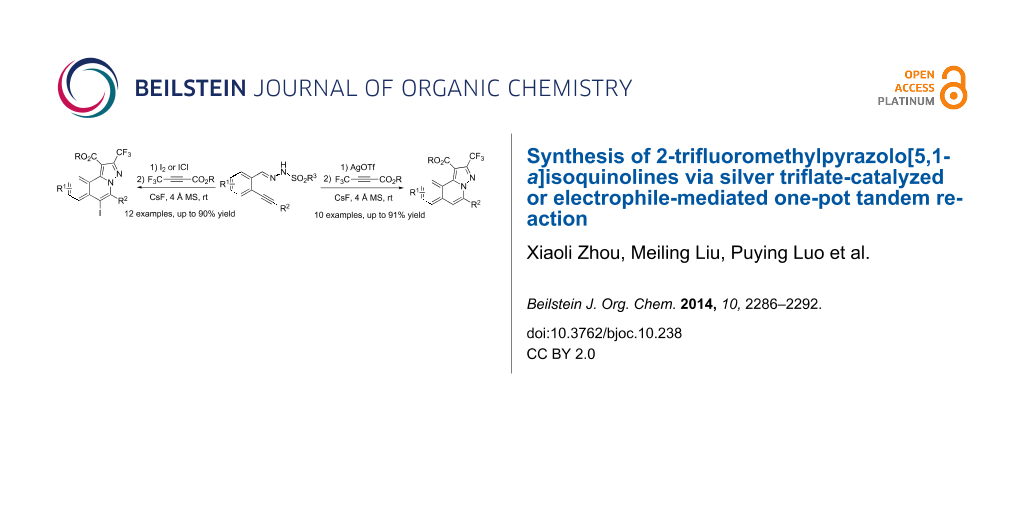
Abstract
An efficient one-pot tandem cyclization/[3 + 2] cycloaddition reaction of N’-(2-alkynylbenzylidene)hydrazides with ethyl 4,4,4-trifluorobut-2-ynoate under silver triflate-catalyzed or electrophile-mediated conditions is described. Various trifluoromethylated pyrazolo[5,1-a]isoquinolines were afforded in moderate to excellent yield by this developed method.
Graphical Abstract
Introduction
Isoquinolines and isoquinoline-derived heterocycles are prevalent structural motifs in natural products and pharmaceuticals that exhibit remarkable biological activities [1,2]. Therefore, great attention has been directed toward the development of efficient methods for the selective functionalization of the isoquinoline cores. Among these, pyrazolo[5,1-a]isoquinoline is an important class of isoquinoline derivatives. Recently, much effort has been spent on the synthesis of these compounds due to their promising biological activities [3-18]. For instance, in 2010, Wu and co-workers found some pyrazolo[5,1-a]isoquinoline derivatives showing activities for the inhibition of CDC25B, TC-PTP, and PTP1B [4].
It has been proved that the physical, chemical, and biological activity of organic molecules can be dramatically improved by substitution of hydrogen with fluorine atoms because of the strong electronegativity, the small size, the strength of the C–F bond, and the low polarizability of the fluorine atom. Statistically, more than 20% of the pharmaceuticals and 40% of the agrochemicals contain one or more fluorine atoms. Thus, there has been considerable interest in developing an efficient method for the synthesis of fluorinated heterocycles. Perfluoroalkynoate is a versatile and powerful building block for generating functionalized perfluoroalkylated compounds, especially fluorinated heterocycles, by tandem reactions [19-26]. For example, 2-perfluoroalkynoates have been widely used in synthesizing fluorinated heterocycles, such as benzodiazepines [22], chromenes [21,25], and 2-oxopyridine-fused 1,3-diazaheterocycles [26].
As part of our ongoing efforts in developing synthetic approaches for the functionalization of isoquinoline cores [11,14] and the synthesis of novel fluorinated heterocycles [27] with potential biological applications, herein, we describe an efficient method for the one-pot synthesis of trifluoromethylated pyrazolo[5,1-a]isoquinoline derivates via a Lewis acid (AgOTf) or an electrophile- (I2 or ICl) promoted annulation of N’-(2-alkynylbenzylidene)hydrazides followed by an 1,3-dipolar cycloaddition.
Results and Discussion
Based on Wu’s work on the silver triflate-catalyzed tandem reaction of N’-(2-alkynylbenzylidene)hydrazides with dimethyl acetylenedicarboxylate [28], we started our research by examining the reaction of N’-(2-alkynylbenzylidene)hydrazides 1a (0.3 mmol) and ethyl 4,4,4-trifluorobut-2-ynoate (2, 0.6 mmol) using NaOAc (0.45 mmol) as base, in the presence of AgOTf (5 mol %) and 4 Å MS (75 mg) in CH2Cl2 (3 mL) at room temperature overnight. Surprisingly, the unexpected [3 + 2] cycloaddition product pyrazolo[5,1-a]isoquinoline derivative 3a instead of the isoquinoline-based azomethine ylide [28] was obtained in good yield (84%, Table 1, entry 1). Similar yields were obtained when NaHCO3 or K2CO3 were used as base (83% and 86% yield, respectively, Table 1, entries 2 and 3). Several other inorganic or organic bases were examined, and the results showed that CsF was the best choice (91% yield, Table 1, entry 8). The control experiment also showed that the base was important for the reaction to proceed (8% yield, Table 1, entry 13). Subsequently, a range of solvents, such as acetonitrile, toluene, THF, dioxane and DMA were screened, and the results revealed that CH2Cl2 was the best one, and most of the others were exhibited good yields (Table 1, entry 8 and entries 14–18). The yield (65%) was reduced obviously when the loading of base was decreased to 1.0 equiv (Table 1, entry 19).
Table 1: Screening of conditions for the silver triflate-catalyzed reaction of N’-(2-alkynylbenzylidene)hydrazide 1a with ethyl 4,4,4-trifluorobut-2-ynoate 2a.
|
|
|||
| Entry | Base (1.5 equiv) | Solvent | Yield (%)b |
|---|---|---|---|
| 1 | NaOAc | CH2Cl2 | 84 |
| 2 | NaHCO3 | CH2Cl2 | 83 |
| 3 | K2CO3 | CH2Cl2 | 86 |
| 4 | Cs2CO3 | CH2Cl2 | 63 |
| 5 | NaOH | CH2Cl2 | 51 |
| 6 | t-BuOK | CH2Cl2 | trace |
| 7 | KF | CH2Cl2 | 80 |
| 8 | CsF | CH2Cl2 | 91 |
| 9 | DABCO | CH2Cl2 | 60 |
| 10 | NEt3 | CH2Cl2 | 61 |
| 11 | DBU | CH2Cl2 | 50 |
| 12 | Pyridine | CH2Cl2 | 37 |
| 13 | – | CH2Cl2 | 8 |
| 14 | CsF | CH3CN | 65 |
| 15 | CsF | toluene | 69 |
| 16 | CsF | THF | 71 |
| 17 | CsF | dioxane | 76 |
| 18 | CsF | DMA | 87 |
| 19 | CsF (1.0 equiv) | CH2Cl2 | 65 |
aReaction conditions: N’-(2-alkynylbenzylidene)hydrazide 1a (0.3 mmol), AgOTf (5 mol %), solvent (3 mL), ethyl 4,4,4-trifluorobut-2-ynoate (2, 0.6 mmol, 2.0 equiv), base (1.5 equiv), 4 Å MS (75 mg), rt, overnight. bIsolated yield based on 1a.
To explore the scope of this tandem cyclization/[3 + 2] cycloaddition reaction, a range of N’-(2-alkynylbenzylidene)hydrazides 1a–j were prepared from the corresponding aldehydes and applied to the synthesis of trifluoromethylated pyrazolo[5,1-a]isoquinoline derivatives 3 under the optimized conditions (Table 1, entry 8). As shown in Table 2, for most cases, N’-(2-alkynylbenzylidene)hydrazides 1 reacted with ethyl 4,4,4-trifluorobut-2-ynoate 2 affording the corresponding products 3 in good to excellent yields. For instance, substrate 1b bearing an electron-donating substituent (methyl) reacted with 2 under the present reaction conditions gave the desired product 3b in good yield (86%, Table 2, entry 2). The structure of 3b was verified by 1H and 13C NMR, HRMS, as well as X-ray diffraction analysis (Figure 1, for details, see Supporting Information File 1). As expected, the substrates 1e–h with electron-withdrawing substituents are suitable partners in this process and the corresponding pyrazolo[5,1-a]isoquinolines 3e–h were obtained in good yields. Fortunately, alkyl-substituted N’-(2-alkynylbenzylidene)hydrazide was demonstrated to be good partner in the transformation. For instance, N’-(2-alkynylbenzylidene)hydrazide 1i reacted with 2, leading to the desired pyrazolo[5,1-a]isoquinoline 3i in 90% yield (Table 2, entry 9).
Table 2: Silver triflate-catalyzed tandem reactions of N’-(2-alkynylbenzylidene)hydrazides 1 with ethyl 4,4,4-trifluorobut-2-ynoate 2.
|
|
|||||||
| Entry | R1, R2/1 | Product 3 | Yield (%)a | Entry | R1, R2/1 | Product 3 | Yield (%)a |
|---|---|---|---|---|---|---|---|
| 1 |
R1 = H
R2 = Ph 1a |
3a |
91 | 6 |
R1 = H
R2 = 4-FC6H4 1f |
3f |
80 |
| 2 |
R1 = H
R2 = 4-MeC6H4 1b |
3b |
86 | 7 |
R1 = H
R2 = 4-AcC6H4 1g |
3g |
87 |
| 3 |
R1 = H
R2 = 4-MeOC6H4 1c |
3c |
75 | 8 |
R1 = H
R2 = 4-NO2C6H4 1h |
3h |
47 |
| 4 |
R1 = H
R2 = 4-EtOC6H4 1d |
3d |
89 | 9 |
R1 = H
R2 = cyclopropyl 1i |
3i |
90 |
| 5 |
R1 = H
R2 = 4-ClC6H4 1e |
3e |
85 | 10 |
R1 = 3-F
R2 = Ph 1j |
3j |
44 |
aIsolated yields based on N’-(2-alkynylbenzylidene)hydrazides 1.
Subsequently, based on our previous reports on electrophile-mediated electrophilic cyclization reaction [28,29], one-pot tandem electrophilic cyclization/[3 + 2] cycloaddition of N’-(2-alkynylbenzylidene)hydrazides 1, electrophiles (I2 or ICl), and ethyl 4,4,4-trifluorobut-2-ynoate (2) were carried out under mild conditions. The results are summarized in Table 3. For all cases, this tandem reaction worked well leading to the corresponding iodinated fluorine-containing pyrazolo[5,1-a]isoquinolines 4 in moderate to excellent yields. Various functional groups, such as methyl, methoxy, ethoxy, halogen, acetyl, nitro, and cyclopropyl groups were tolerated under the reaction conditions. In general, substrates bearing electron-donating substituents show better reactivity than those with electron-withdrawing substituents. For instance, methyl-substituted N’-(2-alkynylbenzylidene)hydrazide 1b reacted with iodine and ethyl 4,4,4-trifluorobut-2-ynoate (2) in the presence of CsF and 4 Å MS leading to the desired product 4b in 90% yield (Table 3, entry 3). A relatively lower yield was obtained when nitro substituted N’-(2-alkynylbenzylidene)hydrazide 1h was used, and the desired product 4g was obtained in 50% yield (Table 3, entry 9). Alkyl-substituted product 4h was obtained in good yields when substrate 1i reacted with iodine or ICl (Table 3, entries 10 and 11), the structure of 4h was verified by 1H and 13C NMR, HRMS, as well as X-ray diffraction analysis (Figure 1, for details, see Supporting Information File 1). Based on this one-pot tandem electrophilic cyclization/[3 + 2] cycloaddition reactions, highly functionalized pyrazolo[5,1-a]isoquinolines can be obtained via palladium-catalyzed cross-coupling reaction.
Table 3: One-pot tandem reactions of N’-(2-alkynylbenzylidene)hydrazides 1, electrophiles, and ethyl 4,4,4-trifluorobut-2-ynoate (2)a.
|
|
|||||||||
| Entry | 1 | X2 | 4 | Yield (%)b | Entry | 1 | X2 | 4 | Yield (%)b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | I2 |
4a |
71 | 7 | 1f | I2 |
4e |
62 |
| 2 | 1a | ICl | 4a | 78 | 8 | 1g | I2 |
4f |
84 |
| 3 | 1b | I2 |
4b |
90 | 9 | 1h | ICl |
4g |
50 |
| 4 | 1b | ICl | 4b | 82 | 10 | 1i | I2 |
4h |
85 |
| 5 | 1c | I2 |
4c |
80 | 11 | 1i | ICl | 4h | 62 |
| 6 | 1d | I2 |
4d |
88 | 12 | 1j | I2 |
4i |
50 |
aReaction conditions: N’-(2-alkynylbenzylidene)hydrazide 1a (0.3 mmol), I2 or ICl (1.3 equiv), solvent (3 mL), ethyl 4,4,4-trifluorobut-2-ynoate (2, 0.6 mmol, 2.0 equiv), base (1.5 equiv), 4 Å MS (75 mg), rt, overnight. bIsolated yields based on N’-(2-alkynylbenzylidene)hydrazides 1.
Conclusion
In conclusion, we have developed an efficient one-pot tandem cyclization/[3 + 2] cycloaddition reaction of N’-(2-alkynylbenzylidene)hydrazides with ethyl 4,4,4-trifluorobut-2-ynoate under silver triflate-catalyzed or electrophiles-mediated conditions. Highly functionalized pyrazolo[5,1-a]isoquinolines can be synthesized in moderate to excellent yield by this developed method.
Experimental
General
All reactions were performed in test tubes under N2-atmosphere. Flash column chromatography was performed with silica gel (200–300 mesh). Analytical thin-layer chromatography was performed on glass plates pre-coated with 0.25 mm 230–400 mesh silica gel impregnated with a fluorescent indicator (254 nm). Thin-layer chromatography plates were visualized by exposure to ultraviolet light. Organic solutions were concentrated on rotary evaporators at 25–35 °C. Commercial reagents and solvents were used as received. 1H and 13C NMR spectra were recorded on a Bruker AV 400 at 400 MHz (1H) and 100 MHz (13C) at ambient temperature. Chemical shifts are reported in parts per million (ppm) on the delta scale (δ) and referenced to tetramethylsilane (0 ppm). HRMS analyses were performed in ESI mode on a Bruker mass spectrometer.
General procedure for the silver triflate-catalyzed one-pot tandem reaction of N’-(2-alkynylbenzylidene)hydrazide 1 with ethyl 4,4,4-trifluorobut-2-ynoate 2: A mixture of N’-(2-alkynylbenzylidene)hydrazide 1 (0.30 mmol, 1.0 equiv) and silver triflate (5 mol %) in anhydrous dichloromethane (3.0 mL) was stirred at room temperature overnight. Then a solution of ethyl 4,4,4-trifluorobut-2-ynoate (2, 0.60 mmol, 2.0 equiv) in dichloroethane (2.0 mL), 4 Å MS (75 mg) and CsF (0.45 mmol, 1.5 equiv) were added and stirred for another 3 h. After completion of the reaction as indicated by TLC, the reaction mixture was purified by flash column chromatography on silica gel to provide the corresponding product 3.
General procedure for the electrophile-mediated one-pot tandem reaction of N’-(2-alkynylbenzylidene)hydrazide 1 with ethyl 4,4,4-trifluorobut-2-ynoate (2): A mixture of N’-(2-alkynylbenzylidene)hydrazide 1 (0.30 mmol, 1.0 equiv) and electrophiles (I2 or ICl) (0.36 mmol, 1.2 equiv) in anhydrous dichloromethane (3.0 mL) was stirred at room temperature overnight. Then a solution of ethyl 4,4,4-trifluorobut-2-ynoate (2, 0.60 mmol, 2.0 equiv) in dichloroethane (2.0 mL), 4 Å MS (75 mg) and CsF (0.45 mmol, 1.5 equiv) were added and stirred for another 3 h. After completion of the reaction as indicated by TLC, the reaction mixture was purified by flash column chromatography on silica gel to provide the corresponding product 4. For details, see Supporting Information File 1.
Supporting Information
| Supporting Information File 1: Characterization data and NMR spectra. | ||
| Format: PDF | Size: 1.4 MB | Download |
| Supporting Information File 2: X-ray data for compound 3b. | ||
| Format: CIF | Size: 14.7 KB | Download |
| Supporting Information File 3: X-ray data for compound 4h. | ||
| Format: CIF | Size: 14.0 KB | Download |
Acknowledgements
Financial Supported from the National Natural Science Foundation of China (21262016), Jiangxi Educational Committee (GJJ12169), the Project of Jiangxi Youth Scientist (20122BCB23012), and Natural Science Foundation of Jiangxi Province of China (20133ACB20008, 20132BAB203006) is gratefully acknowledged.
References
-
Bentley, K. W. The Isoquinoline Alkaloids; Harwood Academic Publishers: Australia, 1998; Vol. 1.
Return to citation in text: [1] -
Trotter, B. W.; Nanda, K. K.; Kett, N. R.; Regan, C. P.; Lynch, J. J.; Stump, G. L.; Kiss, L.; Wang, J.; Spencer, R. H.; Kane, S. A.; White, R. B.; Zhang, R.; Anderson, K. D.; Liverton, N. J.; McIntyre, C. J.; Beshore, D. C.; Hartman, G. D.; Dinsmore, C. J. J. Med. Chem. 2006, 49, 6954–6957. doi:10.1021/jm060927v
Return to citation in text: [1] -
Mousseau, J. J.; Fortier, A.; Charette, A. B. Org. Lett. 2010, 12, 516–519. doi:10.1021/ol902710f
Return to citation in text: [1] -
Chen, Z.; Wu, J. Org. Lett. 2010, 12, 4856–4859. doi:10.1021/ol101988q
Return to citation in text: [1] [2] -
Li, X.; Zhao, M. J. Org. Chem. 2011, 76, 8530–8536. doi:10.1021/jo201530r
Return to citation in text: [1] -
Li, S.; Wu, J. Org. Lett. 2011, 13, 712–715. doi:10.1021/ol102939r
Return to citation in text: [1] -
Huple, D. B.; Chen, C.-H.; Das, A.; Liu, R.-S. Adv. Synth. Catal. 2011, 353, 1877–1882. doi:10.1002/adsc.201100263
Return to citation in text: [1] -
Zhao, J.; Li, P.; Wu, C.; Chen, H.; Ai, W.; Sun, R.; Ren, H.; Larock, R. C.; Shi, F. Org. Biomol. Chem. 2012, 10, 1922–1930. doi:10.1039/c2ob06611d
Return to citation in text: [1] -
Chen, Z.; Gao, L.; Ye, S.; Ding, Q.; Wu, J. Chem. Commun. 2012, 48, 3975–3977. doi:10.1039/c2cc30413a
Return to citation in text: [1] -
Huang, P.; Yang, Q.; Chen, Z.; Ding, Q.; Xu, J.; Peng, Y. J. Org. Chem. 2012, 77, 8092–8098. doi:10.1021/jo3013429
Return to citation in text: [1] -
Ding, Q.; Wang, D.; Sang, X.; Lin, Y.; Peng, Y. Tetrahedron 2012, 68, 8869–8874. doi:10.1016/j.tet.2012.08.039
Return to citation in text: [1] [2] -
Xu, S.-X.; Hao, L.; Wang, T.; Ding, Z.-C.; Zhan, Z.-P. Org. Biomol. Chem. 2013, 11, 294–298. doi:10.1039/c2ob27016a
Return to citation in text: [1] -
Liu, H.; Liu, G.; Qiu, G.; Pu, S.; Wu, J. Tetrahedron 2013, 69, 1476–1480. doi:10.1016/j.tet.2012.12.018
Return to citation in text: [1] -
Ding, Q.; Wang, D.; Luo, P.; Liu, M.; Pu, S.; Zhou, L. Beilstein J. Org. Chem. 2013, 9, 1949–1956. doi:10.3762/bjoc.9.231
Return to citation in text: [1] [2] -
Xiao, Q.; Zheng, D.; Ding, Q.; Wu, J. Tetrahedron 2013, 69, 5119–5122. doi:10.1016/j.tet.2013.04.076
Return to citation in text: [1] -
Pan, X.; Luo, Y.; Wu, J. J. Org. Chem. 2013, 78, 5756–5760. doi:10.1021/jo400523v
Return to citation in text: [1] -
Yuvaraj, P.; Reddy, B. S. R. Tetrahedron Lett. 2014, 55, 806–810. doi:10.1016/j.tetlet.2013.11.116
Return to citation in text: [1] -
Yao, L.; Yu, X.; Mo, C.; Wu, J. Org. Biomol. Chem. 2012, 10, 9447–9451. doi:10.1039/c2ob26824h
Return to citation in text: [1] -
Wei, J.; Chen, J.; Xu, J.; Cao, L.; Deng, H.; Sheng, W.; Zhang, H.; Cao, W. J. Fluorine Chem. 2012, 133, 146–154. doi:10.1016/j.jfluchem.2011.09.009
Return to citation in text: [1] -
Qian, J.; Cao, W.; Zhang, H.; Chen, J.; Zhu, S. J. Fluorine Chem. 2007, 128, 207–210. doi:10.1016/j.jfluchem.2006.12.006
Return to citation in text: [1] -
Lu, L.; Wei, J.; Chen, J.; Zhang, J.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. Tetrahedron 2009, 65, 9152–9156. doi:10.1016/j.tet.2009.09.030
Return to citation in text: [1] [2] -
Xu, J.; Wei, J.; Bian, L.; Zhang, J.; Chen, J.; Deng, H.; Wu, X.; Zhang, H.; Cao, W. Chem. Commun. 2011, 47, 3607–3609. doi:10.1039/c0cc05039c
Return to citation in text: [1] [2] -
Lu, L.; Cao, W.; Chen, J.; Zhang, H.; Zhang, J.; Chen, H.; Wei, J.; Deng, H.; Shao, M. J. Fluorine Chem. 2009, 130, 295–300. doi:10.1016/j.jfluchem.2008.11.002
Return to citation in text: [1] -
Yu, H.; Han, J.; Chen, J.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. Eur. J. Org. Chem. 2012, 3142–3150. doi:10.1002/ejoc.201200180
Return to citation in text: [1] -
Bian, L.; Xu, J.; Xie, L.; Chen, J.; Deng, H.; Shao, M.; Ding, T.; Zhang, H.; Cao, W. Tetrahedron 2013, 69, 6121–6128. doi:10.1016/j.tet.2013.05.053
Return to citation in text: [1] [2] -
Wang, Z.; Sun, T.; Chen, J.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. Tetrahedron 2013, 69, 4270–4275. doi:10.1016/j.tet.2013.03.080
Return to citation in text: [1] [2] -
Ding, Q.; Ye, C.; Pu, S.; Cao, B. Tetrahedron 2014, 70, 409–416. doi:10.1016/j.tet.2013.11.034
Return to citation in text: [1] -
Chen, Z.; Ding, Q.; Yu, X.; Wu, J. Adv. Synth. Catal. 2009, 351, 1692–1698. doi:10.1002/adsc.200900131
Return to citation in text: [1] [2] [3] -
Ding, Q.; Chen, Z.; Yu, X.; Peng, Y.; Wu, J. Tetrahedron Lett. 2009, 50, 340–342. doi:10.1016/j.tetlet.2008.11.006
Return to citation in text: [1]
| 1. | Bentley, K. W. The Isoquinoline Alkaloids; Harwood Academic Publishers: Australia, 1998; Vol. 1. |
| 2. | Trotter, B. W.; Nanda, K. K.; Kett, N. R.; Regan, C. P.; Lynch, J. J.; Stump, G. L.; Kiss, L.; Wang, J.; Spencer, R. H.; Kane, S. A.; White, R. B.; Zhang, R.; Anderson, K. D.; Liverton, N. J.; McIntyre, C. J.; Beshore, D. C.; Hartman, G. D.; Dinsmore, C. J. J. Med. Chem. 2006, 49, 6954–6957. doi:10.1021/jm060927v |
| 22. | Xu, J.; Wei, J.; Bian, L.; Zhang, J.; Chen, J.; Deng, H.; Wu, X.; Zhang, H.; Cao, W. Chem. Commun. 2011, 47, 3607–3609. doi:10.1039/c0cc05039c |
| 19. | Wei, J.; Chen, J.; Xu, J.; Cao, L.; Deng, H.; Sheng, W.; Zhang, H.; Cao, W. J. Fluorine Chem. 2012, 133, 146–154. doi:10.1016/j.jfluchem.2011.09.009 |
| 20. | Qian, J.; Cao, W.; Zhang, H.; Chen, J.; Zhu, S. J. Fluorine Chem. 2007, 128, 207–210. doi:10.1016/j.jfluchem.2006.12.006 |
| 21. | Lu, L.; Wei, J.; Chen, J.; Zhang, J.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. Tetrahedron 2009, 65, 9152–9156. doi:10.1016/j.tet.2009.09.030 |
| 22. | Xu, J.; Wei, J.; Bian, L.; Zhang, J.; Chen, J.; Deng, H.; Wu, X.; Zhang, H.; Cao, W. Chem. Commun. 2011, 47, 3607–3609. doi:10.1039/c0cc05039c |
| 23. | Lu, L.; Cao, W.; Chen, J.; Zhang, H.; Zhang, J.; Chen, H.; Wei, J.; Deng, H.; Shao, M. J. Fluorine Chem. 2009, 130, 295–300. doi:10.1016/j.jfluchem.2008.11.002 |
| 24. | Yu, H.; Han, J.; Chen, J.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. Eur. J. Org. Chem. 2012, 3142–3150. doi:10.1002/ejoc.201200180 |
| 25. | Bian, L.; Xu, J.; Xie, L.; Chen, J.; Deng, H.; Shao, M.; Ding, T.; Zhang, H.; Cao, W. Tetrahedron 2013, 69, 6121–6128. doi:10.1016/j.tet.2013.05.053 |
| 26. | Wang, Z.; Sun, T.; Chen, J.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. Tetrahedron 2013, 69, 4270–4275. doi:10.1016/j.tet.2013.03.080 |
| 3. | Mousseau, J. J.; Fortier, A.; Charette, A. B. Org. Lett. 2010, 12, 516–519. doi:10.1021/ol902710f |
| 4. | Chen, Z.; Wu, J. Org. Lett. 2010, 12, 4856–4859. doi:10.1021/ol101988q |
| 5. | Li, X.; Zhao, M. J. Org. Chem. 2011, 76, 8530–8536. doi:10.1021/jo201530r |
| 6. | Li, S.; Wu, J. Org. Lett. 2011, 13, 712–715. doi:10.1021/ol102939r |
| 7. | Huple, D. B.; Chen, C.-H.; Das, A.; Liu, R.-S. Adv. Synth. Catal. 2011, 353, 1877–1882. doi:10.1002/adsc.201100263 |
| 8. | Zhao, J.; Li, P.; Wu, C.; Chen, H.; Ai, W.; Sun, R.; Ren, H.; Larock, R. C.; Shi, F. Org. Biomol. Chem. 2012, 10, 1922–1930. doi:10.1039/c2ob06611d |
| 9. | Chen, Z.; Gao, L.; Ye, S.; Ding, Q.; Wu, J. Chem. Commun. 2012, 48, 3975–3977. doi:10.1039/c2cc30413a |
| 10. | Huang, P.; Yang, Q.; Chen, Z.; Ding, Q.; Xu, J.; Peng, Y. J. Org. Chem. 2012, 77, 8092–8098. doi:10.1021/jo3013429 |
| 11. | Ding, Q.; Wang, D.; Sang, X.; Lin, Y.; Peng, Y. Tetrahedron 2012, 68, 8869–8874. doi:10.1016/j.tet.2012.08.039 |
| 12. | Xu, S.-X.; Hao, L.; Wang, T.; Ding, Z.-C.; Zhan, Z.-P. Org. Biomol. Chem. 2013, 11, 294–298. doi:10.1039/c2ob27016a |
| 13. | Liu, H.; Liu, G.; Qiu, G.; Pu, S.; Wu, J. Tetrahedron 2013, 69, 1476–1480. doi:10.1016/j.tet.2012.12.018 |
| 14. | Ding, Q.; Wang, D.; Luo, P.; Liu, M.; Pu, S.; Zhou, L. Beilstein J. Org. Chem. 2013, 9, 1949–1956. doi:10.3762/bjoc.9.231 |
| 15. | Xiao, Q.; Zheng, D.; Ding, Q.; Wu, J. Tetrahedron 2013, 69, 5119–5122. doi:10.1016/j.tet.2013.04.076 |
| 16. | Pan, X.; Luo, Y.; Wu, J. J. Org. Chem. 2013, 78, 5756–5760. doi:10.1021/jo400523v |
| 17. | Yuvaraj, P.; Reddy, B. S. R. Tetrahedron Lett. 2014, 55, 806–810. doi:10.1016/j.tetlet.2013.11.116 |
| 18. | Yao, L.; Yu, X.; Mo, C.; Wu, J. Org. Biomol. Chem. 2012, 10, 9447–9451. doi:10.1039/c2ob26824h |
| 27. | Ding, Q.; Ye, C.; Pu, S.; Cao, B. Tetrahedron 2014, 70, 409–416. doi:10.1016/j.tet.2013.11.034 |
| 28. | Chen, Z.; Ding, Q.; Yu, X.; Wu, J. Adv. Synth. Catal. 2009, 351, 1692–1698. doi:10.1002/adsc.200900131 |
| 11. | Ding, Q.; Wang, D.; Sang, X.; Lin, Y.; Peng, Y. Tetrahedron 2012, 68, 8869–8874. doi:10.1016/j.tet.2012.08.039 |
| 14. | Ding, Q.; Wang, D.; Luo, P.; Liu, M.; Pu, S.; Zhou, L. Beilstein J. Org. Chem. 2013, 9, 1949–1956. doi:10.3762/bjoc.9.231 |
| 28. | Chen, Z.; Ding, Q.; Yu, X.; Wu, J. Adv. Synth. Catal. 2009, 351, 1692–1698. doi:10.1002/adsc.200900131 |
| 29. | Ding, Q.; Chen, Z.; Yu, X.; Peng, Y.; Wu, J. Tetrahedron Lett. 2009, 50, 340–342. doi:10.1016/j.tetlet.2008.11.006 |
| 26. | Wang, Z.; Sun, T.; Chen, J.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. Tetrahedron 2013, 69, 4270–4275. doi:10.1016/j.tet.2013.03.080 |
| 21. | Lu, L.; Wei, J.; Chen, J.; Zhang, J.; Deng, H.; Shao, M.; Zhang, H.; Cao, W. Tetrahedron 2009, 65, 9152–9156. doi:10.1016/j.tet.2009.09.030 |
| 25. | Bian, L.; Xu, J.; Xie, L.; Chen, J.; Deng, H.; Shao, M.; Ding, T.; Zhang, H.; Cao, W. Tetrahedron 2013, 69, 6121–6128. doi:10.1016/j.tet.2013.05.053 |
| 28. | Chen, Z.; Ding, Q.; Yu, X.; Wu, J. Adv. Synth. Catal. 2009, 351, 1692–1698. doi:10.1002/adsc.200900131 |
© 2014 Zhou et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)




![[1860-5397-10-238-1]](/bjoc/content/figures/1860-5397-10-238-1.png?scale=2.0&max-width=1024&background=FFFFFF)