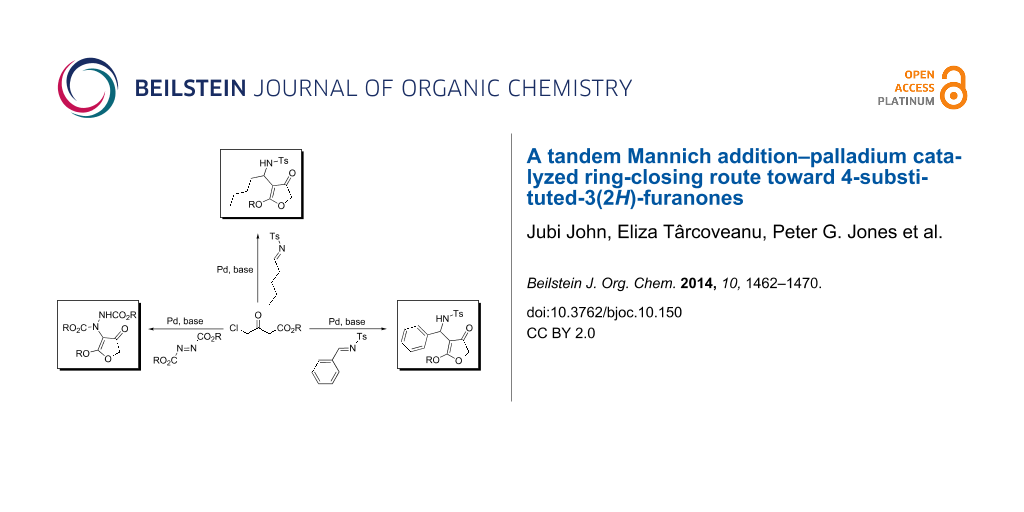
Abstract
A facile route towards highly functionalized 3(2H)-furanones via a sequential Mannich addition–palladium catalyzed ring closing has been elaborated. The reaction of 4-chloroacetoacetate esters with imines derived from aliphatic and aromatic aldehydes under palladium catalysis afforded 4-substituted furanones in good to excellent yields. 4-Hydrazino-3(2H)-furanones could also be synthesized from diazo esters in excellent yields by utilising the developed strategy. We could also efficiently transform the substituted furanones to aza-prostaglandin analogues.
Graphical Abstract
Introduction
Organic chemists welcome the introduction of facile tandem protocols because of the advantages of multiple bond formation in one-pot processes, which in turn makes the process economic and most importantly eco-friendly [1,2]. Conjugate addition is a very efficient tool used by synthetic chemists for the construction of carbon–carbon or carbon–heteroatom bonds [3]. Much effort has been invested to develop different variants of conjugate additions, both in catalyzed and non-catalyzed pathways. α-Halo ketones are known to react with zero-valent palladium like allyl halides to form oxa-π-allylpalladium complexes. Despite this fact, only minor attention has been paid to the formation of oxa-π-allylpalladium species from α-halo ketones and to their reactivity [4-8]. In this report we describe the use of a tandem methodology involving a Mannich addition/palladium-catalyzed ring-closing protocol for the facile synthesis of 4-substituted furanones.
Since 3(2H)-furanones form the main component of many natural products and pharmaceutically important compounds (Figure 1) a number of routes for their synthesis [9-18] to have been reported in the literature.
Figure 1: Bioactive molecules I [19], II [26], III & IV [21,22] with 3(2H)-furanone moiety.
Figure 1: Bioactive molecules I [19], II [26], III & IV [21,22] with 3(2H)-furanone moiety.
Substituted 3(2H)-furanones exhibit a wide range of biological activities such as antiallergic, antiulcer, antiinflammatory, and antitumor activities or selective COX-2 inhibition [19-26]. Synthetic routes for the preparation of these compounds involve transformations of substituted α-hydroxy-1,3-diketones [15,17], substituted furans [27-29], cyclization of allenic hydroxyketones [30], transition metal-catalyzed strategies (Au [31-33], Pt [34,35], Pd [36], Hg [37]), etc. Lately, organo-catalyzed asymmetric routes towards 3(2H)-furanones from 4-haloacetoacetates and nitrostyrene were reported by Lu et al. [38] and Yan et al. [39].
We have recently reported on a palladium-catalyzed tandem methodology for the synthesis of 4-substituted-3(2H)-furanones from activated alkenes and 4-chloroacetoacetates (Scheme 1) [40].
Scheme 1: Pd-catalyzed synthesis of 3(2H)-furanones from activated alkenes [40].
Scheme 1: Pd-catalyzed synthesis of 3(2H)-furanones from activated alkenes [40].
The reaction was found to be general for a wide range of alkenes derived from aromatic and aliphatic aldehydes. The reaction proceeded via Michael addition of the acetoacetate to the alkene with a subsequent palladium-catalyzed ring closure of the primary adduct to form the furanone. In this paper, we report our investigations that extend the reaction to heteroatom-containing electrophiles such as imines and diazo esters.
Results and Discussion
Following the work on nitrostyrenes, Yan et al. also reported a two-step asymmetric route towards 4-substituted-furanones from imines [41]. Recently Fructos et al. have shown that N-p-toluenesulfonyl-protected imines were better candidates for gold catalyzed Mannich addition of acetoacetates when compared to N-Boc (tert-butoxycarbonyl) and N-PMP (p-methoxyphenyl) imines [42]. Hence, we commenced our investigations with the reaction of tosylimine 1a and ethyl 4-chloroacetoacetate (2a) in the presence of 5 mol % of Pd(PPh3)4 and 2.0 equivalents of Na2CO3, in dioxane at 50 °C for 10 hours. The reaction afforded 4-substituted-3(2H)-furanone 3 in 57% yield (Scheme 2). Interestingly, we could not detect the formation of diene-2,5-dicarboxylate 4 (formed by the dimerization of 2a) as a side product, although this was observed previously in the reaction of activated alkenes [40] and 4-chloroacetoacetates.
Scheme 2: Pd-catalyzed synthesis of 3(2H)-furanone from tosylimine 1a.
Scheme 2: Pd-catalyzed synthesis of 3(2H)-furanone from tosylimine 1a.
Table 1 summarizes our efforts towards optimizing various reaction parameters with 1a and 2a as model substrates. Screening of bases revealed that Na2CO3 was more effective than either K2CO3 or KOt-Bu (Table 1, entries 1–3). When KOt-Bu was employed as base, byproduct 4 (formed by the dimerisation of 2a) was formed in higher amounts (Table 1, entry 3). From the tested catalysts, Pd(PPh3)4 and Pd2dba3·CHCl3, and ligands, dppe and P(o-furyl)3, the combination of Pd2dba3·CHCl3 and dppe afforded the furanone 3 in 88% yield (Table 1, entry 8). A solvent screen revealed that dioxane was best for the present transformation from which the product 3 was obtained in 88% yield (Table 1, entries 8–10). It was found that lowering the temperature had a negative influence on the yield as 3 was obtained only in 43% even after 24 hours when the substrates 1a and 2a were stirred in the presence of Pd2dba3·CHCl3/dppe/Na2CO3 in dioxane at room temperature. When the catalyst loading was decreased to 1 mol %, the reaction afforded the furanone 3 in 78% yield after 10 hours (Table 1, entry 14). The catalytic role of palladium in the present transformation was proved by conducting two control experiments. The first reaction was performed only in the presence of base (Table 1, entry 12) and the second one only in the presence of the Pd2dba3·CHCl3/dppe combination (Table 1, entry 13). Furanone 3 was obtained in 65% yield from the first reaction whereas the second reaction afforded only trace amounts of the desired product. Thus we can see that under palladium catalysis there is an increase in the yield of 3(2H)-furanone 3 by 23% by comparing the first control experiment and the reaction depicted in entry 8 of Table 1, thereby proving that palladium is catalyzing the reaction.
Table 1: Optimisation studiesa.
|
|
||||
| Entry | Catalyst | Ligand | Base | Yield 3b(%) |
|---|---|---|---|---|
| 1 | Pd(PPh3)4 | – | Na2CO3 | 57 |
| 2 | Pd(PPh3)4 | – | K2CO3 | 34 |
| 3 | Pd(PPh3)4 | – | KOt-Bu | 19c |
| 4 | Pd(PPh3)4 | P(o-furyl)3 | Na2CO3 | 66 |
| 5 | Pd(PPh3)4 | dppe | Na2CO3 | 70 |
| 6 | Pd2dba3·CHCl3 | – | Na2CO3 | 85 |
| 7 | Pd2dba3·CHCl3 | P(o-furyl)3 | Na2CO3 | 82 |
| 8 | Pd2dba3·CHCl3 | dppe | Na2CO3 | 88 |
| 9 | Pd2dba3·CHCl3 | dppe | Na2CO3 | 80d |
| 10 | Pd2dba3·CHCl3 | dppe | Na2CO3 | 84e |
| 11 | Pd2dba3·CHCl3 | dppe | Na2CO3 | 43f |
| 12 | – | – | Na2CO3 | 65 |
| 13 | Pd2dba3·CHCl3 | dppe | – | trace |
| 14 | Pd2dba3·CHCl3 | dppe | Na2CO3 | 78g |
aReaction conditions: 1a (1.0 equiv), 2a (1.1 equiv), catalyst (5 mol %), ligand (10 mol %), base (2.0 equiv), dioxane (2 mL), 50 °C, 10 h. bIsolated yield. cDimerisation product 4 was formed in 27% yield.dCH3CN instead of dioxane. eTHF instead of dioxane. frt, 24 h. g1 mol % of Pd2dba3·CHCl3, 5 mol % of dppe.
With optimal conditions in hand (imine (1.0 equiv), 4-chloroacetoacetate (1.1 equiv), Pd2dba3·CHCl3 (5 mol %), dppe (10 mol %) Na2CO3 (2.0 equiv) in dioxane at 50 °C for 10 h), studies towards the generality of the reaction were carried out with different imines 1a–c derived from aromatic aldehydes with 4-chloro-acetoacetates 2a,b.
In all the cases substituted 3(2H)-furanones were obtained in good to excellent yields (Figure 2). The reaction was also extended to tosylimines derived from aliphatic aldehydes, pentanal (for 1d) and 3-phenylpropionaldehyde (for 1e) and thus we could rule out any influence of the aromatic moiety on the outcome of the reaction. The corresponding 4-susbtituted-3(2H)-furanones were obtained from these two substrates in good yields (Figure 2). We also tried a reaction with N-Boc protected imine under optimized conditions and the corresponding furanone was obtained in good yield (Figure 2, compound 14). The structure elucidation of the furanone products 3, and 5–14 was accomplished by the usual spectroscopic methods (see Supporting Information File 1) and also by X-ray structure determination [43] for furanones 7 (product formed by the reaction of 1b with 2b) and 10 (product formed by the reaction of 1d with 2a) (Figure 3).
Figure 2: Generalisation with aromatic and aliphatic imines (reaction conditions: 1 (1.0 equiv), 2 (1.1 equiv), Pd2dba3·CHCl3 (5 mol %), dppe (10 mol %), Na2CO3 (2.0 equiv), dioxane (2 mL), 50 °C, 10 h, isolated yield).
Figure 2: Generalisation with aromatic and aliphatic imines (reaction conditions: 1 (1.0 equiv), 2 (1.1 equiv...
![[1860-5397-10-150-3]](/bjoc/content/figures/1860-5397-10-150-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Thermal ellipsoid diagrams (50% probability levels) of 4-substituted-3(2H)-furanones 7 (above) and 10 (below).
Figure 3: Thermal ellipsoid diagrams (50% probability levels) of 4-substituted-3(2H)-furanones 7 (above) and ...
We believe that the mechanism of the present furanone synthesis from imines is similar to that reported for activated alkenes [40]. This tandem protocol has two steps, the first being a Mannich addition of the enolate to the imine. To find out whether Mannich addition is palladium catalyzed, we carried out two reactions of tosylimine 1a with methyl acetoacetate. The first transformation was carried out under basic conditions (Na2CO3 (1.0 equiv), dioxane, 50 °C, 6 h) and the second under optimized conditions. Both reactions afforded the Mannich addition product in similar yields, proving that Mannich addition was uncatalyzed.
Based on these results, we propose a plausible mechanism for 3(2H)-furanone synthesis from tosylimines and 4-chloroacetoacetate (Scheme 3). The reaction starts with the Mannich addition of the enolate 15 to the carbon atom of the imine double bond to form intermediate 16. This step is the same for both the catalyzed and the uncatalyzed pathway. The second step of the catalyzed route involves the oxidative addition of Pd(0)Ln to the C–Cl bond of Mannich adduct 16 to form 17. The oxy-π-allylpalladium intermediate 18 can then be formed from intermediate 17 [4-8]. The final step of the catalyzed mechanism, i.e., the ring closure towards the formation of 3(2H)-furanone is initiated by the abstraction of the acidic proton by the base and consequent ester enolate attack to the end carbon of the oxy-π-allylpalladium intermediate.
Scheme 3: Mechanism of formation of the 3(2H)-furanone derivative from an imine.
Scheme 3: Mechanism of formation of the 3(2H)-furanone derivative from an imine.
Having established an efficient method for the synthesis of highly functionalized furanones from imines, we were interested next in extending the range of the electrophiles by introducing different functionalities at the 4-position of the 3(2H)-furanone. We chose to check the reactivity of diisopropylazodicarboxylate (19a) with ethyl 4-chloroacetoacetate (2a) under palladium catalysis. In the initial reaction, 19a was treated with 2a in the presence of Pd(PPh3)4 as catalyst and base (K2CO3) in dioxane at 50 °C. After 10 hours, the expected product 4-hydrazino-3(2H)-furanone 20 was isolated in 58% yield (Scheme 4).
Scheme 4: Pd-catalyzed synthesis of 3(2H)-furanone from diazoester 19a.
Scheme 4: Pd-catalyzed synthesis of 3(2H)-furanone from diazoester 19a.
Table 2 describes the screening of different reaction conditions to improve the yield of 20. First we tested the efficacy of different bases K2CO3, Na2CO3 and KOt-Bu, of which Na2CO3 proved to be the best (Table 2, entries 1–3). The highest yield for 4-hydrazino-3(2H)-furanone was given when Pd(PPh3)4 alone was used among different catalyst/ligand combinations tested with Pd(PPh3)4 and Pd2dba3.CHCl3 as catalysts and dppe and P(o-furyl)3 as ligands (Table 2, entries 2, and 4–8). The product was obtained in better yields when dioxane was used as the solvent (Table 2, entries 2, 9 and 10). At room temperature the reaction with optimised catalyst/solvent system furnished only 38% of the substituted furanone even after 24 hours (Table 2, entry 11). A control experiment was done without the catalyst/ligand combination but only in the presence of base, which afforded the furanone in 69% yield (Table 2, entry 12). Thus by comparing the control experiment and the reaction depicted in entry 2 of Table 2 we can confirm that palladium catalyzes the reaction.
Table 2: Optimisation studiesa.
|
|
||||
| Entry | Catalyst | Ligand | Base | Yield 20b(%) |
|---|---|---|---|---|
| 1 | Pd(PPh3)4 | – | K2CO3 | 58 |
| 2 | Pd(PPh3)4 | – | Na2CO3 | 90 |
| 3 | Pd(PPh3)4 | – | KOt-Bu | 35c |
| 4 | Pd(PPh3)4 | P(o-furyl)3 | Na2CO3 | 75 |
| 5 | Pd(PPh3)4 | dppe | Na2CO3 | 79 |
| 6 | Pd2dba3.CHCl3 | – | Na2CO3 | 83 |
| 7 | Pd2dba3.CHCl3 | P(o-furyl)3 | Na2CO3 | 80 |
| 8 | Pd2dba3.CHCl3 | dppe | Na2CO3 | 76 |
| 9 | Pd(PPh3)4 | – | Na2CO3 | 58d |
| 10 | Pd(PPh3)4 | – | Na2CO3 | 82e |
| 11 | Pd(PPh3)4 | – | Na2CO3 | 38f |
| 12 | – | – | Na2CO3 | 69 |
aReaction conditions: 19a (1.0 equiv), 2a (1.1 equiv), catalyst (5 mol %), ligand (10 mol %), base (2.0 equiv), dioxane (2 mL), 50 °C, 10 h. bIsolated yield. cDimerization product 4 was formed in 23% yield. dCH3CN instead of dioxane. eTHF instead of dioxane. frt, 24 h.
The generality of the reaction was checked with different diazo esters 19a–c with 4-chloroacetoacetate esters 2a and 2b under the optimized conditions (diazo ester (1.0 equiv), 4-chloroacetoacetate (1.1 equiv), Pd(PPh3)4 (5 mol %), Na2CO3 (2.0 equiv) in dioxane at 50 °C for 10 h). All reactions afforded the corresponding 4-hydrazino-3(2H)-furanones in excellent yields (Figure 4).
Figure 4: Generalisation with diazo esters (reaction conditions: 19 (1.0 equiv), 2 (1.1 equiv), Pd(PPh3)4 (5 mol %), Na2CO3 (2.0 equiv), dioxane (2 mL), 50 °C, 10 h, isolated yield).
Figure 4: Generalisation with diazo esters (reaction conditions: 19 (1.0 equiv), 2 (1.1 equiv), Pd(PPh3)4 (5 ...
The possibility for further functionalization of 4-substitued furanones was checked by treating 11 with n-heptylamine in MeOH at 40 °C. After 10 hours the aza-prostaglandin analogue [26] 27 was isolated in 85% yield (Scheme 5).
Scheme 5: Synthesis of aza-prostaglandin analogue.
Scheme 5: Synthesis of aza-prostaglandin analogue.
Conclusion
We have developed an efficient protocol for the synthesis of 4-substituted 3(2H)-furanones by the reaction of imines or diazo esters with 4-chloroacetoacetates under palladium catalysis. We could extend the reaction to imines derived from aromatic and aliphatic aldehydes. The reaction proceeded via a catalyzed tandem Mannich addition–palladium-catalyzed ring-closing pathway to afford various 3(2H)-furanones in good to excellent yields. We could further apply this route to the preparation of an aza-prostaglandin analogue. The synthesized molecules are currently being screened for biological activities. We have also extended the reaction to triple-bonded electrophiles such as acetylenes, benzyne and nitriles; the results will be reported in due course. Studies are in progress to develop a stereoselective version of the process.
Supporting Information
| Supporting Information File 1: Experimental part and NMR spectra. | ||
| Format: PDF | Size: 3.1 MB | Download |
References
-
Guo, H.-C.; Ma, J.-A. Angew. Chem., Int. Ed. 2006, 45, 354–366. doi:10.1002/anie.200500195
Return to citation in text: [1] -
Tietze, L. F. Chem. Rev. 1996, 96, 115–136. doi:10.1021/cr950027e
Return to citation in text: [1] -
Perlmutter, P. Conjugate Addition Reactions in Organic Synthesis; Pergamon: Oxford, 1992.
Return to citation in text: [1] -
Ogoshi, S.; Morimoto, T.; Nishio, K.; Ohe, K.; Murai, S. J. Org. Chem. 1993, 58, 9–10. doi:10.1021/jo00053a004
Return to citation in text: [1] [2] -
Ikeda, I.; Ohsuka, A.; Tani, K.; Hirao, T.; Kurosawa, H. J. Org. Chem. 1996, 61, 4971–4974. doi:10.1021/jo951425k
Return to citation in text: [1] [2] -
Stille, J. K.; Wong, P. K. J. Org. Chem. 1975, 40, 532–534. doi:10.1021/jo00892a044
Return to citation in text: [1] [2] -
Negishi, E.; de Meijere, A., Eds. Handbook of Organopalladium Chemistry for Organic Synthesis; John Wiley & Sons: New York, 2002; Vol. 1 and 2.
Return to citation in text: [1] [2] -
Patil, N. T.; Yamamoto, Y. Top. Organomet. Chem. 2006, 19, 91–113. doi:10.1007/3418_014
Return to citation in text: [1] [2] -
Curran, D. P.; Singleton, D. H. Tetrahedron Lett. 1983, 24, 2079–2082. doi:10.1016/S0040-4039(00)81849-3
Return to citation in text: [1] -
Wolff, S.; Agosta, W. C. Tetrahedron Lett. 1985, 26, 703–704. doi:10.1016/S0040-4039(00)89113-3
Return to citation in text: [1] -
Saimoto, H.; Hiyama, T.; Nozaki, H. J. Am. Chem. Soc. 1981, 103, 4975–4977. doi:10.1021/ja00406a066
Return to citation in text: [1] -
Jackson, R. F. W.; Raphael, R. A. J. Chem. Soc., Perkin Trans. 1 1984, 535–539. doi:10.1039/p19840000535
Return to citation in text: [1] -
Kupchan, S. M.; Sigel, C. W.; Matz, M. J.; Gilmore, C. J.; Bryan, R. F. J. Am. Chem. Soc. 1976, 98, 2295–2300. doi:10.1021/ja00424a050
Return to citation in text: [1] -
Smith, A. B., III; Guaciaro, M. A.; Schow, S. R.; Wovkulich, P. M.; Toder, B. H.; Hall, T. W. J. Am. Chem. Soc. 1981, 103, 219–222. doi:10.1021/ja00391a054
Return to citation in text: [1] -
Smith, A. B., III; Levenberg, P. A.; Jerris, P. J.; Scarborough, R. M., Jr.; Wovkulich, P. M. J. Am. Chem. Soc. 1981, 103, 1501–1513. doi:10.1021/ja00396a034
Return to citation in text: [1] [2] -
Dreyer, D. L.; Lee, A. Phytochemistry 1972, 11, 763–767. doi:10.1016/0031-9422(72)80045-1
Return to citation in text: [1] -
Jerris, P. J.; Smith, A. B., III. J. Org. Chem. 1981, 46, 577–585. doi:10.1021/jo00316a018
Return to citation in text: [1] [2] -
Haug, T. T.; Kirsch, S. F. In Targets in Heterocyclic Systems; Attanasi, O. A.; Spinelli, D., Eds.; Royal Society of Chemistry: Cambridge, 2009; Vol. 13, pp 57–91.
See for a book chapter on synthesis and chemistry of 3-(2H)-furanone.
Return to citation in text: [1] -
Mack, R. A.; Zazulak, W. I.; Radov, L. A.; Baer, J. E.; Stewart, J. D.; Elzer, P. H.; Kinsolving, C. R.; Georgiev, V. S. J. Med. Chem. 1988, 31, 1910–1918. doi:10.1021/jm00118a008
Return to citation in text: [1] [2] -
Felman, S. W.; Jirkovsky, I.; Memoli, K. A.; Borella, L.; Wells, C.; Russell, J.; Ward, J. J. Med. Chem. 1992, 35, 1183–1190. doi:10.1021/jm00085a003
Return to citation in text: [1] -
Jackson, R. F. W.; Raphael, R. A. Tetrahedron Lett. 1983, 24, 2117–2120. doi:10.1016/S0040-4039(00)81859-6
Return to citation in text: [1] [2] -
Villemin, D.; Jaffrès, P.-A.; Hachémi, M. Tetrahedron Lett. 1997, 38, 537–538. doi:10.1016/S0040-4039(96)02365-9
Return to citation in text: [1] [2] -
Sakamoto, H. T.; Flausino, D.; Castellano, E. E.; Stark, C. B. W.; Gates, P. J.; Lopes, N. P. J. Nat. Prod. 2003, 66, 693–695. doi:10.1021/np020314v
Return to citation in text: [1] -
Shin, S. S.; Byun, Y.; Lim, K. M.; Choi, J. K.; Lee, K.-W.; Moh, J. H.; Kim, J. K.; Jeong, Y. S.; Kim, J. Y.; Choi, Y. H.; Koh, H.-J.; Park, Y.-H.; Oh, Y. I.; Noh, M.-S.; Chung, S. J. Med. Chem. 2004, 47, 792–804. doi:10.1021/jm020545z
Return to citation in text: [1] -
Shamshina, J. L.; Snowden, T. S. Tetrahedron Lett. 2007, 48, 3767–3769. doi:10.1016/j.tetlet.2007.03.166
Return to citation in text: [1] -
Pashkovskii, F. S.; Shchukina, E. M.; Gribovskii, M. G.; Lakhvich, F. A. Russ. J. Org. Chem. 2006, 42, 527–540. doi:10.1134/S1070428006040087
Return to citation in text: [1] [2] [3] -
Henry, D. W.; Silverstein, R. M. J. Org. Chem. 1966, 31, 2391–2394. doi:10.1021/jo01345a506
Return to citation in text: [1] -
Antonioletti, R.; Bonadies, F.; Prencipe, T.; Scettri, A. J. Chem. Soc., Chem. Commun. 1988, 850–851. doi:10.1039/c39880000850
Return to citation in text: [1] -
Winkler, J. D.; Oh, K.; Asselin, S. M. Org. Lett. 2005, 7, 387–389. doi:10.1021/ol047810q
Return to citation in text: [1] -
Poonoth, M.; Krause, N. J. Org. Chem. 2011, 76, 1934–1936. doi:10.1021/jo102416e
Return to citation in text: [1] -
Liu, Y.; Liu, M.; Guo, S.; Tu, H.; Zhou, Y.; Gao, H. Org. Lett. 2006, 8, 3445–3448. doi:10.1021/ol061059z
Return to citation in text: [1] -
Crone, B.; Kirsch, S. F. J. Org. Chem. 2007, 72, 5435–5438. doi:10.1021/jo070695n
Return to citation in text: [1] -
Egi, M.; Azechi, K.; Saneto, M.; Shimizu, K.; Akai, S. J. Org. Chem. 2010, 75, 2123–2126. doi:10.1021/jo100048j
Return to citation in text: [1] -
Kirsch, S. F.; Binder, J. T.; Liébert, C.; Menz, H. Angew. Chem., Int. Ed. 2006, 45, 5878–5880. doi:10.1002/anie.200601836
Return to citation in text: [1] -
Bunnelle, E. M.; Smith, C. R.; Lee, S. K.; Singaram, S. W.; Rhodes, A. J.; Sarpong, R. Tetrahedron 2008, 64, 7008–7014. doi:10.1016/j.tet.2008.02.103
Return to citation in text: [1] -
Silva, F.; Reiter, M.; Mills-Webb, R.; Sawicki, M.; Klär, D.; Bensel, N.; Wagner, A.; Gouverneur, V. J. Org. Chem. 2006, 71, 8390–8394. doi:10.1021/jo061292a
Return to citation in text: [1] -
Marson, C. M.; Edaan, E.; Morrell, J. M.; Coles, S. J.; Hursthouse, M. B.; Davies, D. T. Chem. Commun. 2007, 2494–2496. doi:10.1039/b701548h
Return to citation in text: [1] -
Dou, X.; Han, X.; Lu, Y. Chem.–Eur. J. 2012, 18, 85–89. doi:10.1002/chem.201102796
Return to citation in text: [1] -
Yan, Y.-Y.; Lu, R.-J.; Wang, J.-J.; Xuan, Y.-N.; Yan, M. Tetrahedron 2012, 68, 6123–6130. doi:10.1016/j.tet.2012.05.082
Return to citation in text: [1] -
John, J.; Hopf, H. Eur. J. Org. Chem. 2013, 841–845. doi:10.1002/ejoc.201201253
Return to citation in text: [1] [2] [3] [4] -
Luo, N.-H.; Sun, X.; Yan, Y.-Y.; Nie, S.-Z.; Yan, M. Tetrahedron: Asymmetry 2011, 22, 1536–1541. doi:10.1016/j.tetasy.2011.08.022
Return to citation in text: [1] -
Delgada-Rebollo, M.; Moreno, R.; Fructos, M. R.; Prieto, A. Eur. J. Org. Chem. 2013, 31–34. doi:10.1002/ejoc.201201252
Return to citation in text: [1] -
Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications no. CCDC-922077 (7), -922078 (10). Copies of the data can be obtained free of charge from http://www.ccdc.cam.ac.uk/data_request/cif
Return to citation in text: [1]
| 1. | Guo, H.-C.; Ma, J.-A. Angew. Chem., Int. Ed. 2006, 45, 354–366. doi:10.1002/anie.200500195 |
| 2. | Tietze, L. F. Chem. Rev. 1996, 96, 115–136. doi:10.1021/cr950027e |
| 19. | Mack, R. A.; Zazulak, W. I.; Radov, L. A.; Baer, J. E.; Stewart, J. D.; Elzer, P. H.; Kinsolving, C. R.; Georgiev, V. S. J. Med. Chem. 1988, 31, 1910–1918. doi:10.1021/jm00118a008 |
| 37. | Marson, C. M.; Edaan, E.; Morrell, J. M.; Coles, S. J.; Hursthouse, M. B.; Davies, D. T. Chem. Commun. 2007, 2494–2496. doi:10.1039/b701548h |
| 9. | Curran, D. P.; Singleton, D. H. Tetrahedron Lett. 1983, 24, 2079–2082. doi:10.1016/S0040-4039(00)81849-3 |
| 10. | Wolff, S.; Agosta, W. C. Tetrahedron Lett. 1985, 26, 703–704. doi:10.1016/S0040-4039(00)89113-3 |
| 11. | Saimoto, H.; Hiyama, T.; Nozaki, H. J. Am. Chem. Soc. 1981, 103, 4975–4977. doi:10.1021/ja00406a066 |
| 12. | Jackson, R. F. W.; Raphael, R. A. J. Chem. Soc., Perkin Trans. 1 1984, 535–539. doi:10.1039/p19840000535 |
| 13. | Kupchan, S. M.; Sigel, C. W.; Matz, M. J.; Gilmore, C. J.; Bryan, R. F. J. Am. Chem. Soc. 1976, 98, 2295–2300. doi:10.1021/ja00424a050 |
| 14. | Smith, A. B., III; Guaciaro, M. A.; Schow, S. R.; Wovkulich, P. M.; Toder, B. H.; Hall, T. W. J. Am. Chem. Soc. 1981, 103, 219–222. doi:10.1021/ja00391a054 |
| 15. | Smith, A. B., III; Levenberg, P. A.; Jerris, P. J.; Scarborough, R. M., Jr.; Wovkulich, P. M. J. Am. Chem. Soc. 1981, 103, 1501–1513. doi:10.1021/ja00396a034 |
| 16. | Dreyer, D. L.; Lee, A. Phytochemistry 1972, 11, 763–767. doi:10.1016/0031-9422(72)80045-1 |
| 17. | Jerris, P. J.; Smith, A. B., III. J. Org. Chem. 1981, 46, 577–585. doi:10.1021/jo00316a018 |
| 18. |
Haug, T. T.; Kirsch, S. F. In Targets in Heterocyclic Systems; Attanasi, O. A.; Spinelli, D., Eds.; Royal Society of Chemistry: Cambridge, 2009; Vol. 13, pp 57–91.
See for a book chapter on synthesis and chemistry of 3-(2H)-furanone. |
| 38. | Dou, X.; Han, X.; Lu, Y. Chem.–Eur. J. 2012, 18, 85–89. doi:10.1002/chem.201102796 |
| 4. | Ogoshi, S.; Morimoto, T.; Nishio, K.; Ohe, K.; Murai, S. J. Org. Chem. 1993, 58, 9–10. doi:10.1021/jo00053a004 |
| 5. | Ikeda, I.; Ohsuka, A.; Tani, K.; Hirao, T.; Kurosawa, H. J. Org. Chem. 1996, 61, 4971–4974. doi:10.1021/jo951425k |
| 6. | Stille, J. K.; Wong, P. K. J. Org. Chem. 1975, 40, 532–534. doi:10.1021/jo00892a044 |
| 7. | Negishi, E.; de Meijere, A., Eds. Handbook of Organopalladium Chemistry for Organic Synthesis; John Wiley & Sons: New York, 2002; Vol. 1 and 2. |
| 8. | Patil, N. T.; Yamamoto, Y. Top. Organomet. Chem. 2006, 19, 91–113. doi:10.1007/3418_014 |
| 34. | Kirsch, S. F.; Binder, J. T.; Liébert, C.; Menz, H. Angew. Chem., Int. Ed. 2006, 45, 5878–5880. doi:10.1002/anie.200601836 |
| 35. | Bunnelle, E. M.; Smith, C. R.; Lee, S. K.; Singaram, S. W.; Rhodes, A. J.; Sarpong, R. Tetrahedron 2008, 64, 7008–7014. doi:10.1016/j.tet.2008.02.103 |
| 3. | Perlmutter, P. Conjugate Addition Reactions in Organic Synthesis; Pergamon: Oxford, 1992. |
| 36. | Silva, F.; Reiter, M.; Mills-Webb, R.; Sawicki, M.; Klär, D.; Bensel, N.; Wagner, A.; Gouverneur, V. J. Org. Chem. 2006, 71, 8390–8394. doi:10.1021/jo061292a |
| 15. | Smith, A. B., III; Levenberg, P. A.; Jerris, P. J.; Scarborough, R. M., Jr.; Wovkulich, P. M. J. Am. Chem. Soc. 1981, 103, 1501–1513. doi:10.1021/ja00396a034 |
| 17. | Jerris, P. J.; Smith, A. B., III. J. Org. Chem. 1981, 46, 577–585. doi:10.1021/jo00316a018 |
| 30. | Poonoth, M.; Krause, N. J. Org. Chem. 2011, 76, 1934–1936. doi:10.1021/jo102416e |
| 19. | Mack, R. A.; Zazulak, W. I.; Radov, L. A.; Baer, J. E.; Stewart, J. D.; Elzer, P. H.; Kinsolving, C. R.; Georgiev, V. S. J. Med. Chem. 1988, 31, 1910–1918. doi:10.1021/jm00118a008 |
| 20. | Felman, S. W.; Jirkovsky, I.; Memoli, K. A.; Borella, L.; Wells, C.; Russell, J.; Ward, J. J. Med. Chem. 1992, 35, 1183–1190. doi:10.1021/jm00085a003 |
| 21. | Jackson, R. F. W.; Raphael, R. A. Tetrahedron Lett. 1983, 24, 2117–2120. doi:10.1016/S0040-4039(00)81859-6 |
| 22. | Villemin, D.; Jaffrès, P.-A.; Hachémi, M. Tetrahedron Lett. 1997, 38, 537–538. doi:10.1016/S0040-4039(96)02365-9 |
| 23. | Sakamoto, H. T.; Flausino, D.; Castellano, E. E.; Stark, C. B. W.; Gates, P. J.; Lopes, N. P. J. Nat. Prod. 2003, 66, 693–695. doi:10.1021/np020314v |
| 24. | Shin, S. S.; Byun, Y.; Lim, K. M.; Choi, J. K.; Lee, K.-W.; Moh, J. H.; Kim, J. K.; Jeong, Y. S.; Kim, J. Y.; Choi, Y. H.; Koh, H.-J.; Park, Y.-H.; Oh, Y. I.; Noh, M.-S.; Chung, S. J. Med. Chem. 2004, 47, 792–804. doi:10.1021/jm020545z |
| 25. | Shamshina, J. L.; Snowden, T. S. Tetrahedron Lett. 2007, 48, 3767–3769. doi:10.1016/j.tetlet.2007.03.166 |
| 26. | Pashkovskii, F. S.; Shchukina, E. M.; Gribovskii, M. G.; Lakhvich, F. A. Russ. J. Org. Chem. 2006, 42, 527–540. doi:10.1134/S1070428006040087 |
| 31. | Liu, Y.; Liu, M.; Guo, S.; Tu, H.; Zhou, Y.; Gao, H. Org. Lett. 2006, 8, 3445–3448. doi:10.1021/ol061059z |
| 32. | Crone, B.; Kirsch, S. F. J. Org. Chem. 2007, 72, 5435–5438. doi:10.1021/jo070695n |
| 33. | Egi, M.; Azechi, K.; Saneto, M.; Shimizu, K.; Akai, S. J. Org. Chem. 2010, 75, 2123–2126. doi:10.1021/jo100048j |
| 21. | Jackson, R. F. W.; Raphael, R. A. Tetrahedron Lett. 1983, 24, 2117–2120. doi:10.1016/S0040-4039(00)81859-6 |
| 22. | Villemin, D.; Jaffrès, P.-A.; Hachémi, M. Tetrahedron Lett. 1997, 38, 537–538. doi:10.1016/S0040-4039(96)02365-9 |
| 26. | Pashkovskii, F. S.; Shchukina, E. M.; Gribovskii, M. G.; Lakhvich, F. A. Russ. J. Org. Chem. 2006, 42, 527–540. doi:10.1134/S1070428006040087 |
| 27. | Henry, D. W.; Silverstein, R. M. J. Org. Chem. 1966, 31, 2391–2394. doi:10.1021/jo01345a506 |
| 28. | Antonioletti, R.; Bonadies, F.; Prencipe, T.; Scettri, A. J. Chem. Soc., Chem. Commun. 1988, 850–851. doi:10.1039/c39880000850 |
| 29. | Winkler, J. D.; Oh, K.; Asselin, S. M. Org. Lett. 2005, 7, 387–389. doi:10.1021/ol047810q |
| 40. | John, J.; Hopf, H. Eur. J. Org. Chem. 2013, 841–845. doi:10.1002/ejoc.201201253 |
| 39. | Yan, Y.-Y.; Lu, R.-J.; Wang, J.-J.; Xuan, Y.-N.; Yan, M. Tetrahedron 2012, 68, 6123–6130. doi:10.1016/j.tet.2012.05.082 |
| 40. | John, J.; Hopf, H. Eur. J. Org. Chem. 2013, 841–845. doi:10.1002/ejoc.201201253 |
| 26. | Pashkovskii, F. S.; Shchukina, E. M.; Gribovskii, M. G.; Lakhvich, F. A. Russ. J. Org. Chem. 2006, 42, 527–540. doi:10.1134/S1070428006040087 |
| 40. | John, J.; Hopf, H. Eur. J. Org. Chem. 2013, 841–845. doi:10.1002/ejoc.201201253 |
| 4. | Ogoshi, S.; Morimoto, T.; Nishio, K.; Ohe, K.; Murai, S. J. Org. Chem. 1993, 58, 9–10. doi:10.1021/jo00053a004 |
| 5. | Ikeda, I.; Ohsuka, A.; Tani, K.; Hirao, T.; Kurosawa, H. J. Org. Chem. 1996, 61, 4971–4974. doi:10.1021/jo951425k |
| 6. | Stille, J. K.; Wong, P. K. J. Org. Chem. 1975, 40, 532–534. doi:10.1021/jo00892a044 |
| 7. | Negishi, E.; de Meijere, A., Eds. Handbook of Organopalladium Chemistry for Organic Synthesis; John Wiley & Sons: New York, 2002; Vol. 1 and 2. |
| 8. | Patil, N. T.; Yamamoto, Y. Top. Organomet. Chem. 2006, 19, 91–113. doi:10.1007/3418_014 |
| 40. | John, J.; Hopf, H. Eur. J. Org. Chem. 2013, 841–845. doi:10.1002/ejoc.201201253 |
| 43. | Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications no. CCDC-922077 (7), -922078 (10). Copies of the data can be obtained free of charge from http://www.ccdc.cam.ac.uk/data_request/cif |
| 41. | Luo, N.-H.; Sun, X.; Yan, Y.-Y.; Nie, S.-Z.; Yan, M. Tetrahedron: Asymmetry 2011, 22, 1536–1541. doi:10.1016/j.tetasy.2011.08.022 |
| 42. | Delgada-Rebollo, M.; Moreno, R.; Fructos, M. R.; Prieto, A. Eur. J. Org. Chem. 2013, 31–34. doi:10.1002/ejoc.201201252 |
© 2014 John et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)