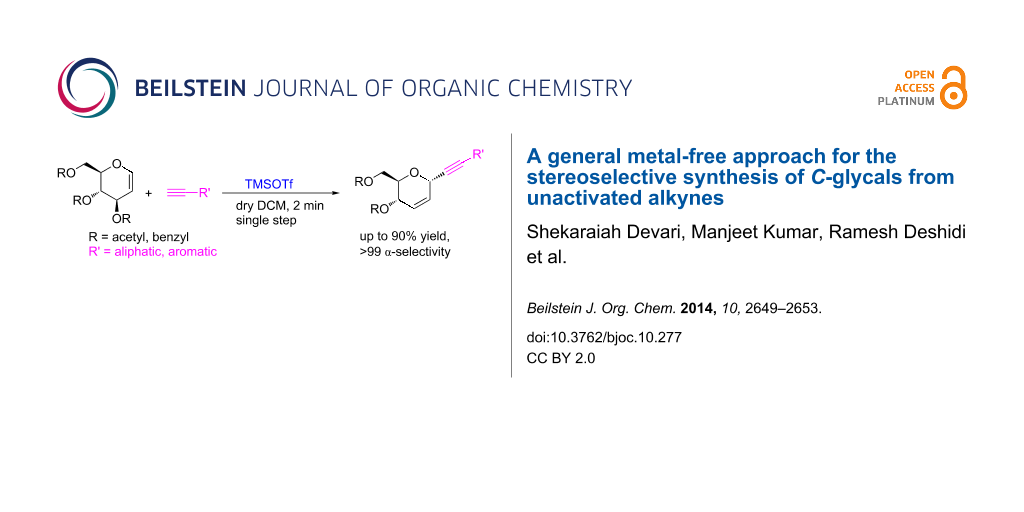
Abstract
A novel metal-free strategy for a rapid and α-selctive C-alkynylation of glycals was developed. The reaction utilizes TMSOTf as a promoter to generate in situ trimethylsilylacetylene for C-alkynylation. Thanks to this methodology, we can access C-glycosides in a single step from a variety of acetylenes , i.e., arylacetylenes and most importantly aliphatic alkynes.
Graphical Abstract
Introduction
C-Glycosides represent an important class of carbohydrate mimics, owing to their presence in a large number of biologically active natural products, such as palytoxin, spongistatin, vitexin, orientin, bergenin and halichondrin [1-5]. Over the years myriad methods have appeared for their efficient and stereoselective synthesis [6-12]. Among these methods, C-alkynylation [13-16] is of particular interest as it is amenable to further modifications into chiral molecules, carbohydrate analogues, and natural products, such as tautomycin [17,18] and ciguatoxin [19-21]. In recent past, significant efforts have been directed toward the C-alkynylation of glycals [9,22,23]. However, all these methods use priorly activated terminal alkynes, e.g., silylacetylene, activated with various Lewis acids such as SnCl4, BF3·OEt2, TiCl4, I2, InBr3, and ZrCl4 [24-30], followed by a Ferrier type rearrangement [31-34] (Scheme 1). A consequence of the prior activation of alkynes is the involvement of multiple steps and thus loss of yields. Recently, Mukherjee and co-workers [35] have reported a one-step C-alkynylation of glycals by using metal in combination with a co-oxidant, i.e., Cu(OTf)2 with ascorbic acid. However, a drawback of the method is its limited applicability to arylacetylenes only and its restricted selectivity to reactions performed in the solvent acetonitrile (Scheme 1).
It is obvious from the preceding discussion that the stereoselective addition of alkynes to the anomeric carbon of sugar nuclei in a single step still represents an interesting challenge. We reasoned that the development of a strategy which in situ activates the terminal alkyne and further catalyzes the reaction without the aid of other Lewis acids might be a solution to this problem. Thus, in continuation of our efforts [36-38], we describe a highly stereoselective TMSOTf catalyzed rapid C-alkynylation of glycals with a wide variety of unactivated alkynes, i.e., arylacetylenes and aliphatic alkynes. The method circumvents the use of metal catalysts/co-oxidants and exhibits short reaction times, i.e., 2 min. This method may find use in a large number of reactions, which are characterized by a requirement of pre-formed trimethylsilylacetylene.
Results and Discussion
Initial investigations involved the use of 3,4,6-tri-O-acetyl-D-glucal (1) and phenylacetylene (2) as model substrates with TMSOTf as a promoter within DCM at −20 °C. To our delight TLC showed full consumption of the starting materials in 2 min and yielded the desired product 3a in 80% yield (Table 1, entry 1). The structure and stereochemistry were elucidated by a comparison of the chemical shifts to that of reported values [35]. Also, the stereochemistry of the resulting product 3a was unambiguously established as α by NOESY spectra, indicating cross peaks between H-1, H-6 and H-4. To further establish the role of the catalyst we repeated the reaction with other Lewis acid catalysts, such as In(OTf)3, Cu(OTf)2, Sc(OTf)3 and BF3·OEt2, but no product could be obtained (Table 1, entries 2–5). Next, we focused our attention on optimizing the suitable amount of the catalyst loading. We observed that a decrease of the catalyst loading below 30 mol % led to no product formation even after 1 h (Table 1, entries 6 and 7). However, an increase to 40 mol % with longer reaction time resulted in a loss of yield due to the degradation of the product (Table 1, entry 8). A further increase in catalyst loading had no significant impact on the overall reaction yield and time (Table 1, entry 9). We also examined the effect of temperature on the reaction, and observed that both an increase (up to rt) and a decrease (to −40 °C) resulted in the loss of yield (Table 1, entries 10–13).
Table 1: Alkynylation reaction of 3,4,6-tri-O-acetyl-D-glucal with phenylacetylenea.
|
|
||||||
| entry | Lewis acid | equiv | T (°C) | time | yieldb | (α/β)c |
|---|---|---|---|---|---|---|
| 1 | TMSOTf | 0.5 | −20 | 2 min | 80 | 99:1 |
| 2 | In(OTf)3 | 0.5 | −20 | 5 h | – | – |
| 3 | Cu(OTf)2 | 0.5 | −20 | 5 h | – | – |
| 4 | Sc(OTf)3 | 0.5 | −20 | 5 h | – | – |
| 5 | BF3·OEt2 | 0.5 | −20 | 5 h | – | – |
| 6 | TMSOTf | 0.2 | −20 | 1 h | – | – |
| 7 | TMSOTf | 0.3 | −20 | 1 h | <10 | ND |
| 8 | TMSOTf | 0.4 | −20 | 15 min | 57 | ND |
| 9 | TMSOTf | 0.8 | −20 | 2 min | 82 | ND |
| 10 | TMSOTf | 0.5 | −40 | 5 min | 63 | ND |
| 11 | TMSOTf | 0.5 | −10 | 2 min | 77 | ND |
| 12 | TMSOTf | 0.5 | 0 | 2 min | 59 | ND |
| 13 | TMSOTf | 0.5 | rt | 2 min | 37 | ND |
aIn all cases 1 equiv of 1 and 1.2 equiv of 2 were used; bisolated yields; cdetermined by 1H, 13C NMR and NOESY spectra; ND = not determined.
The scope of the present method was further expanded to a variety of alkynes and glycals (Scheme 2). It was established that the system was tolerant to a wide variety of electron-donating as well as electron-withdrawing terminal alkynes to give the corresponding products 3a–e in excellent yields and selectivity. It is noteworthy that the earlier reported [35] single-step strategy failed to yield a product with aliphatic alkynes, so that we applied the method to aliphatic alkynes. The reaction with cyclopropylacetylene, hept-1-yne and oct-1-yne maintained a high selectivity and gave the corresponding products 3f–h in 54, 42 and 39% yield, respectively. To further broaden the scope of the reaction, tri-O-benzyl-D-glucal was subjected to the reaction with phenylacetylene, p-methylphenylacetylene, p-(tert-butyl)phenylacetylene and p-pentylphenylacetylene giving the corresponding products 3i–l in 82, 86, 90, and 87% yield, respectively, with >99% selectivity. Also, the reaction with other glycals, i.e., 3,4,6-tri-O-acetyl-D-galactal and 2,4-di-O-acetyl-L-rhamnal with phenylacetylene gave the corresponding products 3m and 3n in 82 and 80% yield, respectively, and with a high selectivity.
Scheme 2: Substrate scope of the reaction process under optimized reaction conditions.
Scheme 2: Substrate scope of the reaction process under optimized reaction conditions.
The present results indicate the activation of terminal alkynes by TMSOTf forming trimethylsilylacetylenes [39]. In order to confirm the formation of trimethylsilylacetylenes, we attempted a control experiment involving the addition of molecular iodine instead of glucal. As expected [40,41], the reaction on heating at 70 °C for 3 h gave the iodinated phenylacetylene (Scheme 3, reaction 1 & Figure S1, Supporting Information File 1). Thus, the triflic acid generated in situ consequent to the formation of trimethylsilylacetylene activates the tri-O-acetyl-D-glucal forming an oxonium ion intermediate, which is attacked by trimethylsilylacetylene to give the corresponding product (Scheme 3, reaction 2). The stereochemistry of the reaction products is possibly determined by the coordination between two π-electron orbitals of the oxocarbonium ion and the acetylene groups, while the stereoelectronic control allows the α-pseudo-axial orbital to form the bond [35].
Scheme 3: Plausible mechanism of the Ferrier rearrangement.
Scheme 3: Plausible mechanism of the Ferrier rearrangement.
Conclusion
In conclusion, we developed a highly efficient and α-selective method for the synthesis of alkynyl glycosides from virtually any alkyne, that is, aliphatic and aromatic. To the best of our knowledge, this is the first report which descibres the in situ generation of trimethylsilylacetylene and its subsequent usage for C-alkynylation without the co-addition of a Lewis acid. The protocol may find application in a large number of reactions catalyzed by Lewis acid wherein pre-formed silylated terminal alkynes are required. Further studies are underway to broaden the scope of the present reaction.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization data, and 1H and 13C NMR spectra of relevant compounds. | ||
| Format: PDF | Size: 2.0 MB | Download |
References
-
Lewis, M. D.; Cha, J. K.; Kishi, Y. J. Am. Chem. Soc. 1982, 104, 4976–4978. doi:10.1021/ja00382a053
Return to citation in text: [1] -
Fraser-Reid, B. Acc. Chem. Res. 1985, 18, 347–354. doi:10.1021/ar00119a004
Return to citation in text: [1] -
Paterson, I.; Keown, L. E. Tetrahedron Lett. 1997, 38, 5727–5730. doi:10.1016/S0040-4039(97)01257-4
Return to citation in text: [1] -
Faul, M. M.; Huff, B. E. Chem. Rev. 2000, 100, 2407–2474. doi:10.1021/cr940210s
Return to citation in text: [1] -
Wiebe, C.; Schlemmer, C.; Weck, S.; Opatz, T. Chem. Commun. 2011, 47, 9212–9214. doi:10.1039/c1cc13078a
Return to citation in text: [1] -
Casiraghi, G.; Zanardi, F.; Rassu, G.; Spanu, P. Chem. Rev. 1995, 95, 1677–1716. doi:10.1021/cr00038a001
Return to citation in text: [1] -
Schuff, B. P.; Mercer, G. J.; Nguyen, H. M. Org. Lett. 2007, 9, 3173–3176. doi:10.1021/ol071268z
Return to citation in text: [1] -
Ryan, D. A.; Gin, D. Y. J. Am. Chem. Soc. 2008, 130, 15228–15229. doi:10.1021/ja804589j
Return to citation in text: [1] -
Koester, D. C.; Werz, D. B. Beilstein J. Org. Chem. 2012, 8, 675–682. doi:10.3762/bjoc.8.75
Return to citation in text: [1] [2] -
Yin, J.; Linker, T. Org. Biomol. Chem. 2012, 10, 2351–2362. doi:10.1039/c2ob06529k
Return to citation in text: [1] -
Ansari, A. A.; Reddy, Y. S.; Vankar, Y. D. Beilstein J. Org. Chem. 2014, 10, 300–306. doi:10.3762/bjoc.10.27
Return to citation in text: [1] -
Tan, H. Y.; Xiang, S.; Leng, W. L.; Liu, X.-W. RSC Adv. 2014, 4, 34816–34822. doi:10.1039/C4RA07429G
Return to citation in text: [1] -
Ichikawa, Y.; Tsuboi, K.; Jiang, Y.; Naganawa, A.; Isobe, M. Tetrahedron Lett. 1995, 36, 7101–7104. doi:10.1016/0040-4039(95)01436-L
Return to citation in text: [1] -
Yeager, A. R.; Min, G. K.; Porco, J. A., Jr.; Schaus, S. E. Org. Lett. 2006, 8, 5065–5068. doi:10.1021/ol0618252
Return to citation in text: [1] -
Vieira, A. S.; Fiorante, P. F.; Hough, T. L. S.; Ferreira, F. P.; Lüdtke, D. S.; Stefani, H. A. Org. Lett. 2008, 10, 5215–5218. doi:10.1021/ol8022177
Return to citation in text: [1] -
Gorja, D. R.; Kumar, K. S.; Mukkanti, K.; Pal, M. Beilstein J. Org. Chem. 2011, 7, 338–345. doi:10.3762/bjoc.7.44
Return to citation in text: [1] -
Chen, X.; Zheng, Y.; Shen, Y. Chem. Rev. 2007, 107, 1777–1830. doi:10.1021/cr050029r
Return to citation in text: [1] -
Adler, J. T.; Cook, M.; Luo, Y.; Pitt, S. C.; Ju, J.; Li, W.; Shen, B.; Kunnimalaiyaan, M.; Chen, H. Mol. Cancer Ther. 2009, 8, 914–920. doi:10.1158/1535-7163.MCT-08-0712
Return to citation in text: [1] -
Inoue, M.; Sasaki, M.; Tachibana, K. J. Org. Chem. 1999, 64, 9416–9429. doi:10.1021/jo990989b
Return to citation in text: [1] -
Inoue, M.; Hirama, M. Acc. Chem. Res. 2004, 37, 961–968. doi:10.1021/ar0301577
Return to citation in text: [1] -
Inoue, M.; Miyazaki, K.; Ishihara, Y.; Tatami, A.; Ohnuma, Y.; Kawada, Y.; Komano, K.; Yamashita, S.; Lee, N.; Hirama, M. J. Am. Chem. Soc. 2006, 128, 9352–9354. doi:10.1021/ja063041p
Return to citation in text: [1] -
Koester, D. C.; Leibeling, M.; Neufeld, R.; Werz, D. B. Org. Lett. 2010, 12, 3934–3937. doi:10.1021/ol101625p
Return to citation in text: [1] -
Parkan, K.; Pohl, R.; Kotora, M. Chem. – Eur. J. 2014, 20, 4414–4419. doi:10.1002/chem.201304304
Return to citation in text: [1] -
Ichikawa, Y.; Isobe, M.; Konobe, M.; Goto, T. Carbohydr. Res. 1987, 171, 193–199. doi:10.1016/S0008-6215(00)90886-3
Return to citation in text: [1] -
Kozikowski, A. P.; Park, P. U. J. Org. Chem. 1990, 55, 4668–4682. doi:10.1021/jo00302a036
Return to citation in text: [1] -
Tsukiyama, T.; Peters, S. C.; Isobe, M. Synlett 1993, 413–414. doi:10.1055/s-1993-22476
Return to citation in text: [1] -
Isobe, M.; Nishizawa, R.; Hosokawa, S.; Nishikawa, T. Chem. Commun. 1998, 2665–2676. doi:10.1039/a804940h
Return to citation in text: [1] -
Hosokawa, S.; Kirschbaum, B.; Isobe, M. Tetrahedron Lett. 1998, 39, 1917–1920. doi:10.1016/S0040-4039(98)00047-1
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Raju, A. K.; Rao, C. V. Tetrahedron Lett. 2002, 43, 5437–5440. doi:10.1016/S0040-4039(02)01081-X
Return to citation in text: [1] -
Isobe, M.; Phoosaha, W.; Saeeng, R.; Kira, K.; Yenjai, C. Org. Lett. 2003, 5, 4883–4885. doi:10.1021/ol035957w
Return to citation in text: [1] -
Ferrier, R. J.; Zubkov, O. A. Transformation of glycals into 2,3-unsaturated glycosyl derivatives. In Organic Reactions; Overman, L. E., Ed.; John Wiley and Sons: New York, NY, 2003; Vol. 62, pp 569–736. doi:10.1002/0471264180.or062.04
Return to citation in text: [1] -
Ferrier, R. J.; Hoberg, J. O. Adv. Carbohydr. Chem. Biochem. 2003, 58, 55–119.
Return to citation in text: [1] -
Ansari, A. A.; Lahiri, R.; Vankar, Y. D. ARKIVOC 2013, No. ii, 316–362. doi:10.3998/ark.5550190.0014.223
Return to citation in text: [1] -
Gómez, A. M.; Lobo, F.; Uriel, C.; López, J. C. Eur. J. Org. Chem. 2013, 7221–7262. doi:10.1002/ejoc.201300798
Return to citation in text: [1] -
Kusunuru, A. K.; Tatina, M.; Yousuf, S. K.; Mukherjee, D. Chem. Commun. 2013, 49, 10154–10156. doi:10.1039/c3cc44250k
Return to citation in text: [1] [2] [3] [4] -
Mukherjee, D.; Shah, B. A.; Gupta, P.; Taneja, S. C. J. Org. Chem. 2007, 72, 8965–8968. doi:10.1021/jo070363i
Return to citation in text: [1] -
Kumar, B.; Aga, M. A.; Rouf, A.; Shah, B. A.; Taneja, S. C. J. Org. Chem. 2011, 76, 3506–3510. doi:10.1021/jo102333x
Return to citation in text: [1] -
Deshidi, R.; Kumar, M.; Devari, S.; Shah, B. A. Chem. Commun. 2014, 50, 9533–9535. doi:10.1039/C4CC03783A
Return to citation in text: [1] -
Rahaim, R. J., Jr.; Shaw, J. T. J. Org. Chem. 2008, 73, 2912–2915. doi:10.1021/jo702557d
Return to citation in text: [1] -
Hashmi, A. S. K.; Döpp, R.; Lothschütz, C.; Rudolph, M.; Riedel, D.; Rominger, F. Adv. Synth. Catal. 2010, 352, 1307–1314. doi:10.1002/adsc.201000159
Return to citation in text: [1] -
Usanov, D. L.; Yamamoto, H. J. Am. Chem. Soc. 2011, 133, 1286–1289. doi:10.1021/ja1102822
Return to citation in text: [1]
| 1. | Lewis, M. D.; Cha, J. K.; Kishi, Y. J. Am. Chem. Soc. 1982, 104, 4976–4978. doi:10.1021/ja00382a053 |
| 2. | Fraser-Reid, B. Acc. Chem. Res. 1985, 18, 347–354. doi:10.1021/ar00119a004 |
| 3. | Paterson, I.; Keown, L. E. Tetrahedron Lett. 1997, 38, 5727–5730. doi:10.1016/S0040-4039(97)01257-4 |
| 4. | Faul, M. M.; Huff, B. E. Chem. Rev. 2000, 100, 2407–2474. doi:10.1021/cr940210s |
| 5. | Wiebe, C.; Schlemmer, C.; Weck, S.; Opatz, T. Chem. Commun. 2011, 47, 9212–9214. doi:10.1039/c1cc13078a |
| 19. | Inoue, M.; Sasaki, M.; Tachibana, K. J. Org. Chem. 1999, 64, 9416–9429. doi:10.1021/jo990989b |
| 20. | Inoue, M.; Hirama, M. Acc. Chem. Res. 2004, 37, 961–968. doi:10.1021/ar0301577 |
| 21. | Inoue, M.; Miyazaki, K.; Ishihara, Y.; Tatami, A.; Ohnuma, Y.; Kawada, Y.; Komano, K.; Yamashita, S.; Lee, N.; Hirama, M. J. Am. Chem. Soc. 2006, 128, 9352–9354. doi:10.1021/ja063041p |
| 35. | Kusunuru, A. K.; Tatina, M.; Yousuf, S. K.; Mukherjee, D. Chem. Commun. 2013, 49, 10154–10156. doi:10.1039/c3cc44250k |
| 17. | Chen, X.; Zheng, Y.; Shen, Y. Chem. Rev. 2007, 107, 1777–1830. doi:10.1021/cr050029r |
| 18. | Adler, J. T.; Cook, M.; Luo, Y.; Pitt, S. C.; Ju, J.; Li, W.; Shen, B.; Kunnimalaiyaan, M.; Chen, H. Mol. Cancer Ther. 2009, 8, 914–920. doi:10.1158/1535-7163.MCT-08-0712 |
| 13. | Ichikawa, Y.; Tsuboi, K.; Jiang, Y.; Naganawa, A.; Isobe, M. Tetrahedron Lett. 1995, 36, 7101–7104. doi:10.1016/0040-4039(95)01436-L |
| 14. | Yeager, A. R.; Min, G. K.; Porco, J. A., Jr.; Schaus, S. E. Org. Lett. 2006, 8, 5065–5068. doi:10.1021/ol0618252 |
| 15. | Vieira, A. S.; Fiorante, P. F.; Hough, T. L. S.; Ferreira, F. P.; Lüdtke, D. S.; Stefani, H. A. Org. Lett. 2008, 10, 5215–5218. doi:10.1021/ol8022177 |
| 16. | Gorja, D. R.; Kumar, K. S.; Mukkanti, K.; Pal, M. Beilstein J. Org. Chem. 2011, 7, 338–345. doi:10.3762/bjoc.7.44 |
| 39. | Rahaim, R. J., Jr.; Shaw, J. T. J. Org. Chem. 2008, 73, 2912–2915. doi:10.1021/jo702557d |
| 6. | Casiraghi, G.; Zanardi, F.; Rassu, G.; Spanu, P. Chem. Rev. 1995, 95, 1677–1716. doi:10.1021/cr00038a001 |
| 7. | Schuff, B. P.; Mercer, G. J.; Nguyen, H. M. Org. Lett. 2007, 9, 3173–3176. doi:10.1021/ol071268z |
| 8. | Ryan, D. A.; Gin, D. Y. J. Am. Chem. Soc. 2008, 130, 15228–15229. doi:10.1021/ja804589j |
| 9. | Koester, D. C.; Werz, D. B. Beilstein J. Org. Chem. 2012, 8, 675–682. doi:10.3762/bjoc.8.75 |
| 10. | Yin, J.; Linker, T. Org. Biomol. Chem. 2012, 10, 2351–2362. doi:10.1039/c2ob06529k |
| 11. | Ansari, A. A.; Reddy, Y. S.; Vankar, Y. D. Beilstein J. Org. Chem. 2014, 10, 300–306. doi:10.3762/bjoc.10.27 |
| 12. | Tan, H. Y.; Xiang, S.; Leng, W. L.; Liu, X.-W. RSC Adv. 2014, 4, 34816–34822. doi:10.1039/C4RA07429G |
| 40. | Hashmi, A. S. K.; Döpp, R.; Lothschütz, C.; Rudolph, M.; Riedel, D.; Rominger, F. Adv. Synth. Catal. 2010, 352, 1307–1314. doi:10.1002/adsc.201000159 |
| 41. | Usanov, D. L.; Yamamoto, H. J. Am. Chem. Soc. 2011, 133, 1286–1289. doi:10.1021/ja1102822 |
| 35. | Kusunuru, A. K.; Tatina, M.; Yousuf, S. K.; Mukherjee, D. Chem. Commun. 2013, 49, 10154–10156. doi:10.1039/c3cc44250k |
| 35. | Kusunuru, A. K.; Tatina, M.; Yousuf, S. K.; Mukherjee, D. Chem. Commun. 2013, 49, 10154–10156. doi:10.1039/c3cc44250k |
| 31. | Ferrier, R. J.; Zubkov, O. A. Transformation of glycals into 2,3-unsaturated glycosyl derivatives. In Organic Reactions; Overman, L. E., Ed.; John Wiley and Sons: New York, NY, 2003; Vol. 62, pp 569–736. doi:10.1002/0471264180.or062.04 |
| 32. | Ferrier, R. J.; Hoberg, J. O. Adv. Carbohydr. Chem. Biochem. 2003, 58, 55–119. |
| 33. | Ansari, A. A.; Lahiri, R.; Vankar, Y. D. ARKIVOC 2013, No. ii, 316–362. doi:10.3998/ark.5550190.0014.223 |
| 34. | Gómez, A. M.; Lobo, F.; Uriel, C.; López, J. C. Eur. J. Org. Chem. 2013, 7221–7262. doi:10.1002/ejoc.201300798 |
| 35. | Kusunuru, A. K.; Tatina, M.; Yousuf, S. K.; Mukherjee, D. Chem. Commun. 2013, 49, 10154–10156. doi:10.1039/c3cc44250k |
| 24. | Ichikawa, Y.; Isobe, M.; Konobe, M.; Goto, T. Carbohydr. Res. 1987, 171, 193–199. doi:10.1016/S0008-6215(00)90886-3 |
| 25. | Kozikowski, A. P.; Park, P. U. J. Org. Chem. 1990, 55, 4668–4682. doi:10.1021/jo00302a036 |
| 26. | Tsukiyama, T.; Peters, S. C.; Isobe, M. Synlett 1993, 413–414. doi:10.1055/s-1993-22476 |
| 27. | Isobe, M.; Nishizawa, R.; Hosokawa, S.; Nishikawa, T. Chem. Commun. 1998, 2665–2676. doi:10.1039/a804940h |
| 28. | Hosokawa, S.; Kirschbaum, B.; Isobe, M. Tetrahedron Lett. 1998, 39, 1917–1920. doi:10.1016/S0040-4039(98)00047-1 |
| 29. | Yadav, J. S.; Reddy, B. V. S.; Raju, A. K.; Rao, C. V. Tetrahedron Lett. 2002, 43, 5437–5440. doi:10.1016/S0040-4039(02)01081-X |
| 30. | Isobe, M.; Phoosaha, W.; Saeeng, R.; Kira, K.; Yenjai, C. Org. Lett. 2003, 5, 4883–4885. doi:10.1021/ol035957w |
| 9. | Koester, D. C.; Werz, D. B. Beilstein J. Org. Chem. 2012, 8, 675–682. doi:10.3762/bjoc.8.75 |
| 22. | Koester, D. C.; Leibeling, M.; Neufeld, R.; Werz, D. B. Org. Lett. 2010, 12, 3934–3937. doi:10.1021/ol101625p |
| 23. | Parkan, K.; Pohl, R.; Kotora, M. Chem. – Eur. J. 2014, 20, 4414–4419. doi:10.1002/chem.201304304 |
| 36. | Mukherjee, D.; Shah, B. A.; Gupta, P.; Taneja, S. C. J. Org. Chem. 2007, 72, 8965–8968. doi:10.1021/jo070363i |
| 37. | Kumar, B.; Aga, M. A.; Rouf, A.; Shah, B. A.; Taneja, S. C. J. Org. Chem. 2011, 76, 3506–3510. doi:10.1021/jo102333x |
| 38. | Deshidi, R.; Kumar, M.; Devari, S.; Shah, B. A. Chem. Commun. 2014, 50, 9533–9535. doi:10.1039/C4CC03783A |
© 2014 Devari et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)