Abstract
Sequential cycloisomerizations of diynyl o-benzaldehyde substrates to access novel polycyclic cyclopropanes are reported. The reaction sequence involves initial Cu(I)-mediated cycloisomerization/nucleophilic addition to an isochromene followed by diastereoselective Pt(II)-catalyzed enyne cycloisomerization.
Graphical Abstract
Introduction
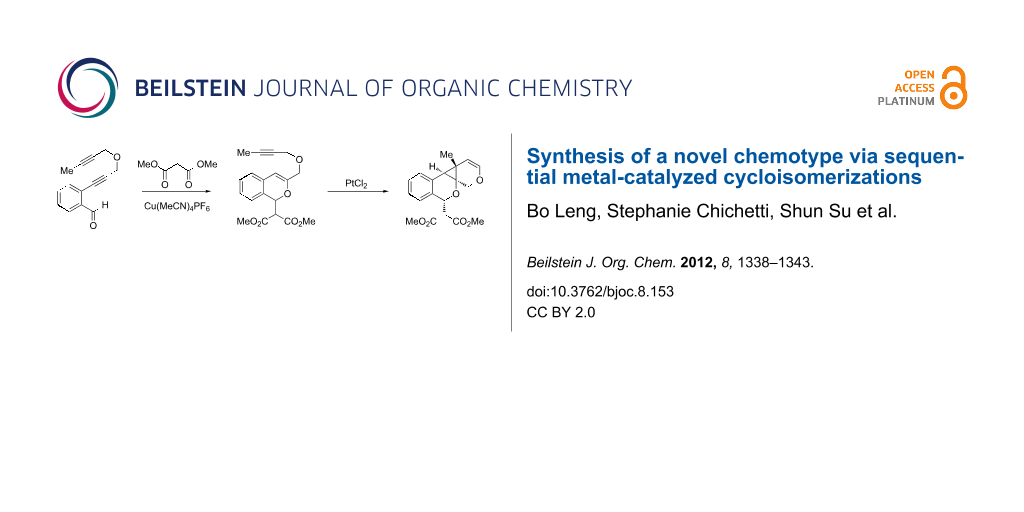
Our laboratory has an ongoing interest in discovering transformations that afford novel chemotypes [1-4]. To this end, we have developed a reaction screening paradigm that enables the discovery of new reaction processes and chemotypes [5]. For example, we have conducted multidimensional reaction screens using alkynyl o-benzaldehyde scaffolds, which revealed a number of reactions affording novel polycyclic scaffolds, including Au(III)-catalyzed addition of diethyl malonate to 1 to afford isochromene 2 (Scheme 1). The chemotypes discovered in initial pilot studies have been further developed into library scaffolds and identified as biologically interesting structures [6]. Herein, we report the expanded utility of alkynyl o-benzaldehydes through a sequential metal-catalyzed cycloisomerization process to afford a novel polycyclic cyclopropane chemotype.
Scheme 1: Cycloisomerization/nucleophilic addition of alkynyl benzaldehyde 1 to isochromene 2.
Scheme 1: Cycloisomerization/nucleophilic addition of alkynyl benzaldehyde 1 to isochromene 2.
Results and Discussion
In an effort to further explore the utility of alkynyl o-benzaldehydes as scaffolds for reaction screening, we designed a focused reaction screen with diynyl benzaldehyde [7] substrate 3. Based on the cycloisomerization/addition reactions previously studied (Scheme 1), it was not clear at the outset of our study whether an o-alkynyl benzaldehyde containing an additional alkynyl moiety (3) would react to form an isochromene derivative or whether additional polycyclization would occur [8]. Accordingly, a reaction screen was conducted, evaluating a number of metal catalysts in the presence of diethyl malonate. From this focused reaction screen we identified three types of reactivity: (1) no reaction; (2) alkyne hydration (4); and (3) cycloisomerization leading to isochromene (5) (Figure 1). Many catalysts resulted in no reaction, including ones that might have been expected to catalyze cycloisomerization, such as AgOTf. Two catalysts, Cu(OTf)2 and Pd(MeCN)2Cl2, afforded only hydration of the alkyne. Interestingly, hydration was regioselective, which is possibly due to direction from the ether oxygen. We were most interested in metal catalysts that effected cycloisomerization of 3 to alkynyl isochromene 5, which is an interesting enyne substrate with potential for further reactivity [9,10]. In the reaction screen of alkynyl benzaldehyde substrate 3, we found that in the absence of optimization Cu(MeCN)4PF6 [11-13] afforded the highest isolated yield of 5 (60%) (Scheme 2).
Figure 1: Reaction screen with diynyl benzaldehyde 3.
Figure 1: Reaction screen with diynyl benzaldehyde 3.
Scheme 2: Sequential cycloisomerizations of substrate 3. Condition A: PtCl2 (10 mol %), Cu(MeCN)4PF6 (10 mol %), toluene, 80 °C, 8 h (40%). Condition B: Step 1: Cu(MeCN)4PF6 (10 mol %), rt, 1 h. Step 2: PtCl2 (10 mol %), 80 °C, toluene, 5 h (89%).
Scheme 2: Sequential cycloisomerizations of substrate 3. Condition A: PtCl2 (10 mol %), Cu(MeCN)4PF6 (10 mol ...
As the production of isochromene 5 offered a unique opportunity for additional cycloisomerization processes, we elected to explore this manifold of reactivity. Based on reports by Echavarren and co-workers [14,15], we treated enyne 5 with PtCl2 at 80 °C in toluene [16,17]. The reaction afforded polycyclic cyclopropane 6 in good yield (65%) as a single diastereomer (Scheme 2a). Interestingly, reaction of 3 in the presence of only PtCl2 afforded exclusively isochromene 5 in low yield. Further studies revealed that a multicatalytic reaction system [18] utilizing both Cu(I) and Pt(II) [19] catalysts afforded the desired cyclopropane 6 in moderate yield (40%) along with ketone 7 (45%), derived from [4 + 2] cycloaddition of the benzopyrylium intermediate with the pendent alkyne [20] (Scheme 2b). However, better yields were observed when the initial cycloisomerization was carried out in the presence of Cu(MeCN)4PF6 followed by the addition of PtCl2 to the reaction mixture (Scheme 2b). Optimization of the one-pot conditions afforded exclusively 6 in good yield (89%). X-ray crystal analysis confirmed the structure and relative stereochemistry of polycyclic cyclopropane 6 (Figure 2, Supporting Information File 1).
![[1860-5397-8-153-2]](/bjoc/content/figures/1860-5397-8-153-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: X-ray crystal structure of cyclopropane 6.
Figure 2: X-ray crystal structure of cyclopropane 6.
We next focused on an evaluation of the general scope of the reaction with regard to aryl and alkyne substitution. Reaction utilizing an electron-poor trifluoromethyl-substituted diynyl benzaldehyde 8 was successful, producing product 9 in moderate yield (Table 1, entry 1). m-Methyl- and naphthyl-containing substrates 10 and 12 afforded polycyclic cyclopropanes 11 and 13 in 48 and 51% yields, respectively (Table 1, entries 2 and 3).
We next explored substitution of the pendant alkyne. Reaction with cyclohexane diyne 14 afforded the fused cyclopropane 15 in moderate yield (60%), while methyl ether 16 afforded cyclopropane 17 in 62% yield. Phenyl substitution (18) also resulted in a moderate yield (59%, Table 1, entry 6). Substituting the oxygen with N-tosyl (20) afforded N-tosyl cyclopropane 21 in good yield (82%). Substitution at the internal methylene (22) resulted in a diverted reaction pathway (vida infra) affording product 23 exclusively in moderate yield (65%).
A proposed mechanistic pathway for diastereoselective, sequential cycloisomerizations is shown in Scheme 3. We propose the initial cycloisomerization and nucleophilic addition of diynyl benzaldehyde 3 and dimethyl malonate is catalyzed by Cu(I) to afford isochromene 24 [20-22]. Pt(II) π-coordination of the pendant alkyne of 24 followed by cyclization of the enol ether affords the seven-membered-ring metal-“ate” intermediate 25. The cyclization occurs at the face opposite the malonate substituent (Nu, 24a) to minimize steric interactions relative to 24b, leading to the observed diastereoselectivity (Scheme 3, inset) [23,24]. Subsequent cyclopropane formation through addition of the vinyl metal to the oxonium intermediate affords metallocarbenoid 26, which may then undergo a 1,2-hydride shift to intermediate 27 followed by elimination of the metal catalyst [25] to afford the observed cyclopropane product 6.
Scheme 3: Proposed reaction pathway for diastereoselective, sequential cycloisomerization.
Scheme 3: Proposed reaction pathway for diastereoselective, sequential cycloisomerization.
An alternative reaction pathway may be invoked for the ethyl-substituted substrate 22 leading to product 23 (Scheme 4). After initial cyclization of the enol ether with the Pt-activated alkyne, the resulting metal-“ate” intermediate 28 may undergo preferential elimination and proto-demetallation to afford 1,5-diene 29. A second elimination results in the ring-opened triene 30. Subsequent 6π-electrocyclization affords alcohol 31, which aromatizes through loss of water to afford the observed isochromane 23.
Scheme 4: Proposed alternative reaction pathway affording 23.
Scheme 4: Proposed alternative reaction pathway affording 23.
Conclusion
We have described sequential cycloisomerizations of diynyl o-benzaldehyde substrates to access novel polycyclic cyclopropanes. The reaction sequence involves initial Cu(I)-mediated cycloisomerization/nucleophilic addition to an isochromene followed by diastereoselective Pt(II)-catalyzed enyne cycloisomerization. The chemistry reported herein illustrates the power of sequential cycloisomerization processes to provide access to novel chemotypes and chemical diversity from readily accessible building blocks [26]. Further transformations of the novel polycyclic cyclopropanes as well as additional studies employing reaction screening for metal-mediated processes is ongoing and will be reported in future publications.
Experimental
General Information: All nuclear magnetic resonance spectra were recorded on either a Varian or Bruker spectrometer. 1H NMR spectra were recorded at 400 MHz at ambient temperature with CDCl3 as solvent, unless otherwise stated. 13C NMR spectra were recorded at 100.0 MHz at ambient temperature with CDCl3 as solvent, unless otherwise stated. Chemical shifts are reported in parts per million relative to CDCl3 (1H, δ 7.27; 13C, δ 77.0) and acetone-d6 (1H, δ 2.05; 13C, δ 30.8). Data for 1H NMR are reported as follows: chemical shift, multiplicity (ovrlp = overlapping, s = singlet, d = doublet, t = triplet, q = quartet, qt = quintuplet, m = multiplet), coupling constant in hertz, and integration. All 13C NMR spectra were recorded with complete proton decoupling. Analytical LC was performed on a 2.1 × 50 mm, 1.7 μM C18 column. Analytical thin-layer chromatography was performed by using 0.25 mm silica gel 60-F plates. Otherwise, flash chromatography was performed by using 200–400 mesh silica gel. Yields refer to chromatographically and spectroscopically pure materials, unless otherwise stated. Acetonitrile, CH2Cl2, THF, and toluene were purified by passing through two packed columns of neutral alumina. All reactions were performed under an argon atmosphere in oven-dried or flame-dried glassware.
General procedure for the synthesis of alkynyl o-benzaldehydes: 2-(3-(but-2-ynyloxy)prop-1-ynyl)benzaldehyde. To a solution of 2-bromobenzaldehyde (2.0 g, 10.8 mmol) and 1-(prop-2-ynyloxy)but-2-yne (1.4 g, 13 mmol) in Et3N (68 mL), was added tetrakis(triphenylphosphine)palladium(0) (0.38 g, 0.32 mmol). The reaction mixture was stirred at room temperature for 5 min. Copper(I) iodide (0.075 g, 0.4 mmol) was added, and the mixture was heated to 60 °C overnight. The mixture was concentrated in vacuo and purified by flash chromatography (SiO2, petroleum ether/EtOAc 4:1) to afford diynyl benzaldehyde 3 (1.5 g, 7.1 mmol, 66%) as a viscous yellow oil. 1H NMR (400 MHz, CDCl3) δ 10.22 (s, 1H), 7.91 (d, J = 7.6 Hz, 1H), 7.57 (m, 2H), 7.46 (m, 1H), 4.54 (s, 2H), 4.29 (q, J = 2.4 Hz, 2H), 1.89 (t, J = 2.4 Hz, 3 H); 13C NMR (100 MHz, CDCl3) δ 191.6, 136.2, 133.8, 133.6, 129.0, 127.3, 126.1, 91.9, 83.7, 82.2, 74.2, 57.6, 57.1, 3.7; IR (thin film) νmax: 2920, 2852, 1697, 1594, 1477, 1450, 1350, 1274, 1244, 1193, 1138, 1076, 765 cm−1.
General one-pot procedure for sequential cycloisomerization: To a flame-dried round-bottom flask was added 3 (10 mg, 0.046 mmol), dimethyl malonate (5.8 μL, 0.05 mmol) and toluene (1.0 mL). To the reaction mixture was added tetrakis(acetonitrile)copper(I) hexafluorophosphate (1.7 mg, 0.005 mmol), and the reaction mixture was stirred at room temperature for 1 h. Platinum(II) chloride (1.2 mg, 0.005 mmol) was added and the reaction mixture was heated to 80 °C for 5 h. The reaction mixture was concentrated in vacuo and purified by flash chromatography (SiO2, petroleum ether/EtOAc 9:1 to 4:1) to afford the desired cycloisomerization product 6 (14 mg, 0.041 mmol, 89%) as a white solid. 1H NMR (400 MHz, CDCl3) δ 7.25 (m, 2H), 7.08 (m, 1H), 6.98 (d, J = 4.2 Hz, 1H), 6.11 (d, J = 5.6 Hz, 1H), 5.28 (d, J = 10.4 Hz, 1H), 5.07 (d, J = 5.6 Hz, 1H), 4.33 (d, J = 10.0 Hz, 1H), 3.92 (d, J = 10.8 Hz, 1H), 3.83 (s, 3H), 3.66 (d, J = 10.0 Hz, 1H), 3.49 (s, 3H), 2.51 (s, 1H), 0.73 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 167.3, 166.4, 141.0, 135.8, 133.7, 130.8, 130.3, 128.9, 126.2, 111.1, 75.0, 63.9, 62.7, 59.4, 53.2, 52.7, 30.5, 26.4, 12.2; IR (thin film) νmax: 2953, 2926, 2870, 1761, 1741, 1679, 1639, 1493, 1435, 1341, 1253, 1194, 1144, 1073, 1018, 912, 774, 749 cm−1; HRMS–ESI+ (m/z): [M + Na]+ calcd for C19H20O6, 367.1158; found, 367.1189.
Supporting Information
| Supporting Information File 1: Characterization data, spectra, and crystal structure data. | ||
| Format: PDF | Size: 1.6 MB | Download |
References
-
Han, C.; Rangarajan, S.; Voukides, A. C.; Beeler, A. B.; Johnson, R.; Porco, J. A., Jr. Org. Lett. 2009, 11, 413–416. doi:10.1021/ol802729f
Return to citation in text: [1] -
Goodell, J. R.; McMullen, J. P.; Zaborenko, N.; Maloney, J. R.; Ho, C.-X.; Jensen, K. F.; Porco, J. A., Jr.; Beeler, A. B. J. Org. Chem. 2009, 74, 6169–6180. doi:10.1021/jo901073v
Return to citation in text: [1] -
Liang, B.; Kalidindi, S.; Porco, J. A., Jr.; Stephenson, C. R. J. Org. Lett. 2010, 12, 572–575. doi:10.1021/ol902764k
Return to citation in text: [1] -
Kinoshita, H.; Ingham, O. J.; Ong, W. W.; Beeler, A. B.; Porco, J. A., Jr. J. Am. Chem. Soc. 2010, 132, 6412–6418. doi:10.1021/ja100346w
Return to citation in text: [1] -
Beeler, A. B.; Su, S.; Singleton, C. A.; Porco, J. A., Jr. J. Am. Chem. Soc. 2007, 129, 1413–1419. doi:10.1021/ja0674744
Return to citation in text: [1] -
Brown, L. E.; Cheng, K. C.-C.; Wei, W.-G.; Yuan, P.; Dai, P.; Trilles, R.; Ni, F.; Yuan, J.; MacArthur, R.; Guha, R.; Johnson, R. L.; Suc, X.-Z.; Dominguez, M. M.; Snyder, J. K.; Beeler, A. B.; Schaus, S. E.; Inglese, J.; Porco, J. A., Jr. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 6775–6780. doi:10.1073/pnas.1017666108
Return to citation in text: [1] -
Kim, N.; Kim, Y.; Park, W.; Sung, D.; Gupta, A. K.; Oh, C. H. Org. Lett. 2005, 7, 5289–5291. doi:10.1021/ol052229v
See for gold-catalyzed cycloisomerization of o-alkynylbenzaldehydes with a pendant alkyne.
Return to citation in text: [1] -
Michelet, V.; Toullec, P. Y.; Genêt, J.-P. Angew. Chem., Int. Ed. 2008, 47, 4268–4315. doi:10.1002/anie.200701589
See for a review of cycloisomerizations.
Return to citation in text: [1] -
Porcel, S.; Echavarren, A. M. Angew. Chem., Int. Ed. 2007, 46, 2672–2676. doi:10.1002/anie.200605041
Return to citation in text: [1] -
Nieto-Oberhuber, C.; Muñoz, M. P.; Buñuel, E.; Nevado, C.; Cárdenas, D. J.; Echavarren, A. M. Angew. Chem., Int. Ed. 2004, 43, 2402–2406. doi:10.1002/anie.200353207
Return to citation in text: [1] -
Patil, N. T.; Yamamoto, Y. J. Org. Chem. 2004, 69, 5139–5142. doi:10.1021/jo049416b
See for Cu(I)-mediated cycloisomerization/alcohol addition of an o-alkynylbenzaldehyde.
Return to citation in text: [1] -
Chernyak, D.; Gadamsetty, S. B.; Gevorgyan, V. Org. Lett. 2008, 10, 2307–2310. doi:10.1021/ol8008705
See for Cu(I)-mediated cycloisomerization.
Return to citation in text: [1] -
Rauniyar, V.; Wang, Z. J.; Burks, H. E.; Toste, F. D. J. Am. Chem. Soc. 2011, 133, 8486–8489. doi:10.1021/ja202959n
Return to citation in text: [1] -
Nieto-Oberhuber, C.; López, S.; Muñoz, M. P.; Jiménez-Núñez, E.; Buñuel, E.; Cárdenas, D. J.; Echavarren, A. M. Chem.–Eur. J. 2006, 12, 1694–1702. doi:10.1002/chem.200501089
Return to citation in text: [1] -
Nevado, C.; Ferrer, C.; Echavarren, A. M. Org. Lett. 2004, 6, 3191–3194. doi:10.1021/ol0486573
Return to citation in text: [1] -
Cariou, K.; Ronan, B.; Mignani, S.; Fensterbank, L.; Malacria, M. Angew. Chem., Int. Ed. 2007, 46, 1881–1884. doi:10.1002/anie.200604026
Return to citation in text: [1] -
Hardin, A. R.; Sarpong, R. Org. Lett. 2007, 9, 4547–4550. doi:10.1021/ol701973s
Return to citation in text: [1] -
Ambrosini, L. M.; Lambert, T. H. ChemCatChem 2010, 2, 1373–1380. doi:10.1002/cctc.200900323
Return to citation in text: [1] -
Asao, N.; Chan, C. S.; Takahashi, K.; Yamamoto, Y. Tetrahedron 2005, 61, 11322–11326. doi:10.1016/j.tet.2005.09.012
Return to citation in text: [1] -
Dyker, G.; Hildebrandt, D.; Liu, J.; Merz, K. Angew. Chem., Int. Ed. 2003, 42, 4399–4402. doi:10.1002/anie.200352160
Return to citation in text: [1] [2] -
Huang, Q.; Larock, R. C. J. Org. Chem. 2003, 68, 980–988. doi:10.1021/jo0261303
Return to citation in text: [1] -
Belmont, P.; Parker, E. Eur. J. Org. Chem. 2009, 6075–6089. doi:10.1002/ejoc.200900790
Return to citation in text: [1] -
Soriano, E.; Marco-Contelles, J. J. Org. Chem. 2007, 72, 2651–2654. doi:10.1021/jo062594f
Return to citation in text: [1] -
Fehr, C.; Winter, B.; Magpantay, I. Chem.–Eur. J. 2009, 15, 9773–9784. doi:10.1002/chem.200901292
Return to citation in text: [1] -
Zhang, L.; Sun, J.; Kozmin, S. A. Adv. Synth. Catal. 2006, 348, 2271–2296. doi:10.1002/adsc.200600368
Return to citation in text: [1] -
Painter, T. O.; Wang, L.; Majumder, S.; Xie, X.-Q.; Brummond, K. M. ACS Comb. Sci. 2011, 13, 166–174. doi:10.1021/co100052s
See for an illustrative recent application of cycloisomerizations in diversity-oriented synthesis (DOS).
Return to citation in text: [1]
| 1. | Han, C.; Rangarajan, S.; Voukides, A. C.; Beeler, A. B.; Johnson, R.; Porco, J. A., Jr. Org. Lett. 2009, 11, 413–416. doi:10.1021/ol802729f |
| 2. | Goodell, J. R.; McMullen, J. P.; Zaborenko, N.; Maloney, J. R.; Ho, C.-X.; Jensen, K. F.; Porco, J. A., Jr.; Beeler, A. B. J. Org. Chem. 2009, 74, 6169–6180. doi:10.1021/jo901073v |
| 3. | Liang, B.; Kalidindi, S.; Porco, J. A., Jr.; Stephenson, C. R. J. Org. Lett. 2010, 12, 572–575. doi:10.1021/ol902764k |
| 4. | Kinoshita, H.; Ingham, O. J.; Ong, W. W.; Beeler, A. B.; Porco, J. A., Jr. J. Am. Chem. Soc. 2010, 132, 6412–6418. doi:10.1021/ja100346w |
| 8. |
Michelet, V.; Toullec, P. Y.; Genêt, J.-P. Angew. Chem., Int. Ed. 2008, 47, 4268–4315. doi:10.1002/anie.200701589
See for a review of cycloisomerizations. |
| 25. | Zhang, L.; Sun, J.; Kozmin, S. A. Adv. Synth. Catal. 2006, 348, 2271–2296. doi:10.1002/adsc.200600368 |
| 7. |
Kim, N.; Kim, Y.; Park, W.; Sung, D.; Gupta, A. K.; Oh, C. H. Org. Lett. 2005, 7, 5289–5291. doi:10.1021/ol052229v
See for gold-catalyzed cycloisomerization of o-alkynylbenzaldehydes with a pendant alkyne. |
| 26. |
Painter, T. O.; Wang, L.; Majumder, S.; Xie, X.-Q.; Brummond, K. M. ACS Comb. Sci. 2011, 13, 166–174. doi:10.1021/co100052s
See for an illustrative recent application of cycloisomerizations in diversity-oriented synthesis (DOS). |
| 6. | Brown, L. E.; Cheng, K. C.-C.; Wei, W.-G.; Yuan, P.; Dai, P.; Trilles, R.; Ni, F.; Yuan, J.; MacArthur, R.; Guha, R.; Johnson, R. L.; Suc, X.-Z.; Dominguez, M. M.; Snyder, J. K.; Beeler, A. B.; Schaus, S. E.; Inglese, J.; Porco, J. A., Jr. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 6775–6780. doi:10.1073/pnas.1017666108 |
| 20. | Dyker, G.; Hildebrandt, D.; Liu, J.; Merz, K. Angew. Chem., Int. Ed. 2003, 42, 4399–4402. doi:10.1002/anie.200352160 |
| 21. | Huang, Q.; Larock, R. C. J. Org. Chem. 2003, 68, 980–988. doi:10.1021/jo0261303 |
| 22. | Belmont, P.; Parker, E. Eur. J. Org. Chem. 2009, 6075–6089. doi:10.1002/ejoc.200900790 |
| 5. | Beeler, A. B.; Su, S.; Singleton, C. A.; Porco, J. A., Jr. J. Am. Chem. Soc. 2007, 129, 1413–1419. doi:10.1021/ja0674744 |
| 23. | Soriano, E.; Marco-Contelles, J. J. Org. Chem. 2007, 72, 2651–2654. doi:10.1021/jo062594f |
| 24. | Fehr, C.; Winter, B.; Magpantay, I. Chem.–Eur. J. 2009, 15, 9773–9784. doi:10.1002/chem.200901292 |
| 16. | Cariou, K.; Ronan, B.; Mignani, S.; Fensterbank, L.; Malacria, M. Angew. Chem., Int. Ed. 2007, 46, 1881–1884. doi:10.1002/anie.200604026 |
| 17. | Hardin, A. R.; Sarpong, R. Org. Lett. 2007, 9, 4547–4550. doi:10.1021/ol701973s |
| 19. | Asao, N.; Chan, C. S.; Takahashi, K.; Yamamoto, Y. Tetrahedron 2005, 61, 11322–11326. doi:10.1016/j.tet.2005.09.012 |
| 14. | Nieto-Oberhuber, C.; López, S.; Muñoz, M. P.; Jiménez-Núñez, E.; Buñuel, E.; Cárdenas, D. J.; Echavarren, A. M. Chem.–Eur. J. 2006, 12, 1694–1702. doi:10.1002/chem.200501089 |
| 15. | Nevado, C.; Ferrer, C.; Echavarren, A. M. Org. Lett. 2004, 6, 3191–3194. doi:10.1021/ol0486573 |
| 20. | Dyker, G.; Hildebrandt, D.; Liu, J.; Merz, K. Angew. Chem., Int. Ed. 2003, 42, 4399–4402. doi:10.1002/anie.200352160 |
| 11. |
Patil, N. T.; Yamamoto, Y. J. Org. Chem. 2004, 69, 5139–5142. doi:10.1021/jo049416b
See for Cu(I)-mediated cycloisomerization/alcohol addition of an o-alkynylbenzaldehyde. |
| 12. |
Chernyak, D.; Gadamsetty, S. B.; Gevorgyan, V. Org. Lett. 2008, 10, 2307–2310. doi:10.1021/ol8008705
See for Cu(I)-mediated cycloisomerization. |
| 13. | Rauniyar, V.; Wang, Z. J.; Burks, H. E.; Toste, F. D. J. Am. Chem. Soc. 2011, 133, 8486–8489. doi:10.1021/ja202959n |
| 9. | Porcel, S.; Echavarren, A. M. Angew. Chem., Int. Ed. 2007, 46, 2672–2676. doi:10.1002/anie.200605041 |
| 10. | Nieto-Oberhuber, C.; Muñoz, M. P.; Buñuel, E.; Nevado, C.; Cárdenas, D. J.; Echavarren, A. M. Angew. Chem., Int. Ed. 2004, 43, 2402–2406. doi:10.1002/anie.200353207 |
| 18. | Ambrosini, L. M.; Lambert, T. H. ChemCatChem 2010, 2, 1373–1380. doi:10.1002/cctc.200900323 |
© 2012 Leng et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)