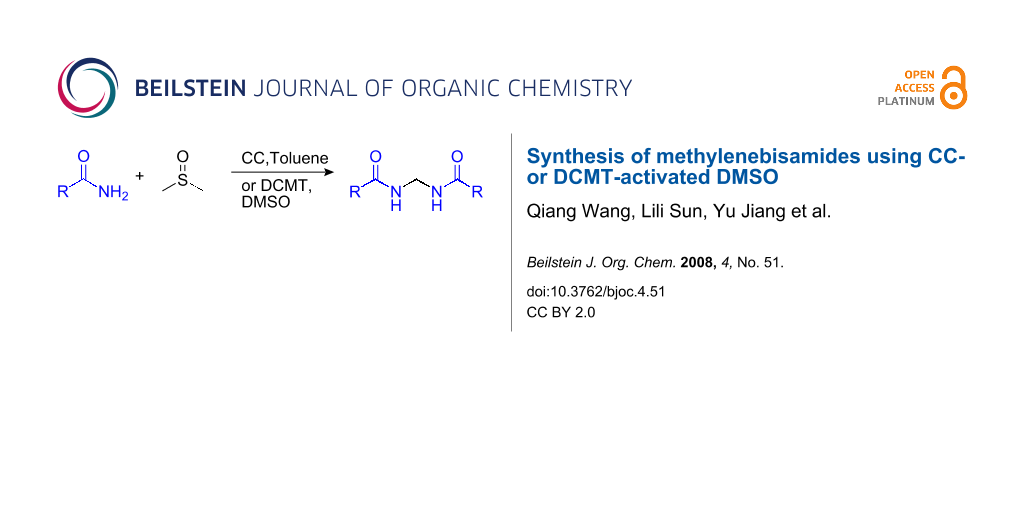
Abstract
Bisamides are key fragments for the introduction of gem-diaminoalkyl residues into retroinverso pseudopeptide derivatives and in the synthesis of peptidomimetic compounds. The literature methods for these types of compounds have certain drawbacks. In particular, when amides react with electrophile-activated DMSO, the yields are rather low. We have found new electrophiles, 2,4,6-trichloro[1,3,5]triazine (CC) and 2,4-dichloro-6-methoxy[1,3,5]triazine (DCMT), which activate DMSO in the presence of amides to yield methylenebisamides in good to fair yields. The amides can be aromatic or aliphatic. The operation is simple and the reagents are inexpensive.
Graphical Abstract
Introduction
Sulfoxides are activated by electrophiles to produce reactive sulfonium salts. These electrophiles include DCC [1,2], acetic anhydride [3], trifluoroacetic anhydride [4], acetyl chloride [5], phosphorus pentoxide [6], polyphosphoric acid [7], sulfuric acid and sulfur trioxide [6] etc. A few reactions such as the Pummerer reaction [8] and Swern oxidation [9] can be performed via the reactive sulfonium salts formed from DMSO and electrophiles.
The reactions of amides with DMSO in presence of electrophiles (sulfur trioxide, acetic anhydride, phosphorus pentoxide) give N,N′-methylenebisamides and N-acylsulfilimines, but rather low yields of N,N′-methylenebisamides (20%) [6]. From our previous research on the chlorination [10] and etherification [11] of benzyl alcohols and from other references [12-14], we believe the reaction between 2,4,6-trichloro[1,3,5]triazine (cyanogen chloride, or CC) and DMSO produces a reactive sulfonium salt intermediate. Therefore, it was of interest to study the reaction of nucleophilic reagents such as amides, alcohols, and phenols with DMSO activated by CC.
The amide moiety is an important constituent of many biologically significant compounds. Bisamides are of considerable interest in the synthesis of peptidomimetic compounds [15]. In particular, bisamides are key fragments for the introduction of gem-diaminoalkyl residues in retro-inverso pseudopeptide derivatives [16] by treating the corresponding amide with iodobenzene bistrifluoroacetate [17]. N,N′-Methylenebisamides are usually prepared by the reactions of amides with aldehydes [18-20], hexamethylenetetramine [21] or activated DMSO [6], or by the reaction of nitriles with formaldehyde [22] or activated sulfoxides [23]. However, each method has certain limitations with regards to scope and reaction conditions, for example, longer reaction time [20], lower yield [6], purification problems [21,23] and drastic reaction conditions [19,22].
In this paper, we report the reaction of amides with DMSO activated by CC or 2,4-dichloro-6-methoxy[1,3,5]triazine (DCMT) [24].
Results and Discussion
Initially, we chose CC as the activation reagent and benzamide as a model substrate to optimize the reaction conditions (Scheme 1, Table 1). No product was observed when the reaction was carried out in CH3CN (Table 1, entry 1). The reaction proceeded in CHCl3, DMSO, EtOAc and toluene (Table 1, entries 2–5), but considerable amounts of undesired products were formed in CHCl3, DMSO and EtOAc (Table 1, entries 2–4). Better results were obtained when the reactions were performed in toluene (Table 1, entry 5). Encouraged by these results, we studied the effects of temperature on the reaction in toluene. Elevating the temperature to 70 °C resulted in an improved reaction rate. As for the influence of CC and DMSO dosage on the reaction, it was found that decreasing the amount of CC to 0.9 equiv resulted in reduced yield, while increasing the amount to 1.5 equiv did not make the reaction system complex and the yield was not notably different. Excess amount of DMSO (7.0 equiv) was used partially because of its ability to dissolve the amides.
Scheme 1: Synthesis of N,N′-methylenedibenzamide using CC-activated DMSO.
Scheme 1: Synthesis of N,N′-methylenedibenzamide using CC-activated DMSO.
Table 1: Optimization studies for the synthesis of N,N′-methylenedibenzamide using CC-activated DMSOa.
| Entry | Solvent | Temp (°C) | t (h) | Yield (%)b |
|---|---|---|---|---|
| 1 | CH3CN | RT | 24 | 0 |
| 2 | CHCl3 | RT | 4 | 10c |
| 3 | DMSO | RT | 24 | 26 |
| 4 | EtOAc | RT | 4 | 30 |
| 5 | Toluene | RT | 4 | 70 |
| 6 | Toluene | RT to 70 | 2 | 71d |
a1.0 equiv amide, 1.2 equiv CC, 7.0 equiv dry DMSO, dry toluene (8.0 mL). bIsolated yield; cS,S-Dimethyl-N-benzoylsulfilimine (40% yield) as major product; dStirred for 30 min at RT, then 70 °C for 1.5 h.
After the optimization of the reaction conditions, various substrates were subjected to the conditions (Scheme 2, Table 2). The electronic effect of the substituents on aromatic rings was observed. For example, a strong electron-donating or electron-withdrawing group on the aromatic ring resulted in decreased reaction yields (Table 2, entries 5, 8), while a substituent on the para-position or meta-position led to a moderate yield (Table 2, entries 4, 7). The effect of substituents on the reaction rate was not remarkable. However, the method was not efficient for the aliphatic amides: considerable amounts of by-products were formed, probably due to the greater nucleophilicity of the aliphatic amides.
Scheme 2: Synthesis of N,N′-methylenebisamide using CC-activated DMSO.
Scheme 2: Synthesis of N,N′-methylenebisamide using CC-activated DMSO.
Table 2: Scope of the synthesis of N,N′-methylenebisamide using CC-activated DMSOa.
| Entry | R | t (h) | Yield (%)b |
|---|---|---|---|
| 1 | Ph | 2 | 71 |
| 2 | 4-CH3C6H4 | 4 | 86 |
| 3 | 4-MeOC6H4 | 2 | 72 |
| 4 | 2-MeOC6H4 | 1.5 | 67 |
| 5 | 3,5-(MeO)2C6H3 | 4 | 30 |
| 6 | 4-ClC6H4 | 5 | 74 |
| 7 | 3-ClC6H4 | 4 | 60 |
| 8 | 4-NO2C6H4 | 4 | 20 |
| 9 | PhCH=CH | 3 | 50 |
| 10 | PhCH2 | – | –c |
| 11 | PhOCH2 | – | –c |
| 12 | (CH3)3C | – | –c |
| 13 | CH3(CH2)5CH2 | – | –c |
a1.0 equiv amide, 1.2 equiv CC, 7.0 equiv dry DMSO, dry toluene (8.0 mL). bIsolated yield; cComplicated products.
In order to improve the yield of aliphatic amides, less active DCMT was used as activating reagent instead of CC. We also optimized the reaction conditions using benzamide as a benchmark (Scheme 3, Table 3). The reaction did not proceed when low boiling solvents such as CH3CN, CHCl3 and ClCH2CH2Cl were used (Table 3, entries 1–3). It was performed efficiently in high boiling solvents (1,4-dioxane, xylene and DMSO) (Table 3, entries 4–6). The results indicate that the effect of temperature on the reaction is remarkable. The electronic effect of the substituents on aromatic rings was similar to the results when CC was used as an activating reagent (Scheme 4, Table 4, entries 5, 8). The system of DCMT/DMSO was efficient for aliphatic amides (Scheme 4, Table 4, entries 10–13).
Scheme 3: Synthesis of N,N′-methylenedibenzamide using DCMT-activated DMSO.
Scheme 3: Synthesis of N,N′-methylenedibenzamide using DCMT-activated DMSO.
Table 3: Optimization studies for the synthesis of N,N′-methylenedibenzamide using DCMT-activated DMSOa.
| Entry | Solvent | Temp (°C) | t (h) | Yield (%)a |
|---|---|---|---|---|
| 1 | CH3CN | Reflux | 24 | 0 |
| 2 | CHCl3 | Reflux | 24 | 0 |
| 3 | 1,2-Dichloroethane | Reflux | 24 | 0 |
| 4 | Dioxane | 100 | 3 | 75 |
| 5 | Xylene | 100 | 3 | 70 |
| 6 | DMSO | 100 | 1 | 75 |
a1.0 equiv amide, 1.5 equiv DCMT, dry DMSO (4.0 mL), 100 °C. b Isolated yield.
Scheme 4: Synthesis of N,N′-methylenebisamide using DCMT-activated DMSO.
Scheme 4: Synthesis of N,N′-methylenebisamide using DCMT-activated DMSO.
Table 4: Scope of the synthesis of N,N′-methylenebisamide using DCMT-activated DMSOa.
| Entry | R | t (h) | Yield (%)b |
|---|---|---|---|
| 1 | Ph | 1 | 75 |
| 2 | 4-CH3C6H4 | 1 | 77 |
| 3 | 4-MeOC6H4 | 1 | 70 |
| 4 | 2-MeOC6H4 | 0.5 | 52 |
| 5 | 3,5-(MeO)2C6H3 | 3c | 50 |
| 6 | 4-ClC6H4 | 1 | 88 |
| 7 | 3-ClC6H4 | 1.5 | 54 |
| 8 | 4-NO2C6H4 | 3 | 28 |
| 9 | PhCH=CH | 1 | 55 |
| 10 | PhCH2 | 4 | 45 |
| 11 | PhOCH2 | 2 | 62 |
| 12 | (CH3)3C | 1 | 60 |
| 13 | CH3(CH2)5CH2 | 3 | 20d |
a1.0 equiv amide, 1.5 equiv DCMT, dry DMSO (4.0 mL), 100 °C. b Isolated yield; cThe reaction performed at 70 °C; dN-(Methylthiomethyl)octanamide (40% yield) as major product.
Based on these experiments, a possible mechanism [6,23,25] is shown in Scheme 5. Intermediate 1 reacted with amides by two pathways. When the reaction of benzamide and CC-activated DMSO was carried out in chloroform, S,S-dimethyl-N-benzoylsulfilimine (4) was isolated as a major product, most probably formed by the attack of the amide on the sulfonium ion 1. When the reaction is carried out in toluene, intermediate 1 decomposes into intermediate 2. Thioether 3 is formed by the addition of the amide to intermediate 2. N-(Methylthiomethyl)octanamide (40% yield) was isolated when octanamide was treated with DCMT in DMSO. Similarly, N-(1-(methylthio)-2-oxo-2-phenylethyl)benzamide (30% yield) was isolated when benzamide reacted with DCMT and 2-(methylsulfinyl)-1-phenylethanone. Thioether 3 is a good nucleophile and capable to substitute the chloride of CC or DCMT to generate sulfonium salt 5. The amide substitutes the thioether of 5 to form methylenebisamides 6.
Scheme 5: Plausible reaction mechanism of amide with CC- or DCMT-activated DMSO.
Scheme 5: Plausible reaction mechanism of amide with CC- or DCMT-activated DMSO.
Conclusion
In conclusion, we have developed a simple and efficient procedure to produce methylenebisamides in good yield via the reaction of amides with CC- or DCMT-activated DMSO. The procedure reported herein is operationally simple, and requires inexpensive and commercially available reagents. A plausible mechanism of the reaction which involves two sulfonium salt intermediates was proposed and supported by the experiments.
Experimental
All chemicals were obtained from commercial sources or prepared according to standard methods [24]. All chemicals and solvents used in the reactions were dried by standard procedures prior to use. IR spectra were recorded on a Bio-Rad Exalibur FTS3000 spectrometer. The 1H NMR (500 MHz) and 13C NMR (125 MHz) were recorded on a Varian Oxford 500 spectrometer. Chemical shifts (δ) are reported relative to TMS (1H) in DMSO-d6 or CDCl3 (13C). Mass spectra were obtained using an LCQ Advantage MAX ion trap mass spectrometer equipped with electrospray ionization (ESI) ion source or a Thermo Finnigan TRACE-DSQ spectrometer. Elemental analyses for C, H and N were performed on a Yanaco CHNCORNER MF-3 elemental analyzer, and the analytical results were within ±0.4% of the theoretical values.
Typical experimental procedure
N,N′-Methylenedibenzamide (Table 2, entry 1)
A mixture of amide (121.4 mg, 1.0 mmol, 1 equiv), CC (222 mg, 1.2 mmol, 1.2 equiv) and dry DMSO (0.5 mL, 7.0 mmol, 7.0 equiv) in dry toluene (8.0 mL) was stirred for 30 min at room temperature. The reaction temperature was then kept at 70 °C for 1.5 h until completion. The mixture was neutralized with saturated aqueous NaHCO3 (20 mL), then extracted with EtOAc (3 × 20 mL). The extract was washed with brine (4 × 15 mL), dried over anhydrous Na2SO4. The solvent was concentrated in vacuo to give the crude product, which was further purified by silica gel column chromatography (PE/EA = 1/1) to afford N,N′-methylenedibenzamide (90.1 mg, 71% yield).
N,N′-Methylenedibenzamide (Table 4, entry 1)
A mixture of amide (121.4 mg, 1.0 mmol, 1 equiv) and DCMT (270 mg, 1.5 mmol, 1.5 equiv) in dry DMSO (4.0 mL) was stirred at 100 °C and monitored by TLC until completion (1.0 h). The working up was similar to Table 2, entry 1 (95.3 mg, 75% yield).
References
-
Pfitzner, K. E.; Moffatt, J. G. J. Am. Chem. Soc. 1965, 87, 5661–5670. doi:10.1021/ja00952a026
Return to citation in text: [1] -
Lerch, U.; Moffatt, J. G. J. Org. Chem. 1971, 36, 3861–3869. doi:10.1021/jo00824a004
Return to citation in text: [1] -
Albright, J. D.; Goldman, L. J. Am. Chem. Soc. 1965, 87, 4214–4216. doi:10.1021/ja01096a055
Return to citation in text: [1] -
Hiraki, Y.; Kamiya, M.; Tanikaga, R.; Ono, N.; Kaji, A. Bull. Chem. Soc. Jpn. 1977, 50, 447–452. doi:10.1246/bcsj.50.447
Return to citation in text: [1] -
Harris, T. D.; Boekelheide, V. J. Org. Chem. 1976, 41, 2770–2772. doi:10.1021/jo00878a028
Return to citation in text: [1] -
Varkey, T. E.; Whitfield, G. F.; Swern, D. J. Org. Chem. 1974, 39, 3365–3372. doi:10.1021/jo00937a013
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Claus, P.; Vycudilik, W. Tetrahedron Lett. 1968, 9, 3607–3610. doi:10.1016/S0040-4039(00)75512-2
Return to citation in text: [1] -
De Lucchi, O.; Miotti, U.; Modena, G. Org. React. 1991, 40, 157–405.
Return to citation in text: [1] -
Epstein, W. W.; Sweat, F. W. Chem. Rev. 1967, 67, 247–260. doi:10.1021/cr60247a001
Return to citation in text: [1] -
Sun, L.; Pei, G.; Niu, H.; Wang, Q.; Li, C. Synthesis 2008, in press.
Return to citation in text: [1] -
Sun, L.; Pei, G.; Li, C. Synthesis 2008, in press.
Return to citation in text: [1] -
Albright, J. D. J. Org. Chem. 1974, 39, 1977–1979. doi:10.1021/jo00927a054
Return to citation in text: [1] -
Blotny, G. Tetrahedron 2006, 62, 9507–9522. doi:10.1016/j.tet.2006.07.039
Return to citation in text: [1] -
Giacomelli, G.; Porcheddu, A.; Luca, L. D. Curr. Org. Chem. 2004, 8, 1497–1519. doi:10.2174/1385272043369845
Return to citation in text: [1] -
Alemán, C.; Puiggali, J. J. Org. Chem. 1995, 60, 910–924. doi:10.1021/jo00109a023
Return to citation in text: [1] -
Pallai, P. V.; Struthers, R. S.; Goodman, M.; Moroder, L.; Wunsch, E.; Vale, W. Biochemistry 1985, 24, 1933–1941. doi:10.1021/bi00329a020
Return to citation in text: [1] -
Rodriguez, M.; Dubreuil, P.; Bali, J.-P.; Martinez, J. J. Med. Chem. 1987, 30, 758–763. doi:10.1021/jm00388a002
Return to citation in text: [1] -
Fernández, A. H.; Alvarez, R. M.; Abajo, T. M. Synthesis 1996, 1299–1301. doi:10.1055/s-1996-4385
Return to citation in text: [1] -
Gilbert, E. E. Synthesis 1972, 30–32. doi:10.1055/s-1972-21820
Return to citation in text: [1] [2] -
Brian, R. C.; Lamberton, A. H. J. Chem. Soc. 1949, 1633–1635. doi:10.1039/jr9490001633
Return to citation in text: [1] [2] -
Sauer, C. W.; Bruni, R. J. J. Am. Chem. Soc. 1955, 77, 2559–2560. doi:10.1021/ja01614a064
Return to citation in text: [1] [2] -
Magat, E. E.; Faris, B. F.; Reith, J. E.; Salisbury, L. F. J. Am. Chem. Soc. 1951, 73, 1028–1031. doi:10.1021/ja01147a042
Return to citation in text: [1] [2] -
Bochkareva, N. N.; Trub, E. P. J. Gen. Chem. USSR 1984, 54, 619–625.
Return to citation in text: [1] [2] [3] -
Ortéga, F.; Bastide, J. Bioorg. Chem. 1997, 25, 261–274. doi:10.1006/bioo.1997.1071
Return to citation in text: [1] [2] -
Kise, H.; Whitfield, G. F.; Swern, D. J. Org. Chem. 1972, 37, 1121–1125. doi:10.1021/jo00973a010
Return to citation in text: [1]
| 24. | Ortéga, F.; Bastide, J. Bioorg. Chem. 1997, 25, 261–274. doi:10.1006/bioo.1997.1071 |
| 1. | Pfitzner, K. E.; Moffatt, J. G. J. Am. Chem. Soc. 1965, 87, 5661–5670. doi:10.1021/ja00952a026 |
| 2. | Lerch, U.; Moffatt, J. G. J. Org. Chem. 1971, 36, 3861–3869. doi:10.1021/jo00824a004 |
| 6. | Varkey, T. E.; Whitfield, G. F.; Swern, D. J. Org. Chem. 1974, 39, 3365–3372. doi:10.1021/jo00937a013 |
| 16. | Pallai, P. V.; Struthers, R. S.; Goodman, M.; Moroder, L.; Wunsch, E.; Vale, W. Biochemistry 1985, 24, 1933–1941. doi:10.1021/bi00329a020 |
| 5. | Harris, T. D.; Boekelheide, V. J. Org. Chem. 1976, 41, 2770–2772. doi:10.1021/jo00878a028 |
| 17. | Rodriguez, M.; Dubreuil, P.; Bali, J.-P.; Martinez, J. J. Med. Chem. 1987, 30, 758–763. doi:10.1021/jm00388a002 |
| 4. | Hiraki, Y.; Kamiya, M.; Tanikaga, R.; Ono, N.; Kaji, A. Bull. Chem. Soc. Jpn. 1977, 50, 447–452. doi:10.1246/bcsj.50.447 |
| 12. | Albright, J. D. J. Org. Chem. 1974, 39, 1977–1979. doi:10.1021/jo00927a054 |
| 13. | Blotny, G. Tetrahedron 2006, 62, 9507–9522. doi:10.1016/j.tet.2006.07.039 |
| 14. | Giacomelli, G.; Porcheddu, A.; Luca, L. D. Curr. Org. Chem. 2004, 8, 1497–1519. doi:10.2174/1385272043369845 |
| 3. | Albright, J. D.; Goldman, L. J. Am. Chem. Soc. 1965, 87, 4214–4216. doi:10.1021/ja01096a055 |
| 15. | Alemán, C.; Puiggali, J. J. Org. Chem. 1995, 60, 910–924. doi:10.1021/jo00109a023 |
| 9. | Epstein, W. W.; Sweat, F. W. Chem. Rev. 1967, 67, 247–260. doi:10.1021/cr60247a001 |
| 6. | Varkey, T. E.; Whitfield, G. F.; Swern, D. J. Org. Chem. 1974, 39, 3365–3372. doi:10.1021/jo00937a013 |
| 7. | Claus, P.; Vycudilik, W. Tetrahedron Lett. 1968, 9, 3607–3610. doi:10.1016/S0040-4039(00)75512-2 |
| 6. | Varkey, T. E.; Whitfield, G. F.; Swern, D. J. Org. Chem. 1974, 39, 3365–3372. doi:10.1021/jo00937a013 |
| 6. | Varkey, T. E.; Whitfield, G. F.; Swern, D. J. Org. Chem. 1974, 39, 3365–3372. doi:10.1021/jo00937a013 |
| 18. | Fernández, A. H.; Alvarez, R. M.; Abajo, T. M. Synthesis 1996, 1299–1301. doi:10.1055/s-1996-4385 |
| 19. | Gilbert, E. E. Synthesis 1972, 30–32. doi:10.1055/s-1972-21820 |
| 20. | Brian, R. C.; Lamberton, A. H. J. Chem. Soc. 1949, 1633–1635. doi:10.1039/jr9490001633 |
| 21. | Sauer, C. W.; Bruni, R. J. J. Am. Chem. Soc. 1955, 77, 2559–2560. doi:10.1021/ja01614a064 |
| 24. | Ortéga, F.; Bastide, J. Bioorg. Chem. 1997, 25, 261–274. doi:10.1006/bioo.1997.1071 |
| 6. | Varkey, T. E.; Whitfield, G. F.; Swern, D. J. Org. Chem. 1974, 39, 3365–3372. doi:10.1021/jo00937a013 |
| 23. | Bochkareva, N. N.; Trub, E. P. J. Gen. Chem. USSR 1984, 54, 619–625. |
| 25. | Kise, H.; Whitfield, G. F.; Swern, D. J. Org. Chem. 1972, 37, 1121–1125. doi:10.1021/jo00973a010 |
| 21. | Sauer, C. W.; Bruni, R. J. J. Am. Chem. Soc. 1955, 77, 2559–2560. doi:10.1021/ja01614a064 |
| 23. | Bochkareva, N. N.; Trub, E. P. J. Gen. Chem. USSR 1984, 54, 619–625. |
| 19. | Gilbert, E. E. Synthesis 1972, 30–32. doi:10.1055/s-1972-21820 |
| 22. | Magat, E. E.; Faris, B. F.; Reith, J. E.; Salisbury, L. F. J. Am. Chem. Soc. 1951, 73, 1028–1031. doi:10.1021/ja01147a042 |
| 20. | Brian, R. C.; Lamberton, A. H. J. Chem. Soc. 1949, 1633–1635. doi:10.1039/jr9490001633 |
| 6. | Varkey, T. E.; Whitfield, G. F.; Swern, D. J. Org. Chem. 1974, 39, 3365–3372. doi:10.1021/jo00937a013 |
| 22. | Magat, E. E.; Faris, B. F.; Reith, J. E.; Salisbury, L. F. J. Am. Chem. Soc. 1951, 73, 1028–1031. doi:10.1021/ja01147a042 |
© 2008 Wang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)