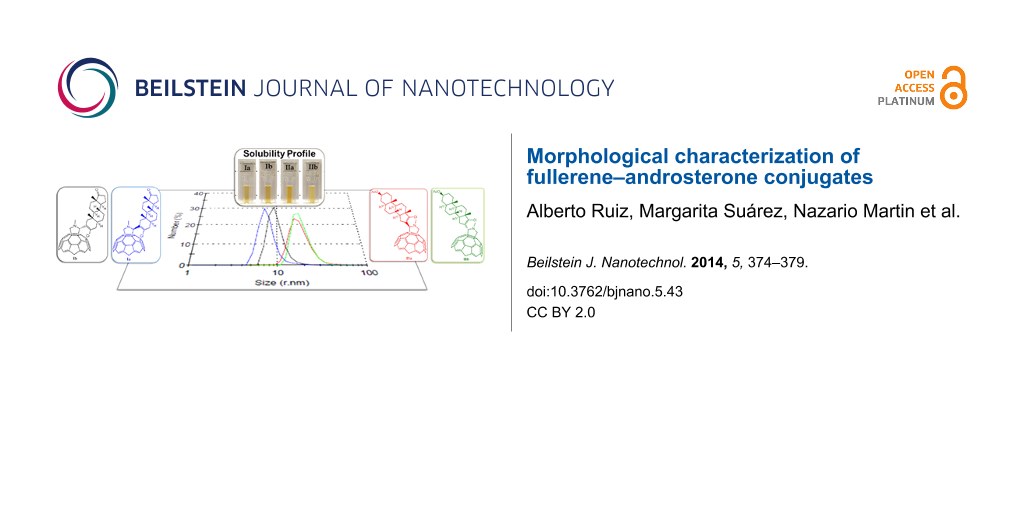
Abstract
Here we report on the self-organization characteristics in water of two diastereomer pairs of fullerene–androsterone hybrids that have the hydrophobic C60 appendage in the A and D ring of the androsterone moiety, respectively. The morphology and particle size in aqueous solution were determined by transmission electron microscopy (TEM) and dynamic light scattering (DLS), with satisfactory agreement between both techniques. In general, these fullerene derivatives are shown to organize into spherical nano-scale structures with diameters in the ranges of 10–20 and 30–50 nm, respectively.
Introduction
Since the discovery of [60]fullerene [1], the efforts of the scientific community have been focused on the preparation of suitably functionalized fullerene derivatives with physical and biological properties interesting for biomedicine and materials science [2]. The covalent linkage of C60 to moieties, such as porphyrins [3], anionic polymethine cyanine [4], and other bioactive molecules such as amino acids [5], peptides [6,7], nucleotides, sugars and steroids [8,9], have allowed the solubilization of these hybrid derivatives in aqueous media, thus enhancing certain biological activities. For the potential use of C60 derivatives as drug delivery systems, the size of the particles is important. In general, fast drug release was observed for small particles, although these particles tended to aggregate. For this reason, a compromise between the size and the stability of dispersion is necessary in order to develop efficient systems [10]. On the other hand, androsterone [11] is an important and well-known metabolite of testosterone, the most prevalent androgen in males. The conjugation of steroids to other chemically or biologically relevant molecules is a common approach in the search for new biomedical and chemical applications. The coupling of C60 with a steroid changes the physicochemical properties of this molecule, improving its solubility and biocompatibility, thus facilitating further bioactivity studies [12]. The morphology and aggregation properties of some fullerene derivatives have been previously established. Brettreich et al., demonstrated that hexa-adducts of [60]fullerene functionalized with both hydrophobic and hydrophilic moieties, form spherical vesicles known as “buckysomes” in aqueous media [13]. The same behavior has also been observed by Conyers et al. [14] and Martin et al. [15] for other fullerene derivatives. As representative examples, the pentamethyl[60]fullerene salt Me5C60K and the pentaphenyl[60]fullerene salt Ph5C60K have a tendency to form closed submicron spheres [16,17]. To the best of our knowledge, all reported C60–steroid derivatives have been spectroscopically characterized. However, the aggregation properties of these entities in water have not been studied to date.
Here we report on the morphological characterization of four fullerene–androsterone conjugates in water. The morphology and particle size of the C60–androsterone hybrids in aqueous solution were determined by transmission electron microscopy (TEM) and dynamic light scattering (DLS).
Results and Discussion
The major drawback of [60]fullerene is its low solubility in water and others solvents, except toluene or o-DCB. The water-soluble C60–androsterone conjugates Ia, Ib, IIa and IIb (Figure 1) were prepared in a multistep synthetic procedure by following the recently reported methodology [18]. The C60 unit was covalently connected to the steroid moiety via a Prato reaction to obtain a diasteromeric mixture of I, with the hydrophobic C60 appendage in the A ring of the steroid moiety (Ia and Ib), and II, in which the steroid D ring was modified (IIa and IIb). These two mixtures of diastereomers were easily separated by flash chromatography, which allowed pure derivatives to be obtained as stable brown solids [17]. The aggregation properties of C60–androsterone conjugates (Ia,b and IIa,b) were subsequently studied by TEM and DLS techniques.
Figure 1: Chemical structure of the different C60–androsterone hybrids.
Figure 1: Chemical structure of the different C60–androsterone hybrids.
Transmission electron microscopy
Uranyl acetate negative stain TEM was performed on the four C60–steroid derivatives (Ia,b and IIa,b) in aqueous solution. In general, the TEM imaging of the C60–androsterone nanoparticles showed that they are polydisperse and mainly spherical, with slight angular features (Figure 2 and Supporting Information File 1). In order to ensure a representative analysis of each sample, several areas of the grids were observed. The two diastereomeric pairs (Ia,b and IIa,b) showed spherical self-assembly due to the non-covalent interactions present in these compounds, i.e., van der Waals forces, hydrogen bonding, hydrophilic/hydrophobic interactions, π–π stacking interactions, and donor–acceptor interactions. The aggregation behavior of these compounds was associated to the hydrophobic nature of the C60 core as the main attractive force, furthermore, the presence of different polar groups, carbonyl and acetoxy group for Ia,b and IIa,b respectively, in the appendage of these monoadducts, may be responsible of the difference size between both diastereomeric pairs, considering the different strength of the hydrogen bonds between these groups and water. Similar behavior has been observed in C60 monoadducts with one or two hydrophilic heads [19-22].
![[2190-4286-5-43-2]](/bjnano/content/figures/2190-4286-5-43-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Representatives uranyl acetate negative stain transmission electron micrographs (TEM) of fullerene–androsterone conjugates.
Figure 2: Representatives uranyl acetate negative stain transmission electron micrographs (TEM) of fullerene–...
For each sample, the diameters of 250 nanoparticles were determined from the TEM images by using the ImageJ software. Figure 3 shows the size distribution of the 250 nanoparticles for both diastereomeric pairs, with each bar representing a diameter range of 10 nm. The fullerene–androsterone conjugates Ia,b predominantly showed nanoparticles in the 11–20 nm size range, about 15% of the population of Ia nanoparticles were in 5–10 nm range. Furthermore, larger aggregates from 21 to 60 nm were seen, and isolated nanoparticles that measure 71–80 nm were occasionally observed for diasteromer Ia. Particles larger than 70 nm were not observed for the diasteromer Ib (Figure 3A). The other diasteromer pairs IIa,b showed slightly larger nanoparticles than Ia,b, as well as a more heterogeneous population distribution, although with a predominant size in the range of 31–50 nm (Figure 3B).
![[2190-4286-5-43-3]](/bjnano/content/figures/2190-4286-5-43-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Size distributions of 250 nanoparticles of C60–androsterone (A) Ia,b and (B) IIa,b adsorbed on the TEM grids.
Figure 3: Size distributions of 250 nanoparticles of C60–androsterone (A) Ia,b and (B) IIa,b adsorbed on the ...
The size and shape of these aggregates can be attributed to the hydrophilic moiety that is linked to [60]fullerene in the C60–androsterone derivatives (Ia,b and IIa,b). The steroid linked to fullerene promotes hydrophilicity and steric repulsion to offset the C60 fragment, and therefore these derivatives tend to self-organize as unilamellar vesicles, and form spherical aggregates with a variety of sizes, but with a well-defined spherical shape. In general, when the C60 moiety was attached into the D rind (IIa,b) instead of the A ring (Ia,b) of the androsterone moiety, larger aggregates were obtained. These results are a consequence of the difference in hydrophilicity of the androsterone moieties in both diasteromeric pairs. The presence of a carbonyl group in Ia,b instead of a less polar group such as acetoxy in IIa,b, promoted the formation of smaller vesicles.
Dynamic light scattering
DLS measurements gave further information about the particle size of the C60–androsterone conjugates. The concentration range studied was 0.1–0.4 mg·mL−1. The histograms of the four C60–androsterone derivatives Ia,b and IIa,b in Figure 4 show the average particle size distribution in water at 0.1 mg·mL−1, in which the particles of 5–12 nm and a more broad range of 12–26 nm of hydrodynamic radius are shown for Ia,b and IIa,b, respectively. The four samples analyzed had polydispersity index values of 0.393 to 0.454, which are consistent with polydisperse samples.
![[2190-4286-5-43-4]](/bjnano/content/figures/2190-4286-5-43-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Histogram analyses of the particle size distribution by number, and solubility profile in water at 0.1 mg·mL−1 of fullerene–steroid derivatives Ia,b and IIa,b.
Figure 4: Histogram analyses of the particle size distribution by number, and solubility profile in water at ...
The histograms of particle size distribution by volume at the same concentration, of the four C60–androsterone derivatives Ia,b and IIa,b in Figure 5 show at least three distinct populations of particles. In general, the derivatives II showed mean populations of particles with slightly higher size (r = 14–30 nm) than derivatives I (r = 6–22 nm). Similar DLS results were obtained for the C60–androsterone conjugates (Ia,b and IIa,b) at 0.4 mg·mL−1 (see Supporting Information File 1).
![[2190-4286-5-43-5]](/bjnano/content/figures/2190-4286-5-43-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Histogram analyses of the particle size distribution by volume of fullerene–steroid derivatives Ia,b and IIa,b.
Figure 5: Histogram analyses of the particle size distribution by volume of fullerene–steroid derivatives Ia,b...
Although DLS and TEM techniques showed the same trend to the aggregation behaviour of both diasteromeric pairs Ia,b and IIa,b, slightly but systematic differences in vesicle size were detected. The dynamic light scattering (DLS) measures the hydrodynamic diameter of the particle including the solvation layers, while TEM gives the most direct information about the size distribution and shapes of the primary particles. DLS and TEM have different basis and can lead to discrepancies in sizing.
Conclusion
The molecular self-assembly characteristics of C60–androsterone derivatives Ia,b and IIa,b in water involve the formation of nano-sized spherical vesicles with most particles falling in the size ranges of 11–20 nm and 31–50 nm for Ia,b and IIa,b, respectively, as estimated by TEM. DLS measurements showed that all samples formed aggregates in water. The tendency of the sphere size distribution results is in good agreement with TEM micrographs. Both TEM and DLS results revealed that IIa,b derivatives are slightly larger. It should be noted that the introduction of the C60 cage in a different position on the androsterone moiety induces morphologically distinct nanostructures in water. Thus these particles have the same shape but differ in size.
Experimental
The fullerene–androsterone conjugates (Ia,b and IIa,b) were synthesized as previously described [18]. The nanoparticle solutions were made by dissolving the corresponding sample (10 mg) in H2O (10 mL) with ultrasonication for 2 h, followed by centrifugation (10 min, 3000 rpm) and the transparent brown suspension was transferred to a clean vessel. The resulting suspension was analysed by TEM and DLS.
Transmission electron microscopy
The aggregates were visualized using uranyl acetate negative staining. One drop of the clear solution was transferred to a TEM grid (copper grid, 3.0 mm, 200 mesh, coated with Formvar film), together with a drop of uranyl acetate (2% water solution) for 1 min, and allowed to dry. Analysis of stained grids was performed with a JEOL JEM 2100 (Tokyo, Japan) Transmission Electron Microscope.
Dynamic light scattering
Light scattering experiments were performed on a Zetasizer Nano S ZS90 (Malvern Instruments, USA). Data were collected at 25 °C by monitoring the scattered light intensity. The hydrodynamic radius and the polydispersity index of the samples were calculated by using the CONTIN analysis software. Each light scattering measurement was performed at least three times.
Supporting Information
| Supporting Information File 1: Additional TEM images and DLS data for the C60–androsterone conjugates Ia,b and IIa,b. | ||
| Format: PDF | Size: 2.1 MB | Download |
References
-
Kroto, H. W.; Heath, J. R.; O'Brien, S. C.; Curl, R. F.; Smalley, R. E. Nature 1985, 318, 162–163. doi:10.1038/318162a0
Return to citation in text: [1] -
Izquierdo, M.; Filippone, S.; Martin-Domenech, A.; Martin, N. New reactivity in fullerene chemistry. In Handbook of Carbon Nano Materials; D'Souza, F.; Kadish, K. M., Eds.; World Scientific: Singapore, 2011; pp 33–58.
Return to citation in text: [1] -
Feng, L.; Slanina, Z.; Sato, S.; Yoza, K.; Tsuchiya, T.; Mizorogi, N.; Akasaka, T.; Nagase, S.; Martin, N.; Guldi, D. M. Angew. Chem., Int. Ed. 2011, 50, 5909–5912. doi:10.1002/anie.201100432
Return to citation in text: [1] -
Villegas, C.; Krokos, E.; Bouit, P.-A.; Delgado, J. L.; Guldi, D. M.; Martin, N. Energy Environ. Sci. 2011, 4, 679–684. doi:10.1039/c0ee00497a
Return to citation in text: [1] -
Hu, Z.; Guan, W.; Wang, W.; Huang, L.; Xing, H.; Zhu, Z. Cell Biol. Int. 2007, 31, 798–804. doi:10.1016/j.cellbi.2007.01.013
Return to citation in text: [1] -
Toniolo, C.; Bianco, A.; Maggini, M.; Scorrano, G.; Prato, M.; Marastoni, M.; Tomatis, R.; Spisani, S.; Palu, G.; Blair, E. D. J. Med. Chem. 1994, 37, 4558–4562. doi:10.1021/jm00052a015
Return to citation in text: [1] -
Bjelakovic, M.; Todorović, N.; Milić, D. Eur. J. Org. Chem. 2012, 5291–5300. doi:10.1002/ejoc.201200274
Return to citation in text: [1] -
Zhou, Z.; Lin, Z.-X.; Liang, D.; Hu, J.-Q. Tetrahedron 2013, 69, 43–49. doi:10.1016/j.tet.2012.10.071
Return to citation in text: [1] -
Coro, J.; Rodríguez, H.; Rivera, D. G.; Suárez, M.; Molero, D.; Herranz, M. Á.; Martínez-Álvarez, R.; Filippone, S.; Martín, N. Eur. J. Org. Chem. 2009, 4810–4817. doi:10.1002/ejoc.200900583
Return to citation in text: [1] -
Gavande Sunil, D.; Salunke Hemant, D.; Ughade Prajakta, L.; Baviskar Dheeraj, T.; Jain Dinesh, K. J. J. Pharm. Res. (Gurgaon, India) 2012, 5, 169–173.
Return to citation in text: [1] -
Raeside, J. I.; Renaud, R. L.; Marshall, D. E. J. Steroid Biochem. Mol. Biol. 1992, 42, 113–120. doi:10.1016/0960-0760(92)90017-D
Return to citation in text: [1] -
Li, L.-S.; Hu, Y.-J.; Wu, Y.; Wu, Y.-L.; Yue, J.; Yang, F. J. Chem. Soc., Perkin Trans. 1 2001, 617–621. doi:10.1039/b007498p
Return to citation in text: [1] -
Brettreich, M.; Burghardt, S.; Bottcher, C.; Bayerl, T.; Bayerl, S.; Hirsch, A. Angew. Chem., Int. Ed. 2000, 39, 1845–1848. doi:10.1002/(SICI)1521-3773(20000515)39:10<1845::AID-ANIE1845>3.0.CO;2-Q
Return to citation in text: [1] -
Partha, R.; Lackey, M.; Hirsch, A.; Casscells, S. W.; Conyers, J. L. NanoBiotechnology 2007, 5, No. 6. doi:10.1186/1477-3155-5-6
Return to citation in text: [1] -
Muñoz, A.; Illesca, B. M.; Sánchez-Navarro, M.; Rojo, J.; Martín, N. J. Am. Chem. Soc. 2011, 133, 16758–16761. doi:10.1021/ja206769a
Return to citation in text: [1] -
Burger, C.; Hao, J.; Ying, Q.; Isobe, H.; Sawamura, M.; Nakamura, E.; Chu, B. J. Colloid Interface Sci. 2004, 275, 632–641. doi:10.1016/j.jcis.2004.02.048
Return to citation in text: [1] -
Zhou, S.; Burger, C.; Chu, B.; Sawamura, M.; Nagahama, N.; Toganoh, M.; Hackler, U. E.; Isobe, H.; Nakamura, E. Science 2001, 291, 1944–1947. doi:10.1126/science.291.5510.1944
Return to citation in text: [1] [2] -
Ruiz, A.; Coro, J.; Almagro, L.; Ruíz, J. A.; Molero, D.; Maroto, E. E.; Filippone, S.; Herranz, M. Á.; Martínez-Álvarez, R.; Sancho-García, J. C.; di Meo, F.; Suárez, M.; Martín, N. J. Org. Chem. 2013, 78, 2819–2826. doi:10.1021/jo302528t
Return to citation in text: [1] [2] -
Verma, S.; Hauck, T.; El-Khouly, M. E.; Padmawar, P. A.; Canteenwala, T.; Pritzker, K.; Ito, O.; Chiang, L. Y. Langmuir 2005, 21, 3267–3327. doi:10.1021/la047082f
Return to citation in text: [1] -
Yang, J.; Li, L.; Wang, C. Macromolecules 2003, 36, 6060–6065. doi:10.1021/ma025909b
Return to citation in text: [1] -
Li, H.; Babu, S. S.; Nakanishi, T. Supramolecular Chemistry of Fullerene-Containing Micelles and Gels. In Supramolecular Chemistry of Fullerenes and Carbon Nanotubes; Martín, N.; Nierengarten, J.-F., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp 159–172. doi:10.1002/9783527650125.ch7
Return to citation in text: [1] -
Braun, M.; Hartnagel, U.; Ravanelli, E.; Schade, B.; Böttcher, C.; Vostrowsky, O.; Hirsch, A. Eur. J. Org. Chem. 2004, 1983–2001. doi:10.1002/ejoc.200300663
Return to citation in text: [1]
| 19. | Verma, S.; Hauck, T.; El-Khouly, M. E.; Padmawar, P. A.; Canteenwala, T.; Pritzker, K.; Ito, O.; Chiang, L. Y. Langmuir 2005, 21, 3267–3327. doi:10.1021/la047082f |
| 20. | Yang, J.; Li, L.; Wang, C. Macromolecules 2003, 36, 6060–6065. doi:10.1021/ma025909b |
| 21. | Li, H.; Babu, S. S.; Nakanishi, T. Supramolecular Chemistry of Fullerene-Containing Micelles and Gels. In Supramolecular Chemistry of Fullerenes and Carbon Nanotubes; Martín, N.; Nierengarten, J.-F., Eds.; Wiley-VCH: Weinheim, Germany, 2012; pp 159–172. doi:10.1002/9783527650125.ch7 |
| 22. | Braun, M.; Hartnagel, U.; Ravanelli, E.; Schade, B.; Böttcher, C.; Vostrowsky, O.; Hirsch, A. Eur. J. Org. Chem. 2004, 1983–2001. doi:10.1002/ejoc.200300663 |
| 18. | Ruiz, A.; Coro, J.; Almagro, L.; Ruíz, J. A.; Molero, D.; Maroto, E. E.; Filippone, S.; Herranz, M. Á.; Martínez-Álvarez, R.; Sancho-García, J. C.; di Meo, F.; Suárez, M.; Martín, N. J. Org. Chem. 2013, 78, 2819–2826. doi:10.1021/jo302528t |
| 1. | Kroto, H. W.; Heath, J. R.; O'Brien, S. C.; Curl, R. F.; Smalley, R. E. Nature 1985, 318, 162–163. doi:10.1038/318162a0 |
| 5. | Hu, Z.; Guan, W.; Wang, W.; Huang, L.; Xing, H.; Zhu, Z. Cell Biol. Int. 2007, 31, 798–804. doi:10.1016/j.cellbi.2007.01.013 |
| 18. | Ruiz, A.; Coro, J.; Almagro, L.; Ruíz, J. A.; Molero, D.; Maroto, E. E.; Filippone, S.; Herranz, M. Á.; Martínez-Álvarez, R.; Sancho-García, J. C.; di Meo, F.; Suárez, M.; Martín, N. J. Org. Chem. 2013, 78, 2819–2826. doi:10.1021/jo302528t |
| 4. | Villegas, C.; Krokos, E.; Bouit, P.-A.; Delgado, J. L.; Guldi, D. M.; Martin, N. Energy Environ. Sci. 2011, 4, 679–684. doi:10.1039/c0ee00497a |
| 17. | Zhou, S.; Burger, C.; Chu, B.; Sawamura, M.; Nagahama, N.; Toganoh, M.; Hackler, U. E.; Isobe, H.; Nakamura, E. Science 2001, 291, 1944–1947. doi:10.1126/science.291.5510.1944 |
| 3. | Feng, L.; Slanina, Z.; Sato, S.; Yoza, K.; Tsuchiya, T.; Mizorogi, N.; Akasaka, T.; Nagase, S.; Martin, N.; Guldi, D. M. Angew. Chem., Int. Ed. 2011, 50, 5909–5912. doi:10.1002/anie.201100432 |
| 15. | Muñoz, A.; Illesca, B. M.; Sánchez-Navarro, M.; Rojo, J.; Martín, N. J. Am. Chem. Soc. 2011, 133, 16758–16761. doi:10.1021/ja206769a |
| 2. | Izquierdo, M.; Filippone, S.; Martin-Domenech, A.; Martin, N. New reactivity in fullerene chemistry. In Handbook of Carbon Nano Materials; D'Souza, F.; Kadish, K. M., Eds.; World Scientific: Singapore, 2011; pp 33–58. |
| 16. | Burger, C.; Hao, J.; Ying, Q.; Isobe, H.; Sawamura, M.; Nakamura, E.; Chu, B. J. Colloid Interface Sci. 2004, 275, 632–641. doi:10.1016/j.jcis.2004.02.048 |
| 17. | Zhou, S.; Burger, C.; Chu, B.; Sawamura, M.; Nagahama, N.; Toganoh, M.; Hackler, U. E.; Isobe, H.; Nakamura, E. Science 2001, 291, 1944–1947. doi:10.1126/science.291.5510.1944 |
| 11. | Raeside, J. I.; Renaud, R. L.; Marshall, D. E. J. Steroid Biochem. Mol. Biol. 1992, 42, 113–120. doi:10.1016/0960-0760(92)90017-D |
| 13. | Brettreich, M.; Burghardt, S.; Bottcher, C.; Bayerl, T.; Bayerl, S.; Hirsch, A. Angew. Chem., Int. Ed. 2000, 39, 1845–1848. doi:10.1002/(SICI)1521-3773(20000515)39:10<1845::AID-ANIE1845>3.0.CO;2-Q |
| 10. | Gavande Sunil, D.; Salunke Hemant, D.; Ughade Prajakta, L.; Baviskar Dheeraj, T.; Jain Dinesh, K. J. J. Pharm. Res. (Gurgaon, India) 2012, 5, 169–173. |
| 14. | Partha, R.; Lackey, M.; Hirsch, A.; Casscells, S. W.; Conyers, J. L. NanoBiotechnology 2007, 5, No. 6. doi:10.1186/1477-3155-5-6 |
| 8. | Zhou, Z.; Lin, Z.-X.; Liang, D.; Hu, J.-Q. Tetrahedron 2013, 69, 43–49. doi:10.1016/j.tet.2012.10.071 |
| 9. | Coro, J.; Rodríguez, H.; Rivera, D. G.; Suárez, M.; Molero, D.; Herranz, M. Á.; Martínez-Álvarez, R.; Filippone, S.; Martín, N. Eur. J. Org. Chem. 2009, 4810–4817. doi:10.1002/ejoc.200900583 |
| 6. | Toniolo, C.; Bianco, A.; Maggini, M.; Scorrano, G.; Prato, M.; Marastoni, M.; Tomatis, R.; Spisani, S.; Palu, G.; Blair, E. D. J. Med. Chem. 1994, 37, 4558–4562. doi:10.1021/jm00052a015 |
| 7. | Bjelakovic, M.; Todorović, N.; Milić, D. Eur. J. Org. Chem. 2012, 5291–5300. doi:10.1002/ejoc.201200274 |
| 12. | Li, L.-S.; Hu, Y.-J.; Wu, Y.; Wu, Y.-L.; Yue, J.; Yang, F. J. Chem. Soc., Perkin Trans. 1 2001, 617–621. doi:10.1039/b007498p |
© 2014 Ruiz et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Nanotechnology terms and conditions: (http://www.beilstein-journals.org/bjnano)