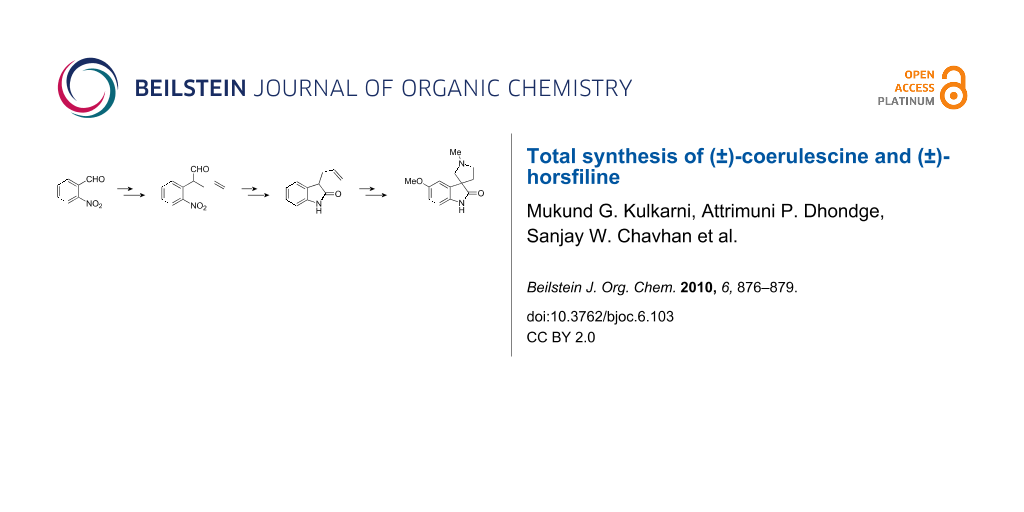
Abstract
Wittig olefination–Claisen rearrangement protocol was applied to obtain 3-allyl oxindole. This oxindole was then converted to (±)-coerulescine and (±)-horsfiline.
Graphical Abstract
Introduction
The spiro[pyrrolidin-3,3′-oxindole] ring system is a widely distributed structural framework present in a number of cytostatic alkaloids. For example, coerulescine (1) and horsfiline (2) represent the simplest prototype members of this subfamily. Coerulescine (1) was isolated from the blue canary grass, Phalaris coerulescens [1,2]. Horsfiline (2) was first isolated in 1991 by Bodo from a Malaysian medical plant, Horsfieldia superba Warb [3]. Several Myristicaceae are used as a source of intoxicating snuffs [1-3]. Other members of this subfamily, such as spirotryprostatins A and B [4,5], elacomine [6] and rychnophylline [7,8], have more complex structures. The majority of these alkaloids have interesting biological activities and pharmacological properties [9]. However, a crucial observation, reported by Danishefsky et al. [10], found that the unnatural analogous 3 and 4 (Figure 1) of the spiro[pyrrolidin-3,3′-oxindole] possessed significant activity against human breast cancer cells. This work led to intense interest in the total synthesis of these alkaloids and their derivatives. Despite previous intensive studies, the total synthesis of coerulescine (1) and horsfiline (2) remain attractive targets for demonstrating the efficacy of newer synthetic protocols.
Figure 1: Spiro[pyrrolidin-3,3'-oxindoles].
Figure 1: Spiro[pyrrolidin-3,3'-oxindoles].
Several synthetic approaches have been developed for the synthesis of the spiro[pyrrolidin-3,3'-oxindole] framework for horsfiline and coerulescine [11-34], both in racemic and enantiomeric forms. These include the following oxidative rearrangements: lead tetraacetate [3], sodium tungstate [11], tert-butyl hypochlorite [12] and N-bromosuccinimide [13]. Other approaches involve the Mannich reaction [14], ring expansion reactions [15,16], 1,3-dipolar [3 + 2] cycloadditions [17-19], intramolecular radical cyclizations [20-24], electrophilic cyclization [25], asymmetric nitroolefination reaction [26], palladium asymmetric allylic alkylation [27], palladium-catalyzed domino Heck–cyanation [28], Pd-catalyzed intramolecular cyanoamidation [29,30], NHC-mediated O- to C-carboxyl transfer [31], dimethyldioxirane (DMDO) mediated oxidation [32], and by tandem intramolecular photocycloaddition–retro-Mannich reaction [33].
The Wittig olefination–Claisen rearrangement protocol [35] provides a ready access to 4-pentenals, which have served as versatile intermediates for the synthesis of a number of natural products [36-44]. Therefore, we describe the successful application of the above protocol for the synthesis of the coerulescine (1) and horsfiline (2) (Scheme 1).
Scheme 1: Reagents and conditions: a) CH2=CHCH2OCH2P+ Ph3Cl−, t-BuO− Na+, THF, 0 °C; b) xylene, reflux; c) Jones reagent, acetone, d) H2SO4, EtOH, e) Zn, NH4Cl, EtOH, reflux, f) NaH, (Boc)2O, THF, 0 °C, g) NaH, ethyl chloroformate, THF, 0 °C, h) K2OsO4, NMO, NaIO4·SiO2, DCM, i) MeNH2·HCl, NaCNBH3, THF j) 2.5 M HCl aq. THF, reflux; k) n-BuLi, LAH, THF, l) NBS, NaOMe, CuI, reflux.
Scheme 1: Reagents and conditions: a) CH2=CHCH2OCH2P+ Ph3Cl−, t-BuO− Na+, THF, 0 °C; b) xylene, reflux; c) Jo...
Results and Discussion
The Wittig olefination of o-nitrobenzaldehyde with allyloxymethylenetriphenylphosphorane under standard conditions [35] furnished the corresponding allyl vinyl ether 5 as an inseparable mixture of E and Z isomers. However, the NMR signals of the E and Z isomers in the olefinic region were well separated, which allowed us to estimate the ratio of these isomers as 1:2. The mixture of allyl vinyl ethers was heated in refluxing xylene to effect the Claisen rearrangement to obtain 4-pentenal 6 in 85% yield. Aldehyde 6 was transformed into acid 7 by Jones oxidation, which was immediately converted to the ethyl ester 8. Subsequently, reduction of the compound 8 with Zn and NH4Cl resulted in clean cyclization leading to oxindole 9.
After protecting the amide nitrogen with Boc, the oxindole 10 was treated with NaH, followed by ethyl chloroformate at 0 °C to give 11 in 80% yield. Oxidative cleavage of the allyl group was accomplished by catalytic osmium tetroxide and N-methylmorpholine N-oxide (NMO), followed by cleavage of the diol with sodium metaperiodate on silica in methylene chloride. Reductive amination of the aldehyde 12 was conducted using methylamine hydrochloride and NaBH3CN and gave spiro-oxindole 13. The Boc group of 13 was removed by treatment with 2.5 M HCl to give 14. Finally, chemoselective reduction of amide 14 with n-BuLi and LAH (under the conditions reported in [27]) gave coerulescine. Compound 1, on treatment with N-bromosuccinimide, gave the 5-bromo derivative, which upon heating with sodium methoxide in the presence of cuprous iodide gave horsfiline in 60% yield. The physical data of synthetic coerulescine and horsfiline were comparable in all respects with the literature data.
Conclusion
In summary, we have described a new and efficient synthesis of (±)-coerulescine and (±)-horsfiline.
Supporting Information
| Supporting Information File 1: Experimental and spectral data. | ||
| Format: PDF | Size: 968.0 KB | Download |
References
-
Anderton, N.; Cockrum, P. A.; Colegate, S. M.; Edgar, J. A.; Flower, K.; Vit, I.; Willing, R. I. Phytochemistry 1998, 48, 437–439. doi:10.1016/S0031-9422(97)00946-1
Return to citation in text: [1] [2] -
Colegate, S. M.; Anderton, N.; Edgar, J.; Bourke, C. A.; Oram, R. N. Aust. Vet. J. 1999, 77, 537–538.
Return to citation in text: [1] [2] -
Jossang, A.; Jossang, P.; Hadi, H. A.; Sevenet, T.; Bodo, B. J. Org. Chem. 1991, 56, 6527–6530. doi:10.1021/jo00023a016
Return to citation in text: [1] [2] [3] -
Cui, C.-B.; Kakeya, H.; Osada, H. Tetrahedron 1996, 52, 12651–12666. doi:10.1016/0040-4020(96)00737-5
Return to citation in text: [1] -
Cui, C.-B.; Kakeya, H.; Osada, H. J. Antibiot. 1996, 49, 832–835.
Return to citation in text: [1] -
James, M. N. G.; Williams, G. J. B. Can. J. Chem. 1972, 50, 2407–2412. doi:10.1139/v72-386
Return to citation in text: [1] -
Leclercq, J.; De Pauw-Gillet, M.-C.; Bassleer, R.; Angenot, L. J. Ethnopharmacol. 1986, 15, 305–316. doi:10.1016/0378-8741(86)90169-8
Return to citation in text: [1] -
Dupont, L.; Lamotte-Brasseur, J.; Dideberg, O.; Campsteyn, H.; Vermeire, M.; Angenot, L. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 1977, 33, 1801–1807. doi:10.1107/S0567740877007092
Return to citation in text: [1] -
Cordell, G. A., Ed. The Alkaloids: Chemistry and Biology; Academic Press: San Diego, 1998; Vol. 51.
Return to citation in text: [1] -
Edmondson, S.; Danishefsky, S. J.; Sepp-Lorenzino, L.; Rosen, N. J. Am. Chem. Soc. 1999, 121, 2147–2155. doi:10.1021/ja983788i
Return to citation in text: [1] -
Somei, M.; Noguchi, K.; Yamagami, R.; Kawada, Y.; Yamada, K.; Yamada, F. Heterocycles 2000, 53, 7–10. doi:10.3987/COM-99-8743
Return to citation in text: [1] [2] -
Kuehne, M. E.; Roland, D. M.; Hafter, R. J. Org. Chem. 1978, 43, 3705–3710. doi:10.1021/jo00413a015
Return to citation in text: [1] [2] -
Pellegrini, C.; Strässler, C.; Weber, M.; Borschberg, H.-J. Tetrahedron: Asymmetry 1994, 5, 1979–1992. doi:10.1016/S0957-4166(00)86273-4
Return to citation in text: [1] [2] -
Bascop, S.-I.; Sapi, J.; Laronze, J.-Y.; Lévy, J. Heterocycles 1994, 38, 725–732. doi:10.3987/COM-93-6639
Return to citation in text: [1] [2] -
Fischer, C.; Meyers, C.; Carreira, E. M. Helv. Chim. Acta 2000, 83, 1175–1181. doi:10.1002/1522-2675(20000607)83:6<1175::AID-HLCA1175>3.0.CO;2-D
Return to citation in text: [1] [2] -
Syam Kumar, U. K.; Ila, H.; Junjappa, H. Org. Lett. 2001, 3, 4193–4196. doi:10.1021/ol016824i
Return to citation in text: [1] [2] -
Palmisano, G.; Annunziata, R.; Papeo, G.; Sisti, M. Tetrahedron: Asymmetry 1996, 7, 1–4. doi:10.1016/0957-4166(95)00406-8
Return to citation in text: [1] [2] -
Cravotto, G.; Giovenzana, G. B.; Pilati, T.; Sisti, M.; Palmisano, G. J. Org. Chem. 2001, 66, 8447–8453. doi:10.1021/jo015854w
Return to citation in text: [1] [2] -
Selvakumar, N.; Azhagan, A. M.; Srinivas, D.; Krishna, G. G. Tetrahedron Lett. 2002, 43, 9175–9178. doi:10.1016/S0040-4039(02)02267-0
Return to citation in text: [1] [2] -
Jones, K.; Wilkinson, J. J. Chem. Soc., Chem. Commun. 1992, 1767–1769. doi:10.1039/C39920001767
Return to citation in text: [1] [2] -
Lizos, D. E.; Murphy, J. A. Org. Biomol. Chem. 2003, 1, 117–122. doi:10.1039/b208114h
Return to citation in text: [1] [2] -
Cossy, J.; Cases, M.; Pardo, D. G. Tetrahedron Lett. 1998, 39, 2331–2332. doi:10.1016/S0040-4039(98)00193-2
Return to citation in text: [1] [2] -
Lizos, D.; Tripoli, R.; Murphy, J. A. Chem. Commun. 2001, 2732–2733. doi:10.1039/b108622g
Return to citation in text: [1] [2] -
Murphy, J. A.; Tripoli, R.; Khan, T. A.; Mali, U. M. Org. Lett. 2005, 7, 3287–3289. doi:10.1021/ol051095i
Return to citation in text: [1] [2] -
Chang, M.-Y.; Paib, C.-L.; Kung, Y.-H. Tetrahedron Lett. 2005, 46, 8463–8465. doi:10.1016/j.tetlet.2005.10.015
Return to citation in text: [1] [2] -
Lakshmaiah, G.; Kawabata, T.; Shang, M.; Fuji, K. J. Org. Chem. 1999, 64, 1699–1704. doi:10.1021/jo981577q
Return to citation in text: [1] [2] -
Trost, B. M.; Brennan, M. K. Org. Lett. 2006, 8, 2027–2030. doi:10.1021/ol060298j
Return to citation in text: [1] [2] [3] -
Jaegli, S.; Vors, J.-P.; Neuville, L.; Zhu, J. Synlett 2009, 18, 2997–2999. doi:10.1055/s-0029-1218004
Return to citation in text: [1] [2] -
Reddy, V. J.; Douglas, C. J. Tetrahedron 2010, 66, 4719–4729. doi:10.1016/j.tet.2010.02.096
Return to citation in text: [1] [2] -
Reddy, V. J.; Douglas, C. J. Org. Lett. 2010, 12, 952–955. doi:10.1021/ol902949d
Return to citation in text: [1] [2] -
Thomson, J. E.; Kyle, A. F.; Ling, K. B.; Smith, S. R.; Slawin, A. M. Z.; Smith, A. D. Tetrahedron 2010, 66, 3801–3813. doi:10.1016/j.tet.2010.03.047
Return to citation in text: [1] [2] -
Suárez-Castillo, O. R.; Meléndez-Rodríguez, M.; Contreras-Martínez, Y. M.; Alvarez-Hernández, A.; Morales-Ríos, M. S.; Joseph-Nathan, P. Nat. Prod. Commun. 2009, 4, 797–802.
Return to citation in text: [1] [2] -
White, J. D.; Li, Y.; Ihle, D. C. J. Org. Chem. 2010, 75, 3569–3577. doi:10.1021/jo1002714
Return to citation in text: [1] [2] -
Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209–2219. doi:10.1002/ejoc.200300050
See for a review.
Return to citation in text: [1] -
Kulkarni, M. G.; Davawala, S. I.; Doke, A. K.; Pendharkar, D. S. Synthesis 2004, 2919–2926. doi:10.1055/s-2004-831208
Return to citation in text: [1] [2] -
Kulkarni, M. G.; Dhondge, A. P.; Borhade, A. S.; Gaikwad, D. D.; Chavhan, S. W.; Shaikh, Y. B.; Nigdale, V. B.; Desai, M. P.; Birhade, D. R.; Shinde, M. P. Eur. J. Org. Chem. 2009, 3875–3877. doi:10.1002/ejoc.200900391
Return to citation in text: [1] -
Kulkarni, M. G.; Dhondge, A. P.; Borhade, A. S.; Gaikwad, D. D.; Chavhan, S. W.; Shaikh, Y. B.; Nigdale, V. B.; Desai, M. P.; Birhade, D. R.; Shinde, M. P. Tetrahedron Lett. 2009, 50, 2411–2413. doi:10.1016/j.tetlet.2009.03.012
Return to citation in text: [1] -
Kulkarni, M. G.; Rasne, R. M.; Davawala, S. I.; Doke, A. K. Tetrahedron Lett. 2002, 43, 2297–2298. doi:10.1016/S0040-4039(02)00227-7
Return to citation in text: [1] -
Kulkarni, M. G.; Rasne, R. M. J. Chem. Soc., Perkin Trans. 1 1998, 2479–2480. doi:10.1039/a803188f
Return to citation in text: [1] -
Kulkarni, M. G.; Pendharkar, D. S. J. Chem. Soc., Perkin Trans. 1 1997, 3127–3128. doi:10.1039/a706510h
Return to citation in text: [1] -
Kulkarni, M. G.; Pendharkar, D. S. Tetrahedron 1997, 53, 3167–3172. doi:10.1016/S0040-4020(97)00027-6
Return to citation in text: [1] -
Kulkarni, M. G.; Pendharkar, D. S.; Rasne, R. M. Tetrahedron Lett. 1997, 38, 1459–1462. doi:10.1016/S0040-4039(97)00057-9
Return to citation in text: [1] -
Kulkarni, M. G.; Davawala, S. I.; Shinde, M. P.; Dhondge, A. P.; Borhade, A. S.; Chavhan, S. W.; Gaikwad, D. D. Tetrahedron Lett. 2006, 47, 3027–3029. doi:10.1016/j.tetlet.2006.03.012
Return to citation in text: [1] -
Kulkarni, M. G.; Davawala, S. I.; Dhondge, A. P.; Gaikwad, D. D.; Borhade, A. S.; Chavhan, S. W. Tetrahedron Lett. 2006, 47, 1003–1005. doi:10.1016/j.tetlet.2005.11.134
Return to citation in text: [1]
| 35. | Kulkarni, M. G.; Davawala, S. I.; Doke, A. K.; Pendharkar, D. S. Synthesis 2004, 2919–2926. doi:10.1055/s-2004-831208 |
| 27. | Trost, B. M.; Brennan, M. K. Org. Lett. 2006, 8, 2027–2030. doi:10.1021/ol060298j |
| 1. | Anderton, N.; Cockrum, P. A.; Colegate, S. M.; Edgar, J. A.; Flower, K.; Vit, I.; Willing, R. I. Phytochemistry 1998, 48, 437–439. doi:10.1016/S0031-9422(97)00946-1 |
| 2. | Colegate, S. M.; Anderton, N.; Edgar, J.; Bourke, C. A.; Oram, R. N. Aust. Vet. J. 1999, 77, 537–538. |
| 6. | James, M. N. G.; Williams, G. J. B. Can. J. Chem. 1972, 50, 2407–2412. doi:10.1139/v72-386 |
| 15. | Fischer, C.; Meyers, C.; Carreira, E. M. Helv. Chim. Acta 2000, 83, 1175–1181. doi:10.1002/1522-2675(20000607)83:6<1175::AID-HLCA1175>3.0.CO;2-D |
| 16. | Syam Kumar, U. K.; Ila, H.; Junjappa, H. Org. Lett. 2001, 3, 4193–4196. doi:10.1021/ol016824i |
| 4. | Cui, C.-B.; Kakeya, H.; Osada, H. Tetrahedron 1996, 52, 12651–12666. doi:10.1016/0040-4020(96)00737-5 |
| 5. | Cui, C.-B.; Kakeya, H.; Osada, H. J. Antibiot. 1996, 49, 832–835. |
| 17. | Palmisano, G.; Annunziata, R.; Papeo, G.; Sisti, M. Tetrahedron: Asymmetry 1996, 7, 1–4. doi:10.1016/0957-4166(95)00406-8 |
| 18. | Cravotto, G.; Giovenzana, G. B.; Pilati, T.; Sisti, M.; Palmisano, G. J. Org. Chem. 2001, 66, 8447–8453. doi:10.1021/jo015854w |
| 19. | Selvakumar, N.; Azhagan, A. M.; Srinivas, D.; Krishna, G. G. Tetrahedron Lett. 2002, 43, 9175–9178. doi:10.1016/S0040-4039(02)02267-0 |
| 1. | Anderton, N.; Cockrum, P. A.; Colegate, S. M.; Edgar, J. A.; Flower, K.; Vit, I.; Willing, R. I. Phytochemistry 1998, 48, 437–439. doi:10.1016/S0031-9422(97)00946-1 |
| 2. | Colegate, S. M.; Anderton, N.; Edgar, J.; Bourke, C. A.; Oram, R. N. Aust. Vet. J. 1999, 77, 537–538. |
| 3. | Jossang, A.; Jossang, P.; Hadi, H. A.; Sevenet, T.; Bodo, B. J. Org. Chem. 1991, 56, 6527–6530. doi:10.1021/jo00023a016 |
| 13. | Pellegrini, C.; Strässler, C.; Weber, M.; Borschberg, H.-J. Tetrahedron: Asymmetry 1994, 5, 1979–1992. doi:10.1016/S0957-4166(00)86273-4 |
| 3. | Jossang, A.; Jossang, P.; Hadi, H. A.; Sevenet, T.; Bodo, B. J. Org. Chem. 1991, 56, 6527–6530. doi:10.1021/jo00023a016 |
| 14. | Bascop, S.-I.; Sapi, J.; Laronze, J.-Y.; Lévy, J. Heterocycles 1994, 38, 725–732. doi:10.3987/COM-93-6639 |
| 11. | Somei, M.; Noguchi, K.; Yamagami, R.; Kawada, Y.; Yamada, K.; Yamada, F. Heterocycles 2000, 53, 7–10. doi:10.3987/COM-99-8743 |
| 12. | Kuehne, M. E.; Roland, D. M.; Hafter, R. J. Org. Chem. 1978, 43, 3705–3710. doi:10.1021/jo00413a015 |
| 13. | Pellegrini, C.; Strässler, C.; Weber, M.; Borschberg, H.-J. Tetrahedron: Asymmetry 1994, 5, 1979–1992. doi:10.1016/S0957-4166(00)86273-4 |
| 14. | Bascop, S.-I.; Sapi, J.; Laronze, J.-Y.; Lévy, J. Heterocycles 1994, 38, 725–732. doi:10.3987/COM-93-6639 |
| 15. | Fischer, C.; Meyers, C.; Carreira, E. M. Helv. Chim. Acta 2000, 83, 1175–1181. doi:10.1002/1522-2675(20000607)83:6<1175::AID-HLCA1175>3.0.CO;2-D |
| 16. | Syam Kumar, U. K.; Ila, H.; Junjappa, H. Org. Lett. 2001, 3, 4193–4196. doi:10.1021/ol016824i |
| 17. | Palmisano, G.; Annunziata, R.; Papeo, G.; Sisti, M. Tetrahedron: Asymmetry 1996, 7, 1–4. doi:10.1016/0957-4166(95)00406-8 |
| 18. | Cravotto, G.; Giovenzana, G. B.; Pilati, T.; Sisti, M.; Palmisano, G. J. Org. Chem. 2001, 66, 8447–8453. doi:10.1021/jo015854w |
| 19. | Selvakumar, N.; Azhagan, A. M.; Srinivas, D.; Krishna, G. G. Tetrahedron Lett. 2002, 43, 9175–9178. doi:10.1016/S0040-4039(02)02267-0 |
| 20. | Jones, K.; Wilkinson, J. J. Chem. Soc., Chem. Commun. 1992, 1767–1769. doi:10.1039/C39920001767 |
| 21. | Lizos, D. E.; Murphy, J. A. Org. Biomol. Chem. 2003, 1, 117–122. doi:10.1039/b208114h |
| 22. | Cossy, J.; Cases, M.; Pardo, D. G. Tetrahedron Lett. 1998, 39, 2331–2332. doi:10.1016/S0040-4039(98)00193-2 |
| 23. | Lizos, D.; Tripoli, R.; Murphy, J. A. Chem. Commun. 2001, 2732–2733. doi:10.1039/b108622g |
| 24. | Murphy, J. A.; Tripoli, R.; Khan, T. A.; Mali, U. M. Org. Lett. 2005, 7, 3287–3289. doi:10.1021/ol051095i |
| 25. | Chang, M.-Y.; Paib, C.-L.; Kung, Y.-H. Tetrahedron Lett. 2005, 46, 8463–8465. doi:10.1016/j.tetlet.2005.10.015 |
| 26. | Lakshmaiah, G.; Kawabata, T.; Shang, M.; Fuji, K. J. Org. Chem. 1999, 64, 1699–1704. doi:10.1021/jo981577q |
| 27. | Trost, B. M.; Brennan, M. K. Org. Lett. 2006, 8, 2027–2030. doi:10.1021/ol060298j |
| 28. | Jaegli, S.; Vors, J.-P.; Neuville, L.; Zhu, J. Synlett 2009, 18, 2997–2999. doi:10.1055/s-0029-1218004 |
| 29. | Reddy, V. J.; Douglas, C. J. Tetrahedron 2010, 66, 4719–4729. doi:10.1016/j.tet.2010.02.096 |
| 30. | Reddy, V. J.; Douglas, C. J. Org. Lett. 2010, 12, 952–955. doi:10.1021/ol902949d |
| 31. | Thomson, J. E.; Kyle, A. F.; Ling, K. B.; Smith, S. R.; Slawin, A. M. Z.; Smith, A. D. Tetrahedron 2010, 66, 3801–3813. doi:10.1016/j.tet.2010.03.047 |
| 32. | Suárez-Castillo, O. R.; Meléndez-Rodríguez, M.; Contreras-Martínez, Y. M.; Alvarez-Hernández, A.; Morales-Ríos, M. S.; Joseph-Nathan, P. Nat. Prod. Commun. 2009, 4, 797–802. |
| 33. | White, J. D.; Li, Y.; Ihle, D. C. J. Org. Chem. 2010, 75, 3569–3577. doi:10.1021/jo1002714 |
| 34. |
Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209–2219. doi:10.1002/ejoc.200300050
See for a review. |
| 11. | Somei, M.; Noguchi, K.; Yamagami, R.; Kawada, Y.; Yamada, K.; Yamada, F. Heterocycles 2000, 53, 7–10. doi:10.3987/COM-99-8743 |
| 10. | Edmondson, S.; Danishefsky, S. J.; Sepp-Lorenzino, L.; Rosen, N. J. Am. Chem. Soc. 1999, 121, 2147–2155. doi:10.1021/ja983788i |
| 12. | Kuehne, M. E.; Roland, D. M.; Hafter, R. J. Org. Chem. 1978, 43, 3705–3710. doi:10.1021/jo00413a015 |
| 9. | Cordell, G. A., Ed. The Alkaloids: Chemistry and Biology; Academic Press: San Diego, 1998; Vol. 51. |
| 7. | Leclercq, J.; De Pauw-Gillet, M.-C.; Bassleer, R.; Angenot, L. J. Ethnopharmacol. 1986, 15, 305–316. doi:10.1016/0378-8741(86)90169-8 |
| 8. | Dupont, L.; Lamotte-Brasseur, J.; Dideberg, O.; Campsteyn, H.; Vermeire, M.; Angenot, L. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 1977, 33, 1801–1807. doi:10.1107/S0567740877007092 |
| 3. | Jossang, A.; Jossang, P.; Hadi, H. A.; Sevenet, T.; Bodo, B. J. Org. Chem. 1991, 56, 6527–6530. doi:10.1021/jo00023a016 |
| 26. | Lakshmaiah, G.; Kawabata, T.; Shang, M.; Fuji, K. J. Org. Chem. 1999, 64, 1699–1704. doi:10.1021/jo981577q |
| 20. | Jones, K.; Wilkinson, J. J. Chem. Soc., Chem. Commun. 1992, 1767–1769. doi:10.1039/C39920001767 |
| 21. | Lizos, D. E.; Murphy, J. A. Org. Biomol. Chem. 2003, 1, 117–122. doi:10.1039/b208114h |
| 22. | Cossy, J.; Cases, M.; Pardo, D. G. Tetrahedron Lett. 1998, 39, 2331–2332. doi:10.1016/S0040-4039(98)00193-2 |
| 23. | Lizos, D.; Tripoli, R.; Murphy, J. A. Chem. Commun. 2001, 2732–2733. doi:10.1039/b108622g |
| 24. | Murphy, J. A.; Tripoli, R.; Khan, T. A.; Mali, U. M. Org. Lett. 2005, 7, 3287–3289. doi:10.1021/ol051095i |
| 25. | Chang, M.-Y.; Paib, C.-L.; Kung, Y.-H. Tetrahedron Lett. 2005, 46, 8463–8465. doi:10.1016/j.tetlet.2005.10.015 |
| 35. | Kulkarni, M. G.; Davawala, S. I.; Doke, A. K.; Pendharkar, D. S. Synthesis 2004, 2919–2926. doi:10.1055/s-2004-831208 |
| 36. | Kulkarni, M. G.; Dhondge, A. P.; Borhade, A. S.; Gaikwad, D. D.; Chavhan, S. W.; Shaikh, Y. B.; Nigdale, V. B.; Desai, M. P.; Birhade, D. R.; Shinde, M. P. Eur. J. Org. Chem. 2009, 3875–3877. doi:10.1002/ejoc.200900391 |
| 37. | Kulkarni, M. G.; Dhondge, A. P.; Borhade, A. S.; Gaikwad, D. D.; Chavhan, S. W.; Shaikh, Y. B.; Nigdale, V. B.; Desai, M. P.; Birhade, D. R.; Shinde, M. P. Tetrahedron Lett. 2009, 50, 2411–2413. doi:10.1016/j.tetlet.2009.03.012 |
| 38. | Kulkarni, M. G.; Rasne, R. M.; Davawala, S. I.; Doke, A. K. Tetrahedron Lett. 2002, 43, 2297–2298. doi:10.1016/S0040-4039(02)00227-7 |
| 39. | Kulkarni, M. G.; Rasne, R. M. J. Chem. Soc., Perkin Trans. 1 1998, 2479–2480. doi:10.1039/a803188f |
| 40. | Kulkarni, M. G.; Pendharkar, D. S. J. Chem. Soc., Perkin Trans. 1 1997, 3127–3128. doi:10.1039/a706510h |
| 41. | Kulkarni, M. G.; Pendharkar, D. S. Tetrahedron 1997, 53, 3167–3172. doi:10.1016/S0040-4020(97)00027-6 |
| 42. | Kulkarni, M. G.; Pendharkar, D. S.; Rasne, R. M. Tetrahedron Lett. 1997, 38, 1459–1462. doi:10.1016/S0040-4039(97)00057-9 |
| 43. | Kulkarni, M. G.; Davawala, S. I.; Shinde, M. P.; Dhondge, A. P.; Borhade, A. S.; Chavhan, S. W.; Gaikwad, D. D. Tetrahedron Lett. 2006, 47, 3027–3029. doi:10.1016/j.tetlet.2006.03.012 |
| 44. | Kulkarni, M. G.; Davawala, S. I.; Dhondge, A. P.; Gaikwad, D. D.; Borhade, A. S.; Chavhan, S. W. Tetrahedron Lett. 2006, 47, 1003–1005. doi:10.1016/j.tetlet.2005.11.134 |
| 32. | Suárez-Castillo, O. R.; Meléndez-Rodríguez, M.; Contreras-Martínez, Y. M.; Alvarez-Hernández, A.; Morales-Ríos, M. S.; Joseph-Nathan, P. Nat. Prod. Commun. 2009, 4, 797–802. |
| 33. | White, J. D.; Li, Y.; Ihle, D. C. J. Org. Chem. 2010, 75, 3569–3577. doi:10.1021/jo1002714 |
| 29. | Reddy, V. J.; Douglas, C. J. Tetrahedron 2010, 66, 4719–4729. doi:10.1016/j.tet.2010.02.096 |
| 30. | Reddy, V. J.; Douglas, C. J. Org. Lett. 2010, 12, 952–955. doi:10.1021/ol902949d |
| 31. | Thomson, J. E.; Kyle, A. F.; Ling, K. B.; Smith, S. R.; Slawin, A. M. Z.; Smith, A. D. Tetrahedron 2010, 66, 3801–3813. doi:10.1016/j.tet.2010.03.047 |
| 27. | Trost, B. M.; Brennan, M. K. Org. Lett. 2006, 8, 2027–2030. doi:10.1021/ol060298j |
| 28. | Jaegli, S.; Vors, J.-P.; Neuville, L.; Zhu, J. Synlett 2009, 18, 2997–2999. doi:10.1055/s-0029-1218004 |
© 2010 Kulkarni et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)