Abstract
Efficient and scalable syntheses of enantiomerically pure (2R,1'S,2'R)- and (2S,1'S,2'R)-3-[2-mono(di,tri)fluoromethylcyclopropyl]alanines 9a–c, as well as allo-D-threonine (4) and (2S,3R)-β-methylphenylalanine (3), using the Belokon' approach with (S)- and (R)-2-[(N-benzylprolyl)amino]benzophenone [(S)- and (R)-10] as reusable chiral auxiliaries have been developed. Three new fluoromethyl analogues of the naturally occurring octadepsipeptide hormaomycin (1) with (fluoromethylcyclopropyl)alanine moieties have been synthesized and subjected to preliminary tests of their antibiotic activity.
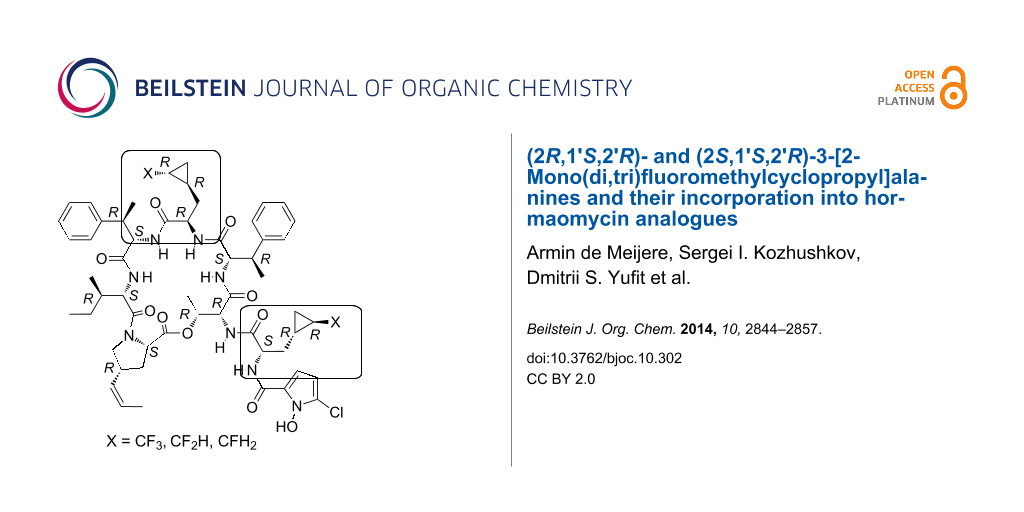
Graphical Abstract
Introduction
The intermolecular signal metabolite hormaomycin (1, Figure 1) was first isolated from a Streptomyces griseoflavus (strain W-384) fermentation broth by Zähner et al. in Tübingen, Germany and structurally identified by Zeeck et al. in Göttingen, Germany in 1989–1990 [3,4]. Once the absolute configuration of all the previously unassigned stereogenic centers in the octapeptidolactone had been established [5,6] and feasible enantioselective syntheses of all the amino acid building blocks had been developed [7,8], a total synthesis of 1 could be embarked on and eventually completed [9]. Several precursor-induced biosyntheses [10,11] and chemical syntheses [11,12] have provided close to 20 hormaomycin analogues that have contributed to an understanding of the biosynthetic pathways, the conformational behavior in solution and the structure–activity relationship.
Figure 1: Structure and absolute configuration of hormaomycin (1), its fluoromethyl-substituted analogues 8a–c and 3-(2-fluoromethylcyclopropyl]alanine building blocks 9a–c. 2: (S)-Ile; 3: (2S,3R)-(β-Me)Phe; 4: (2R)-α-Thr; 5: (1'R,2'R)-(3-Ncp)Ala; 6: (2S,4R)-4-(Z)-(4-PE)Pro; 7: Chpca; 9: (1'S,2'R)-(3-FMcp)Ala.
Figure 1: Structure and absolute configuration of hormaomycin (1), its fluoromethyl-substituted analogues 8a–c...
After the initial observation that hormaomycin (1) has a marked influence on the secondary metabolite production of various streptomycetes including the producing strain itself, and an exceptionally selective inhibitory effect on coryneform bacteria [3,4], an interesting antimalarial activity was discovered [13]. It was this property that led us to consider the synthesis of even more analogues of 1. Bearing in mind that tri(di,mono)fluoromethyl substituents often enhance the activity of pharmacologically relevant compounds [14-17], we embarked on the project to synthesize analogues 8 of hormaomycin (1), in which the (2-nitrocyclopropyl)alanine moieties would be replaced by [2-tri(di,mono)fluoromethylcyclopropyl]alanine residues (Figure 1).
Results and Discussion
As prerequisites for the hormaomycin analogues 8a–c, the correctly configured two diastereomers each of the [2-tri(di,mono)fluoromethylcyclopropyl]alanines 9a–c had to be synthesized in enantiomerically pure form. In view of the successful employment of the enantiomeric nickel(II) complexes (R)-10 and (S)-10 [18-20] in the synthesis of (2R,1R',2R')- and (2S,1R',2R')-3-(2-nitrocyclopropyl)alanine (5) for the native hormaomycin (1) [7,9], a completely analogous route was chosen towards (2R,1'S,2'R)- and (2S,1'S,2'R)-9a–c. This required the racemic trans-(2-fluoromethylcyclopropyl) iodides 11a–c as alkylating agents for the enolates of (R)-10 and (S)-10 (Figure 2).
Figure 2: Structures of the Belokon'-type glycine complexes (BGC) (R)- and (S)-10.
Figure 2: Structures of the Belokon'-type glycine complexes (BGC) (R)- and (S)-10.
The initially intended preparations of the three precursors 14a–c to the iodides 11a–c all starting from the known dimethyl trans-cyclopropane-1,2-dicarboxylate (12) [21] through the monomethyl ester 13 [22], the hydroxymethyl 15 [22] and the formyl derivative 16 [23,24] as outlined in Scheme 1, were only partially successful.
Scheme 1: Intended routes to methyl trans-2-(fluormethyl)cyclopropanecarboxylates 14a–c.
Scheme 1: Intended routes to methyl trans-2-(fluormethyl)cyclopropanecarboxylates 14a–c.
As expected, the hydroxymethyl (15) and formyl derivative 16 underwent smooth conversion into the monofluoro- and difluoromethyl derivatives 14c and 14b, respectively upon treatment with a solution of Deoxo-Fluor in toluene at ambient temperature. Unfortunately, conducting the reaction with carboxylic acid 13 under the same conditions only provided the acid fluoride and not the trifluormethylated compound 14a.
Therefore, an alternative approach to trans-(2-trifluoromethyl)cyclopropanecarboxylic acid 23 by way of the Claisen condensation product of ethyl trifluoroacetate (17) with diethyl succinate (18) [25] was used. The conditions for two of the known further steps [26,27] (Scheme 2) had to be modified to achieve acceptable yields. The reduction of the ketoester 20 to the hydroxyester 21 under the previously described conditions (H2/PtO2) proceeded very slowly, and in several runs (2 h, 24 h, 72 h) the yield of 21 was never better than 60% with up to 25% recovered ketoester 20. However, with powdered sodium borohydride in diethyl ether, the conversion of 20 was quantitative, and the yield of 21 was excellent (98%). In the final step, the attempted 1,3-dehydrotosylation of 22 with potassium tert-butoxide, when carried out in dimethyl sulfoxide as reported [26,27], compound 23 as an intermolecular condensation product of the expected ethyl trans-(2-trifluoromethyl)cyclopropanecarboxylate with DMSO was obtained in 74% yield. Among several other base/solvent combinations tested – NaOEt/EtOH, NaOMe/MeOH, t-BuOK/t-BuOH, NaH/THF, t-BuOK/THF under reflux – the last one gave the best results with up to 47% yield of the trans-(2-trifluoromethyl)cyclopropanecarboxylic acid (24).
Scheme 2: Synthesis of trans-(2-trifluoromethyl)cyclopropanecarboxylic acid (24).
Scheme 2: Synthesis of trans-(2-trifluoromethyl)cyclopropanecarboxylic acid (24).
The conversion of the carboxylic acid 24 and esters 14b,c to the corresponding cyclopropylmethyl alcohols was attempted according to the standard protocol by adding the respective substrate to a twofold excess of LiAlH4 in diethyl ether under reflux. (2-Trifluoromethylcyclopropyl)methanol (25a) thus was obtained in excellent yield (88%), but the difluoromethyl- (25b) and especially monofluoromethylcyclopropylmethanol 25c, respectively, were obtained from the corresponding methyl cyclopropanecarboxylates 14b and 14c, respectively, in very poor yields (3% and 4%, respectively). In the case of monofluoro derivative 14c the main product (38%) was trans-(2-methylcyclopropyl)methanol. In the case of the difluoro compound 14b, a mixture of the mono- (25c) and difluoromethylcyclopropylmethanol 25b along with the non-fluorinated alcohol was obtained in a ratio of approximately 1:1:1.
To avoid this overreduction, inverse addition of 1.1 equiv of LiAlH4 in diethyl ether solution (ca. 1 M) to a solution of the acid 24 or the respective ester 14b,c in diethyl ether (ca. 1 M) was practiced. This way, the desired alcohols 25a–c were obtained in good yields (88, 82 and 76%, respectively). Upon treatment with the iodine/triphenylphosphine reagent in the presence of imidazole, the racemic trans-(2-fluoromethylcyclopropyl)methanols 25a–c were smoothly converted to the corresponding iodides 11a–c in very good yields (Scheme 3).
Scheme 3: Preparation of racemic trans-2-(fluoromethyl)cyclopropylmethyl iodides 11a–c and their conversion to (2S,1'S,2'R)- and (2R,1'S,2'R)-3-(trans-2'-fluoromethylcyclopropyl)alanines 9a–c (only (2S)-enantiomers are shown in the Scheme). Compounds (S)-10 and (R)-10 are shown in Figure 2. For details see Table 1.
Scheme 3: Preparation of racemic trans-2-(fluoromethyl)cyclopropylmethyl iodides 11a–c and their conversion t...
Table 1: Alkylation of the enolates of the Belokon'-type glycine equivalents (S)- and (R)-10 with the racemic trans-2-(fluoromethyl)cyclopropylmethyl iodides rac-11a–c (see Scheme 3). Yields in % based on converted (S)- and (R)-10.
| from (S)-10 | from (R)-10 | |||
|---|---|---|---|---|
| Iodide | 2S,1'S,2'R | 2S,1'R,2'S | 2R,1'S,2'R | 2R,1'R,2'S |
| rac-11a | 46 | 49 | 44 | 42 |
| rac-11b | 45 | 48 | 47 | 45 |
| rac-11c | 44 | 45 | 47 | 42 |
Alkylation of the glycine equivalent enolates derived from (S)- and (R)-2-[(N-benzylprolyl)amino]benzophenone [(S)- and (R)-10] as reusable chiral auxiliaries with the racemic iodides 11a–c, employing the protocol of Larionov and de Meijere [7], in each case led to a mixture of diastereomeric products, which were separated by column chromatography. Unfortunately, the diastereomers could not be separated by fractional crystallization as easily as was previously reported for the corresponding 3-(trans-2-nitrocyclopropyl)alanine derivatives [7]. The absolute configuration of the arbitrarily selected nickel(II) complexes (2S,1'R,2'S)-26a, (2S,1'R,2'S)-26b and (2R,1'R,2'S)-26b were determined by a single crystal X-ray structure analysis (see Figure 3 and Supporting Information File 1) [28].
![[1860-5397-10-302-3]](/bjoc/content/figures/1860-5397-10-302-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Structure and absolute configurations of the nickel(II) complexes (2S,1'R,2'S)-26a, (2S,1'R,2'S)-26b and (2R,1'R,2'S)-26b in the crystals. Less important hydrogen atoms are omitted for clarity.
Figure 3: Structure and absolute configurations of the nickel(II) complexes (2S,1'R,2'S)-26a, (2S,1'R,2'S)-26b...
The isolated nickel complexes 26a–c were decomposed by treatment with refluxing aqueous methanolic hydrogen chloride to give, after ion-exchange chromatography, the corresponding (2S,1'S,2'R)- [see Scheme 3, derived from (S)-10] and (2R,1'S,2'R)-3-(2'-fluoromethylcyclopropyl)alanines [derived from (R)-10] in good to excellent yields. The chiral auxiliary was recovered as the hydrochloride of 2-[(N-benzylprolyl)amino]benzophenone (~95%).
(R)-allo-Threonine (4) is commercially available, but extremely expensive (from 77.80 € for 250 mg to 60.80 € for 25 mg). Therefore a simple and inexpensive access to (R)-allo-threonine was desirable.
The synthesis of 4 from (R)-threonine as a chiral precursor was performed according to the known protocol reported by Tanner et al [29]. Although (R)-threonine is less expensive (21–37 € for 5 g) than the target amino acid, the conversion requires five steps, and the overall yield is not better than 72%.
The Belokon' protocols are among the best to access enantiomerically pure non-proteogenic amino acids. Nickel(II) or copper(II) complexes of Schiff bases derived from glycine and (S)- or (R)-2-N-(N'-benzylprolyl)aminobenzophenone (BPB) [30,31], aminoacetophenone (BPA) [32] or aminobenzaldehyde (BPH) [33] can be used as chiral nucleophilic glycine equivalents in reactions with alkyl halides or carbonyl compounds. The most versatile one is the nickel(II) aminobenzophenone derivative.
It is interesting that nickel(II) complexes of Schiff bases derived from 2-bromoglycine and (S)-BPB can be used as electrophilic glycine equivalents [34]. Alkylations of the nickel(II) complexes of Schiff bases derived from glycine and (S)- or (R)-BPB with alkyl halides virtually yield single stereoisomers, in which the configuration of the newly formed stereogenic center at C-2 of the amino acid moiety is the same as that in the proline moiety of the chiral auxiliary in the starting material.
In reactions of the enolate of this chiral glycine equivalent with aldehydes the situation is more complicated. The reaction of (S)-10 with acetaldehyde under strongly basic conditions led to the (R)-threonine complex 29 (inverse configuration relative to that of the proline moiety of (S)-10 due to epimerization on C-2), but when a weaker base such as triethylamine was employed, a mixture of (R)-threonine 29 and (S)-allo-threonine 31 complexes [35] was obtained.
The hypothesis, that the reaction of the Belokon' glycine complex (BGC) 10 with aldehydes under strongly basic conditions proceeds in two steps and is thermodynamically controlled, was corroborated by experimental tests [36]. The initially formed main product (R,R,R)-28 in the aldol reaction of acetaldehyde with 10 had the same configuration at C-2 as the proline unit in 10. The absolute configuration of this nickel(II) complex was determined by a single crystal X-ray structure analysis (see Figure 4 and Supporting Information File 1) [28]. However, the product ratio changed in time from 95:5 after 30 s through 70:18 after 10 min to 5:95 after 24 h at ambient temperature. This epimerization comes along with a possible rearrangement in the Ni complex. The newly formed hydroxide group of the product 28 can coordinate the Ni atom liberating the carboxylate moiety and thus making the proton at C-2 accessible to base attack (Scheme 4). In order to obtain (R)-allo-threonine (4), it is necessary to carry out the aldol reaction of (R)-10 with an excess of acetaldehyde under strongly basic conditions at low temperature and to quench the reaction after a short time to avoid epimerization of 28.
![[1860-5397-10-302-4]](/bjoc/content/figures/1860-5397-10-302-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Structure and absolute configuration of nickel(II) complex (R,R,R)-28 in the crystal. Hydrogen atoms are omitted for clarity.
Figure 4: Structure and absolute configuration of nickel(II) complex (R,R,R)-28 in the crystal. Hydrogen atom...
Scheme 4: Mechanism of epimerization of the threonine nickel(II) complex 29.
Scheme 4: Mechanism of epimerization of the threonine nickel(II) complex 29.
This modified protocol indeed gave the (R)-allo-threonine (4) in relatively poor yield [7.5% for the Ni complex, 91% (7% overall) for the amino acid], but with high enantiomeric purity in two steps. Bearing in mind that the starting materials are inexpensive and the chiral auxiliary is reusable (≥95% recovery), this protocol represents one of the best routes to the rather expensive (R)-allo-threonine (4).
It is also possible to obtain (R)-allo-threonine starting from (R)-10 and acetaldehyde under thermodynamic control (Et3N as the base, (S)-threonine:(R)-allo-threonine = 1:7), but it is necessary to leave the reaction mixture for two months for the reaction to go to completion [37].
(2S,3R)-3-Methylphenylalanine (L-β-methylphenylalanine, (β-Me)Phe, MeF, 3) also is a constituent of the peptidolactone hormaomycin (1) and is contained in the molecule twice. Thus it is required for the synthesis of hormaomycin and the analogues envisaged here. In addition, a versatile protocol for the preparation of other β-alkylarylalanines for incorporation into hormaomycin analogues as well as into other peptides would be desirable as the incorporation of conformationally constrained α-amino acids such as 3 into peptides is frequently used to study structure–activity relationships [38-40].
Several methods have been developed for the preparation of analogues of β-methylphenylalanine in enantiopure form. These include classical resolution [41], enzymatic resolution in conjunction with HPLC [42], or HPLC separation of derived peptides [43], preparative HPLC separation on a chiral phase column [44], asymmetric synthesis from chiral precursors [45,46] including the stereoselective alkylation of aromatic compounds with triflates of threonine stereoisomers [47], the chiral auxiliary approach [48-51] and enantioselective hydrogenation over a chiral catalyst [52,53].
All these approaches ought to be applicable to prepare unsubstituted β-methylphenylalanine, but most if not all of them have severe drawbacks. Among the chiral auxiliary approaches to β-branched arylalanines, including all four stereoisomers of β-methylphenylalanine, the one employing the "Evans amide" method with a 4-benzyl- or 4-phenyl-2-oxazolidinone moiety, has been used most frequently. Along this route, which requires eight procedural steps (including a transmetallation), the (2S,3R)-3-methyl-3-phenylalanine required for hormaomycin and its analogues, has previously been utilized [9,54,55].
In view of the good performance of the Belokoń protocol for various electrophilic reagents it appeared attractive to apply it for the synthesis of β-methylphenylalanines as well (Scheme 5). Towards this, the (S)-configured glycine nickel(II) complex (S)-10 was alkylated with 1-phenylethyl iodide and some analogues with substituents in the aryl moiety, all in racemic form. The diastereomeric product Ni(II) complexes obtained in each case, could be separated by column chromatography. The pure diastereomers with (2S,3R) configuration were decomposed with an aqueous methanolic HCl solution to furnish the target amino acids, which were purified by ion-exchange chromatography, in good yields (Table 2). (2S,3R)-β-Methylphenylalanine was thus prepared from acetophenone in only four steps in an overall yield of 30%.
Scheme 5: A new general approach to (2S,3R)-β-methylarylalanines 3 by alkylation of the glycine nickel(II) complex (S)-10 with 1-arylethyl iodides 35. For details see Table 2.
Scheme 5: A new general approach to (2S,3R)-β-methylarylalanines 3 by alkylation of the glycine nickel(II) co...
Table 2: Substituted β-methylphenylalanines by alkylation of the glycine nickel(II) complex (S)-10 with 1-arylethyl iodides (yields based on converted 10, d.e. ≥98%). See Scheme 5. The yields of the liberated amino acids 3 based on the respective Ni complexes 32 are in parentheses.
| X | Product | Yield (%) | Product | Yield (%) |
|---|---|---|---|---|
| H | (2S,3S)-32a | 35 | (2S,3R)-32 | 38 (59) |
| o-Cl | (2S,3S)-32-o-Cl | 38 | (2S,3R)-32-o-Cl | 42 |
| m-Cl | (2S,3S)-32-m-Cl | 37 | (2S,3R)-32-m-Cl | 42 (96) |
| p-Cl | (2S,3S)-32-p-Cl | 42 | (2S,3R)-32-p-Cl | 40 (89) |
| p-F | (2S,3S)-32-p-F | 46 | (2S,3R)-32-p-F | 43 |
aThe absolute configurations of the arbitrarily selected nickel(II) complexes (2S,3S)-32, (2S,3R)-32-m-Cl and (2S,3R)-32-p-F were determined by single crystal X-ray structure analyses (see Figure 5 and CCDC-deposited material) [28].
![[1860-5397-10-302-5]](/bjoc/content/figures/1860-5397-10-302-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Structure and absolute configuration of nickel(II) complex (2S,3S)-32 in the crystal. Hydrogen atoms are omitted for clarity.
Figure 5: Structure and absolute configuration of nickel(II) complex (2S,3S)-32 in the crystal. Hydrogen atom...
A similar protocol for the synthesis of 3-alkylphenylalanines was independently developed by Soloshonok et al. [56]. These authors used sodium hydroxide for the deprotonation of 10 and 1-phenylalkyl bromides for the alkylation of the enolate.
Once sufficient quantities of (2S,1'S,2'R)-9a–c and (2R,1'S,2'R)-9a–c (FmcpA), as well as the N-Boc-protected (2S,4R)-4-(Z)-propenylproline [(4-Pe)Pro, 6] [8], the O-MOM-protected 5-chloro-1-hydroxypyrrole-2-carboxylic acid (Chpca, 7) [8], (R)-allo-threonine (allo-Thr, 4) and (2S, 3R)-β-methylphenylalanine [(β-Me)Phe, 3] had been prepared. The assembly of the hormaomycin analogues 8a–c with 3-(2'-fluoromethylcyclopropyl)alanine residues was initiated, employing the same sequence as developed by Zlatopolskiy for the synthesis of hormaomycin 1 [9] and its aza-analogue [12]. To start with, the dicyclopropylmethyl (DCPM) ester of N-Fmoc-protected Ile 37, was condensed with N-Z-protected (βMe)Phe-OH 39. After removal of the Z group from the N-terminus of the resulting dipeptide 42 by catalytic hydrogenation, the product was coupled with N-Fmoc-protected (2R,1'R,2'R)-[3-(mono-, di- or tri-)fluoromethylcyclopropyl]alanines 41a–c to yield tripeptides 47a–c, which, in turn, after deprotection with Et2NH/THF, were coupled with N-Fmoc-protected (βMe)Phe-OH 46 to give N,C-protected tetrapeptides 49a–c.
The N-Boc-protected (4-Pe)Pro-OH 43 and N,C-protected allo-Thr 40 were condensed under 4-pyrrolidinopyridine catalysis to give the ester 45, which, after deallylation under palladium catalysis, was coupled with the tetrapeptides employing the HATU reagent in the presence of HOAt to give the corresponding hexadepsipeptides 51a–c.
From the latter, the DCPM and Boc groups were cleaved off from both termini, leaving the MeZ group intact as proved by an ESIMS spectrum, and the cyclizing peptide condensation was achieved under high dilution conditions with the HATU reagent. The cyclohexadepsipeptides 52a–c were obtained in 54, 60 and 53%, respectively, yield over 8 steps, after HPLC purification (Scheme 6).
Scheme 6: Synthesis of the cyclohexadepsipeptides 52a–c for the hormaomycin analogues 8a–c with 3-(2'-fluoromethylcyclopropyl)alanine residues. a: trifluoromethyl-, b: difluoromethyl-, c: monofluoromethylcyclopropylalanine. Reagents and conditions: i) oxalyl chloride, pyridine/dicyclopropylmethanol, DMAP, CH2Cl2, 0→20 °C, 20 h; ii) ZOSu, NaHCO3, acetone/water, 2 h; iii) 50% Et2NH/THF, 20 °C, 1 h; iv) EDC, HOAt, 2,4,6-collidine, CH2Cl2, 4→20 °C, 14 h; v) FmocOSu, NaHCO3, acetone/water, 3 h; vi) H2, Pd/C, EtOAc, 20 °C, 2 h; vii) MeZOSu, NaHCO3, water/dioxane, 20 °C, 3 h; viii) All-Br, K2CO3, MeCN, 85 °C, 3 h, 60 °C, 16 h; ix) EDC, 4-pyrrolidinopyridine, CH2Cl2, 4→20 °C, 16 h; x) [Pd(PPh3)4], N-methylaniline, DME, 20 °C, 1 h; xi) HATU, HOAt, DIEA, 2,4,6-collidine, CH2Cl2, 4→20 °C, 15 h; xii) 2 M HCl in EtOAc, 20 °C, 20 min; xii) HATU, HOAt, DIEA, 2,4,6-collidine, CH2Cl2, 4→20 °C, 22 h. Boc = tert-butyloxycarbonyl, DCPM = dicyclopropylmethyl, Fmoc = 9-fluorenylmethyloxycarbonyl, DIEA = N,N-diisopropylethylamine, DMAP = 4-dimethylaminopyridine, EDC = N'-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride, HATU = O-(7-azabenzotriazole-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate, HOAt = 7-aza-1-hydroxybenzotriazole, MeZ = (4-methylbenzyl)oxycarbonyl, Z = benzyloxycarbonyl.
Scheme 6: Synthesis of the cyclohexadepsipeptides 52a–c for the hormaomycin analogues 8a–c with 3-(2'-fluorom...
The assemblies of the corresponding hormaomycin analogues were completed after removal of the N-MeZ groups from the cyclic intermediates 52a–c, subsequent coupling with the corresponding N-Teoc-protected (2S,1'R,2'R)-[3-(mono-, di- or tri-)fluoromethylcyclopropyl]alanines 53a–c and, after removal of the Teoc groups, the intermediates 56a–c were in turn coupled with the 1-O-MOM-protected 5-chloro-1-hydroxypyrrole-2-carboxylic acid 54. Eventually, the MOM group was cleaved off by treatment with MgBr2∙Et2O and EtSH in dichloromethane to give, after HPLC purification, the target compounds 8a–c in 72, 82 and 84% yield, respectively (Scheme 7).
Scheme 7: Synthesis of hormaomycin analogues with a: trifluoromethyl-, b: difluoromethyl-, c: monofluoromethylcyclopropylalanine residues. Reagents and conditions: i) anisole, TFA, 20 °C, 2 h; ii) TeocOSu, NaHCO3, N,N-dimethylaminopropylamine, water/acetone, 20 °C, 2 h; iii) HATU, HOAt, DIEA, 2,4,6-collidine, CH2Cl2, 20 °C, 15 h; iv) TFA, 20 °C, 1 h; v) HATU, DIEA, 2,4,6-collidine, CH2Cl2, 20 °C, 4 h; vi) MgBr2·Et2O, EtSH, CH2Cl2, 20 °C, 3.5 h. DIEA = N,N-diisopropylethylamine, HATU = O-(7-azabenzotriazole-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate, HOAt = 7-aza-1-hydroxybenzotriazole, MeZ = (4-methylbenzyl)oxycarbonyl, MOM = methoxymethyl, Teoc = (2-trimethylsilylethyl)oxycarbonyl.
Scheme 7: Synthesis of hormaomycin analogues with a: trifluoromethyl-, b: difluoromethyl-, c: monofluoromethy...
Since the MeZ-protected cyclohexadepsipeptide core of the native hormaomycin was found to have a significant antiparasitic activity, N-acetylated 58 and N-trifluoroacetylated 59 derivatives were prepared by coupling the deprotected cyclic intermediate 52a with acetic and trifluoroacetic acid (Figure 6).
Figure 6: Two derivatives 58 and 59 of cyclohexadepsipeptide 52a containing the (trifluoromethylcyclopropyl)alanine moiety, the aza-analogue 60 of hormamycin (1) itself as well as a simplified analogue 61 for biological testing.
Figure 6: Two derivatives 58 and 59 of cyclohexadepsipeptide 52a containing the (trifluoromethylcyclopropyl)a...
Some antiparasitic activities of hormaomycin, its all-peptide analogue and the new analogues
The syntheses of hormaomycin (1) itself and of its all-peptide aza-analogues 60 and 61 (Figure 6) as developed by Zlatopolskiy et al. [9,12], were reproduced in order to provide large enough quantities for biological tests of their antimalarial activities. Results of the in vitro tests of the antiparasitic activities of hormaomycin (1) [57], its analogues 8a, 52a, 58 and 59, as well as the aza-analogues 60 and 61 are presented in Table 3.
Table 3: In vitro activities of some hormaomycin-derived compounds [58] and established reference drugs against L. donovani (axenic amastigotes), P. falciparum and L6 cells (IC50, concentration in µg/mL).a
| Compound | Leishmania donovani strain MHOM-ET-67/L82 | Plasmodium falciparum strain K1 | L6 cells |
|---|---|---|---|
| Miltefosine | 0.143 | – | – |
| Chloroquine | – | 0.089 | – |
| Podophyllotoxin | – | – | 0.006 |
| 1 | 0.15 | 0.129 | 17.20 |
| 52a | 2.13 | 0.042 | >90 |
| 58 | 1.73 | 0.151 | >90 |
| 8a | 0.205 | 0.183 | 40 |
| 59 | 2.37 | 0.265 | >90 |
| 60 | 4.8 | 0.023 | ? |
| 61 | – | 0.061 | ? |
aIC50 values reported are the averages of two independent assays which varied less than ±50%.
All the newly prepared and tested hormaomycin analogues and the native hormaomycin (1) [57] showed good and selective antimalarial activities. Compounds 8a and 1 additionally showed an activity against L. donovani. However, the activities of the (trifluoromethylcyclopropyl)alanine-containing hormaomycin analogue 8a against L. donovani and P. falciparum were 37 and 45% lower than those of hormaomycin (1) itself. Further exploration of structure–activity relationships of the hormaomycins would be required to prepare new antiparasitic lead compounds.
Conclusion
At first sight, the oligopeptide assembly leading to hormaomycin does not appear to be a very complicated problem. "State of the art" peptide coupling methodology [59,60] allows one to prepare almost any peptides, that do not contain extremely sterically congested fragments such as α,α-dialkyl-substituted amino acids, N-alkyl amino acids or even the more challenging N-aryl amino acids. With a proper choice of the coupling reagent, solvent and other experimental conditions, the oligopeptides in this study were obtained in high yields and with high optical purities. As almost all amino acids, which comprise hormaomycin (1) itself and its anticipated analogues, are β-branched with the exception of 3-(2'-nitrocyclopropyl)alanine and the 3-(2'-fluoromethylcyclopropyl)alanines, HATU as well as the combination of EDC and 7-aza-1-hydroxybenzotriazole (HOAt) [61] were used for each condensation step to ensure high yields. The most unusual fragment in hormaomycin (1) and its analogues is the ester bond between the secondary (4-Pe)Pro moiety and the hydroxy group of allo-Thr. Among several methods described in the literature for the creation of such bonds, the dialkylaminopyridine-promoted carbodiimide-mediated esterification was successfully employed here [62].
As far as the biological activities against L. donovani and P. falciparum are concerned, a certain degree of lowering was observed, but by far no complete loss. Thus, further modifications would be desirable to eventually arrive at a new lead compound.
Experimental
For detailed experimental procedures of all the described syntheses see the Supporting Information File 1.
In vitro antiprotozoal activity assays: The in vitro activities against the protozoan parasites L. donovani and P. falciparum as well as cytotoxicity assessments against L6 cells were determined as reported elsewhere [58]. The following strains, parasite forms and positive controls were used: L. donovani, MHOM/ET/67/L82, axenic amastigote forms, miltefosine, IC50 of 0.143 μg/mL; P. falciparum, K1 (chloroquine and pyrimethamine resistant), erythrocytic stages, chloroquine, IC50 of 0.089 μg/mL and L6 cells, rat skeletal myoblasts, podophyllotoxin, IC50 of 0.006 μg/mL.
Supporting Information
| Supporting Information File 1: Experimental procedures and analytical data. | ||
| Format: PDF | Size: 5.3 MB | Download |
References
-
Arndt, M.; Hilt, G.; Khlebnikov, A. F.; Kozhushkov, S. I.; de Meijere, A. Eur. J. Org. Chem. 2012, 3112–3121. doi:10.1002/ejoc.201200105
-
Khlebnikov, A. F.; Kozhushkov, S. I.; Yufit, D. S.; Schill, H.; Reggelin, M.; Spohr, V.; de Meijere, A. Eur. J. Org. Chem. 2012, 1530–1545. doi:10.1002/ejoc.201101715
-
Andres, N.; Wolf, H.; Zähner, H.; Rössner, E.; Zeeck, A.; König, W. A.; Sinnwell, V. Helv. Chim. Acta 1989, 72, 426–437. doi:10.1002/hlca.19890720303
Return to citation in text: [1] [2] -
Rössner, E.; Zeeck, A.; König, W. A. Angew. Chem. 1990, 102, 84–85. doi:10.1002/ange.19901020122
Angew. Chem., Int. Ed., Engl. 1990, 29, 64–65. doi:10.1002/anie.199000641
Return to citation in text: [1] [2] -
Zindel, J.; Zeeck, A.; König, W. A.; de Meijere, A. Tetrahedron Lett. 1993, 34, 1917–1920. doi:10.1016/S0040-4039(00)91962-2
Return to citation in text: [1] -
Zlatopolskiy, B. D.; Loscha, K.; Alvermann, P.; Kozhushkov, S. I.; Nikolaev, S. V.; Zeeck, A.; de Meijere, A. Chem. – Eur. J. 2004, 10, 4708–4717. doi:10.1002/chem.200400406
Return to citation in text: [1] -
Larionov, O. V.; Savel'eva, T. F.; Kochetkov, K. A.; Ikonnokov, N. S.; Kozhushkov, S. I.; Yufit, D. S.; Howard, J. A. K.; Khrustalev, V. N.; Belokon', Y. N.; de Meijere, A. Eur. J. Org. Chem. 2003, 869–877. doi:10.1002/ejoc.200390131
Return to citation in text: [1] [2] [3] [4] -
Zlatopolskiy, B. D.; Kroll, H.-P.; Melotto, E.; de Meijere, A. Eur. J. Org. Chem. 2004, 4492–4502. doi:10.1002/ejoc.200400480
Return to citation in text: [1] [2] [3] -
Zlatopolskiy, B. D.; de Meijere, A. Chem. – Eur. J. 2004, 10, 4718–4727. doi:10.1002/chem.200400249
Return to citation in text: [1] [2] [3] [4] [5] -
Kozhushkov, S. I.; Zlatopolskiy, B. D.; Brandl, M.; Alvermann, P.; Razdom, M.; Geers, B.; de Meijere, A.; Zeeck, A. Eur. J. Org. Chem. 2005, 854–863. doi:10.1002/ejoc.200400608
Return to citation in text: [1] -
Zlatopolskiy, B. D.; Razdom, M.; Zeeck, A.; de Meijere, A. Eur. J. Org. Chem. 2006, 1525–1534. doi:10.1002/ejoc.200500856
Return to citation in text: [1] [2] -
Reinscheid, U. M.; Zlatopolskiy, B. D.; Griesinger, C.; Zeeck, A.; de Meijere, A. Chem. – Eur. J. 2005, 11, 2929–2945. doi:10.1002/chem.200400977
Return to citation in text: [1] [2] [3] -
Otoguro, K.; Ui, H.; Ishiyama, A.; Arai, N.; Kobayashi, M.; Takahashi, Y.; Masuma, R.; Shiomi, K.; Yamada, H.; Omura, S. J. Antibiot. 2003, 56, 322–324. doi:10.7164/antibiotics.56.322
Return to citation in text: [1] -
O'Hagan, D. Beilstein J. Org. Chem. 2008, 4, No. 11. doi:10.3762/bjoc.4.11
Return to citation in text: [1] -
O'Hagan, D. Beilstein J. Org. Chem. 2010, 6, No. 36. doi:10.3762/bjoc.6.36
Return to citation in text: [1] -
Al-Maharik, N.; O'Hagan, D. Aldrichimica Acta 2011, 44, 65–75.
Return to citation in text: [1] -
Gouverneur, V.; Müller, K., Eds. Fluorine in Pharmaceutical and Medicinal Chemistry; World Scientific Imperial College Press: Singapore, 2012. doi:10.1142/p746
Return to citation in text: [1] -
Belokon', Y. N.; Kochetkov, K. A.; Ikonnikov, N. S.; Strelkova, T. V.; Harutyunyan, S. R.; Saghiyan, A. S. Tetrahedron: Asymmetry 2001, 12, 481–485. doi:10.1016/S0957-4166(01)00071-4
Return to citation in text: [1] -
Saghiyan, A. S.; Geolchanyan, A. V.; Djamgaryan, S. M.; Vardapetyan, S. M.; Tararov, V. I.; Kuz'mina, N. A.; Ikonnikov, N. S.; Belokon', Y. N.; North, M. Russ. Chem. Bull. 2000, 49, 1460–1463. doi:10.1007/BF02495097
Return to citation in text: [1] -
Belokon', Y. N. Janssen Chim. Acta 1992, 10, 4–12.
Return to citation in text: [1] -
Csuk, R.; von Scholz, Y. Tetrahedron 1994, 50, 10431–10442. doi:10.1016/S0040-4020(01)89583-1
Return to citation in text: [1] -
Baldwin, J. E.; Chang, G. E. C. Tetrahedron 1982, 38, 825–835. doi:10.1016/0040-4020(82)80163-4
Return to citation in text: [1] [2] -
Le Corre, M.; Hercouet, A.; Bessieres, B. Tetrahedron: Asymmetry 1995, 6, 683–684. doi:10.1016/0957-4166(95)00060-3
The aldehyde 16 has previously been prepared by oxidation of 15 with pyridinium chlorochromate.
Return to citation in text: [1] -
Braña, M. F.; Guisado, C.; Alguacil, L. F.; Garrido, E.; Pérez-Garcıa, C.; Ruiz-Gayo, M. Bioorg. Med. Chem. Lett. 2002, 12, 3561–3563. doi:10.1016/S0960-894X(02)00793-X
Swern oxidation of 15 to yield the aldehyde 16 has previously been mentioned but without experimental details.
Return to citation in text: [1] -
Brown, P.; Burdon, J.; Smith, T. J.; Tatlow, J. C. Tetrahedron 1960, 10, 164–170. doi:10.1016/S0040-4020(01)97803-2
Return to citation in text: [1] -
Hanack, M.; Meyer, H. Justus Liebigs Ann. Chem. 1968, 720, 81–97. doi:10.1002/jlac.19687200107
Return to citation in text: [1] [2] -
Ratier, M.; Pereyre, M.; Davies, A. G.; Sutcliffe, R. J. Chem. Soc., Perkin Trans. 2 1984, 1907–1915. doi:10.1039/p29840001907
Return to citation in text: [1] [2] -
CCDC-991863 [(2S,1'R,2'S)-26a], -991864 [2S,1'R,2'S)-26b], -991865 [(2R,1'R,2'S)-26b], -991862 [(R,R,R)-28], -991866 [(2S,3S)-32], -1018305 [(2S,3R)-32-m-Cl] and -1018304 [(2S,3R)-32-p-F] contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB21EZ, UK; fax: (+44)1223-336-033; or deposit@ccdc.cam.ac.uk).
Return to citation in text: [1] [2] [3] -
Andersson, P. G.; Guijarro, D.; Tanner, D. J. Org. Chem. 1997, 62, 7364–7375. doi:10.1021/jo970918h
Return to citation in text: [1] -
Belokon', Y. N.; Bakhmutov, V. I.; Chernoglazova, N. I.; Kochetkov, K. A.; Vitt, S. V.; Garbalinskaya, N. S.; Belikov, V. M. J. Chem. Soc., Perkin Trans. 1 1988, 305–312. doi:10.1039/p19880000305
Return to citation in text: [1] -
Belokon', Y. N.; Bulychev, A. G.; Pavlov, V. A.; Fedorova, E. B.; Tsyryapkin, V. A.; Bakhmutov, V. A.; Belikov, V. M. J. Chem. Soc., Perkin Trans. 1 1988, 2075–2083. doi:10.1039/P19880002075
Return to citation in text: [1] -
Belokon', Y. N.; Zel'tzer, I. E.; Ryzhov, M. G.; Saporovskaya, M. B.; Bakhmutov, V. I.; Belikov, V. M. J. Chem. Soc., Chem. Commun. 1982, 180–181. doi:10.1039/C39820000180
Return to citation in text: [1] -
Belokon', Y. N.; Maleyev, V. I.; Vitt, S. V.; Ryzhov, M. G.; Kondrashov, Y. D.; Golubev, S. N.; Vauchskii, Y. P.; Kazika, A. I.; Novikova, M. I.; Krasutskii, P. A.; Yurchenko, A. G.; Dubchak, I. L.; Shklover, V. E.; Struchkov, Y. T.; Bakhmutov, V. I.; Belikov, V. M. J. Chem. Soc., Dalton Trans. 1985, 17–26. doi:10.1039/dt9850000017
Return to citation in text: [1] -
Belokon', Y. N.; Popkov, A. N.; Chernoglazova, N. I.; Saporovskaya, M. B.; Bakhmutov, V. I.; Belikov, V. M. J. Chem. Soc., Chem. Commun. 1988, 1336–1338. doi:10.1039/C39880001336
Return to citation in text: [1] -
Belokon', Y. N.; Bulychev, A. G.; Vitt, S. V.; Struchkov, Y. T.; Batsanov, A. S.; Timofeeva, T. V.; Tsyryapkin, V. A.; Ryzhov, M. G.; Lysova, L. A.; Bakhmutov, V. I.; Belikov, V. M. J. Am. Chem. Soc. 1985, 107, 4252–4259. doi:10.1021/ja00300a030
Return to citation in text: [1] -
Soloshonok, V. A.; Avilov, D. V.; Kukhar, V. P.; Tararov, V. I.; Savel'eva, T. F.; Churkina, T. D.; Ikonnikov, N. S.; Kochetkov, K. A.; Orlova, S. A.; Pysarevsky, A. P.; Struchkov, Y. T.; Raevsky, N. I.; Belokon', Y. N. Tetrahedron: Asymmetry 1995, 6, 1741–1756. doi:10.1016/0957-4166(95)00220-J
Return to citation in text: [1] -
Belokon', Y. N.; Sagyan, A. S.; Djamgaryan, S. A.; Bakhmutov, V. I.; Vitt, S. V.; Batsanov, A. S.; Struchkov, Y. T.; Belikov, V. M. J. Chem. Soc., Perkin Trans. 1 1990, 2301–2310. doi:10.1039/p19900002301
Return to citation in text: [1] -
Balaram, P. Curr. Opin. Struct. Biol. 1992, 2, 845–851. doi:10.1016/0959-440X(92)90110-S
Return to citation in text: [1] -
Hruby, V. J.; Al-Obeidi, F. A.; Kazmierski, W. Biochem. J. 1990, 268, 249–262.
Return to citation in text: [1] -
Hruby, V. J. Acc. Chem. Res. 2001, 34, 389–397. doi:10.1021/ar990063q
Return to citation in text: [1] -
Kataoka, Y.; Seto, Y.; Yamamoto, M.; Yamada, T.; Kuwata, S.; Watanabe, H. Bull. Chem. Soc. Jpn. 1976, 49, 1081–1084. doi:10.1246/bcsj.49.1081
Return to citation in text: [1] -
Alías, M.; López, M. P.; Cativiela, C. Tetrahedron 2004, 60, 885–891. doi:10.1016/j.tet.2003.11.044
Return to citation in text: [1] -
Pastó, M.; Moyano, A.; Pericàs, M. A.; Riera, A. J. Org. Chem. 1997, 62, 8425–8431. doi:10.1021/jo971178f
Return to citation in text: [1] -
Huang, Z.; He, Y.-B.; Raynor, K.; Tallent, M.; Reisine, T.; Goodman, M. J. Am. Chem. Soc. 1992, 114, 9390–9401. doi:10.1021/ja00050a019
Return to citation in text: [1] -
Tourwé, D.; Mannekens, E.; Nguyen Thi Diem, T.; Verheyden, P.; Jaspers, H.; Tóth, G.; Péter, A.; Kertész, I.; Török, G.; Chung, N. N.; Schiller, P. W. J. Med. Chem. 1998, 41, 5167–5176. doi:10.1021/jm981011u
Return to citation in text: [1] -
Davis, F. A.; Liang, C.-H.; Liu, H. J. Org. Chem. 1997, 62, 3796–3797. doi:10.1021/jo9702610
Return to citation in text: [1] -
Effenberger, F.; Weber, T. Angew. Chem. 1987, 99, 146–147. doi:10.1002/ange.19870990217
Angew. Chem., Int. Ed. Engl. 1987, 26, 142–143. doi:10.1002/anie.198701421
Return to citation in text: [1] -
Dharanipragada, R.; Nicolas, E.; Toth, G.; Hruby, V. J. Tetrahedron Lett. 1989, 30, 6841–6844. doi:10.1016/S0040-4039(01)93366-0
Return to citation in text: [1] -
Li, G.; Jarosinski, M. A.; Hruby, V. J. Tetrahedron Lett. 1993, 34, 2561–2564. doi:10.1016/S0040-4039(00)77625-8
Return to citation in text: [1] -
Oppolzer, W.; Tamura, O.; Deerberg, J. Helv. Chim. Acta 1992, 75, 1965–1978. doi:10.1002/hlca.19920750622
Return to citation in text: [1] -
Shapiro, G.; Buechler, D.; Marzi, M.; Schmidt, K.; Gomez-Lor, B. J. Org. Chem. 1995, 60, 4978–4979. doi:10.1021/jo00121a010
Return to citation in text: [1] -
Burk, M. J.; Gross, M. F.; Martinez, J. P. J. Am. Chem. Soc. 1995, 117, 9375–9376. doi:10.1021/ja00141a039
Return to citation in text: [1] -
Burk, M. J.; Bedingfield, K. M.; Kiesman, W. F.; Allen, J. G. Tetrahedron Lett. 1999, 40, 3093–3096. doi:10.1016/S0040-4039(99)00437-2
Return to citation in text: [1] -
Dharanipragada, R.; VanHulle, K.; Bannister, A.; Bear, S.; Kennedy, L.; Hruby, V. J. Tetrahedron 1992, 48, 4733–4748. doi:10.1016/S0040-4020(01)81570-2
Return to citation in text: [1] -
Li, G.; Patel, D.; Hruby, V. J. J. Chem. Soc., Perkin Trans. 1 1994, 3057–3059. doi:10.1039/p19940003057
Return to citation in text: [1] -
Soloshonok, V. A.; Boettiger, T. U.; Bolene, S. B. Synthesis 2008, 2594–2602. doi:10.1055/s-2008-1067172
Return to citation in text: [1] -
A sample of the native hormaomycin (1) was kindly provided by Prof. Axel Zeeck, Göttingen, see [3,4].
Return to citation in text: [1] [2] -
Orhan, I.; Şener, B.; Kaiser, M.; Brun, R.; Tasdemir, D. Mar. Drugs 2010, 8, 47–58. doi:10.3390/md8010047
Return to citation in text: [1] [2] -
Albeiicio, F.; Chinchilla, R.; Dodsworth, D. J.; Nájera, C. Org. Prep. Proced. Int. 2001, 33, 203–303. doi:10.1080/00304940109356592
See for a review on peptide coupling agents.
Return to citation in text: [1] -
Elmore, D. T.; Sovago, I.; Farkas, E.; Penke, B.; Toth, G.; Varadi, G.; Zarandi, M. Amino Acids, Peptides And Proteins. Davies, J. S., Ed.; RSC Publishing: U.K., 2007; Vol. 36, pp 3–346. doi:10.1039/9781847558459
See for a review on peptide coupling agents; covers literature published during 2003.
Return to citation in text: [1] -
Carpino, L. A.; El-Faham, A. Tetrahedron 1999, 55, 6813–6830. doi:10.1016/S0040-4020(99)00344-0
Return to citation in text: [1] -
Kobertz, W. R.; Essigmann, J. M. J. Am. Chem. Soc. 1996, 118, 7101–7107. doi:10.1021/ja960511e
Return to citation in text: [1]
| 36. | Soloshonok, V. A.; Avilov, D. V.; Kukhar, V. P.; Tararov, V. I.; Savel'eva, T. F.; Churkina, T. D.; Ikonnikov, N. S.; Kochetkov, K. A.; Orlova, S. A.; Pysarevsky, A. P.; Struchkov, Y. T.; Raevsky, N. I.; Belokon', Y. N. Tetrahedron: Asymmetry 1995, 6, 1741–1756. doi:10.1016/0957-4166(95)00220-J |
| 28. | CCDC-991863 [(2S,1'R,2'S)-26a], -991864 [2S,1'R,2'S)-26b], -991865 [(2R,1'R,2'S)-26b], -991862 [(R,R,R)-28], -991866 [(2S,3S)-32], -1018305 [(2S,3R)-32-m-Cl] and -1018304 [(2S,3R)-32-p-F] contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB21EZ, UK; fax: (+44)1223-336-033; or deposit@ccdc.cam.ac.uk). |
| 37. | Belokon', Y. N.; Sagyan, A. S.; Djamgaryan, S. A.; Bakhmutov, V. I.; Vitt, S. V.; Batsanov, A. S.; Struchkov, Y. T.; Belikov, V. M. J. Chem. Soc., Perkin Trans. 1 1990, 2301–2310. doi:10.1039/p19900002301 |
| 47. |
Effenberger, F.; Weber, T. Angew. Chem. 1987, 99, 146–147. doi:10.1002/ange.19870990217
Angew. Chem., Int. Ed. Engl. 1987, 26, 142–143. doi:10.1002/anie.198701421 |
| 48. | Dharanipragada, R.; Nicolas, E.; Toth, G.; Hruby, V. J. Tetrahedron Lett. 1989, 30, 6841–6844. doi:10.1016/S0040-4039(01)93366-0 |
| 49. | Li, G.; Jarosinski, M. A.; Hruby, V. J. Tetrahedron Lett. 1993, 34, 2561–2564. doi:10.1016/S0040-4039(00)77625-8 |
| 50. | Oppolzer, W.; Tamura, O.; Deerberg, J. Helv. Chim. Acta 1992, 75, 1965–1978. doi:10.1002/hlca.19920750622 |
| 51. | Shapiro, G.; Buechler, D.; Marzi, M.; Schmidt, K.; Gomez-Lor, B. J. Org. Chem. 1995, 60, 4978–4979. doi:10.1021/jo00121a010 |
| 44. | Huang, Z.; He, Y.-B.; Raynor, K.; Tallent, M.; Reisine, T.; Goodman, M. J. Am. Chem. Soc. 1992, 114, 9390–9401. doi:10.1021/ja00050a019 |
| 45. | Tourwé, D.; Mannekens, E.; Nguyen Thi Diem, T.; Verheyden, P.; Jaspers, H.; Tóth, G.; Péter, A.; Kertész, I.; Török, G.; Chung, N. N.; Schiller, P. W. J. Med. Chem. 1998, 41, 5167–5176. doi:10.1021/jm981011u |
| 46. | Davis, F. A.; Liang, C.-H.; Liu, H. J. Org. Chem. 1997, 62, 3796–3797. doi:10.1021/jo9702610 |
| 42. | Alías, M.; López, M. P.; Cativiela, C. Tetrahedron 2004, 60, 885–891. doi:10.1016/j.tet.2003.11.044 |
| 43. | Pastó, M.; Moyano, A.; Pericàs, M. A.; Riera, A. J. Org. Chem. 1997, 62, 8425–8431. doi:10.1021/jo971178f |
| 38. | Balaram, P. Curr. Opin. Struct. Biol. 1992, 2, 845–851. doi:10.1016/0959-440X(92)90110-S |
| 39. | Hruby, V. J.; Al-Obeidi, F. A.; Kazmierski, W. Biochem. J. 1990, 268, 249–262. |
| 40. | Hruby, V. J. Acc. Chem. Res. 2001, 34, 389–397. doi:10.1021/ar990063q |
| 41. | Kataoka, Y.; Seto, Y.; Yamamoto, M.; Yamada, T.; Kuwata, S.; Watanabe, H. Bull. Chem. Soc. Jpn. 1976, 49, 1081–1084. doi:10.1246/bcsj.49.1081 |
| 52. | Burk, M. J.; Gross, M. F.; Martinez, J. P. J. Am. Chem. Soc. 1995, 117, 9375–9376. doi:10.1021/ja00141a039 |
| 53. | Burk, M. J.; Bedingfield, K. M.; Kiesman, W. F.; Allen, J. G. Tetrahedron Lett. 1999, 40, 3093–3096. doi:10.1016/S0040-4039(99)00437-2 |
| 9. | Zlatopolskiy, B. D.; de Meijere, A. Chem. – Eur. J. 2004, 10, 4718–4727. doi:10.1002/chem.200400249 |
| 54. | Dharanipragada, R.; VanHulle, K.; Bannister, A.; Bear, S.; Kennedy, L.; Hruby, V. J. Tetrahedron 1992, 48, 4733–4748. doi:10.1016/S0040-4020(01)81570-2 |
| 55. | Li, G.; Patel, D.; Hruby, V. J. J. Chem. Soc., Perkin Trans. 1 1994, 3057–3059. doi:10.1039/p19940003057 |
| 28. | CCDC-991863 [(2S,1'R,2'S)-26a], -991864 [2S,1'R,2'S)-26b], -991865 [(2R,1'R,2'S)-26b], -991862 [(R,R,R)-28], -991866 [(2S,3S)-32], -1018305 [(2S,3R)-32-m-Cl] and -1018304 [(2S,3R)-32-p-F] contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB21EZ, UK; fax: (+44)1223-336-033; or deposit@ccdc.cam.ac.uk). |
| 57. | A sample of the native hormaomycin (1) was kindly provided by Prof. Axel Zeeck, Göttingen, see [3,4]. |
| 58. | Orhan, I.; Şener, B.; Kaiser, M.; Brun, R.; Tasdemir, D. Mar. Drugs 2010, 8, 47–58. doi:10.3390/md8010047 |
| 12. | Reinscheid, U. M.; Zlatopolskiy, B. D.; Griesinger, C.; Zeeck, A.; de Meijere, A. Chem. – Eur. J. 2005, 11, 2929–2945. doi:10.1002/chem.200400977 |
| 9. | Zlatopolskiy, B. D.; de Meijere, A. Chem. – Eur. J. 2004, 10, 4718–4727. doi:10.1002/chem.200400249 |
| 12. | Reinscheid, U. M.; Zlatopolskiy, B. D.; Griesinger, C.; Zeeck, A.; de Meijere, A. Chem. – Eur. J. 2005, 11, 2929–2945. doi:10.1002/chem.200400977 |
| 8. | Zlatopolskiy, B. D.; Kroll, H.-P.; Melotto, E.; de Meijere, A. Eur. J. Org. Chem. 2004, 4492–4502. doi:10.1002/ejoc.200400480 |
| 9. | Zlatopolskiy, B. D.; de Meijere, A. Chem. – Eur. J. 2004, 10, 4718–4727. doi:10.1002/chem.200400249 |
| 56. | Soloshonok, V. A.; Boettiger, T. U.; Bolene, S. B. Synthesis 2008, 2594–2602. doi:10.1055/s-2008-1067172 |
| 8. | Zlatopolskiy, B. D.; Kroll, H.-P.; Melotto, E.; de Meijere, A. Eur. J. Org. Chem. 2004, 4492–4502. doi:10.1002/ejoc.200400480 |
| 59. |
Albeiicio, F.; Chinchilla, R.; Dodsworth, D. J.; Nájera, C. Org. Prep. Proced. Int. 2001, 33, 203–303. doi:10.1080/00304940109356592
See for a review on peptide coupling agents. |
| 60. |
Elmore, D. T.; Sovago, I.; Farkas, E.; Penke, B.; Toth, G.; Varadi, G.; Zarandi, M. Amino Acids, Peptides And Proteins. Davies, J. S., Ed.; RSC Publishing: U.K., 2007; Vol. 36, pp 3–346. doi:10.1039/9781847558459
See for a review on peptide coupling agents; covers literature published during 2003. |
| 61. | Carpino, L. A.; El-Faham, A. Tetrahedron 1999, 55, 6813–6830. doi:10.1016/S0040-4020(99)00344-0 |
| 57. | A sample of the native hormaomycin (1) was kindly provided by Prof. Axel Zeeck, Göttingen, see [3,4]. |
| 3. | Andres, N.; Wolf, H.; Zähner, H.; Rössner, E.; Zeeck, A.; König, W. A.; Sinnwell, V. Helv. Chim. Acta 1989, 72, 426–437. doi:10.1002/hlca.19890720303 |
| 4. |
Rössner, E.; Zeeck, A.; König, W. A. Angew. Chem. 1990, 102, 84–85. doi:10.1002/ange.19901020122
Angew. Chem., Int. Ed., Engl. 1990, 29, 64–65. doi:10.1002/anie.199000641 |
| 10. | Kozhushkov, S. I.; Zlatopolskiy, B. D.; Brandl, M.; Alvermann, P.; Razdom, M.; Geers, B.; de Meijere, A.; Zeeck, A. Eur. J. Org. Chem. 2005, 854–863. doi:10.1002/ejoc.200400608 |
| 11. | Zlatopolskiy, B. D.; Razdom, M.; Zeeck, A.; de Meijere, A. Eur. J. Org. Chem. 2006, 1525–1534. doi:10.1002/ejoc.200500856 |
| 23. |
Le Corre, M.; Hercouet, A.; Bessieres, B. Tetrahedron: Asymmetry 1995, 6, 683–684. doi:10.1016/0957-4166(95)00060-3
The aldehyde 16 has previously been prepared by oxidation of 15 with pyridinium chlorochromate. |
| 24. |
Braña, M. F.; Guisado, C.; Alguacil, L. F.; Garrido, E.; Pérez-Garcıa, C.; Ruiz-Gayo, M. Bioorg. Med. Chem. Lett. 2002, 12, 3561–3563. doi:10.1016/S0960-894X(02)00793-X
Swern oxidation of 15 to yield the aldehyde 16 has previously been mentioned but without experimental details. |
| 9. | Zlatopolskiy, B. D.; de Meijere, A. Chem. – Eur. J. 2004, 10, 4718–4727. doi:10.1002/chem.200400249 |
| 25. | Brown, P.; Burdon, J.; Smith, T. J.; Tatlow, J. C. Tetrahedron 1960, 10, 164–170. doi:10.1016/S0040-4020(01)97803-2 |
| 7. | Larionov, O. V.; Savel'eva, T. F.; Kochetkov, K. A.; Ikonnokov, N. S.; Kozhushkov, S. I.; Yufit, D. S.; Howard, J. A. K.; Khrustalev, V. N.; Belokon', Y. N.; de Meijere, A. Eur. J. Org. Chem. 2003, 869–877. doi:10.1002/ejoc.200390131 |
| 8. | Zlatopolskiy, B. D.; Kroll, H.-P.; Melotto, E.; de Meijere, A. Eur. J. Org. Chem. 2004, 4492–4502. doi:10.1002/ejoc.200400480 |
| 22. | Baldwin, J. E.; Chang, G. E. C. Tetrahedron 1982, 38, 825–835. doi:10.1016/0040-4020(82)80163-4 |
| 5. | Zindel, J.; Zeeck, A.; König, W. A.; de Meijere, A. Tetrahedron Lett. 1993, 34, 1917–1920. doi:10.1016/S0040-4039(00)91962-2 |
| 6. | Zlatopolskiy, B. D.; Loscha, K.; Alvermann, P.; Kozhushkov, S. I.; Nikolaev, S. V.; Zeeck, A.; de Meijere, A. Chem. – Eur. J. 2004, 10, 4708–4717. doi:10.1002/chem.200400406 |
| 22. | Baldwin, J. E.; Chang, G. E. C. Tetrahedron 1982, 38, 825–835. doi:10.1016/0040-4020(82)80163-4 |
| 14. | O'Hagan, D. Beilstein J. Org. Chem. 2008, 4, No. 11. doi:10.3762/bjoc.4.11 |
| 15. | O'Hagan, D. Beilstein J. Org. Chem. 2010, 6, No. 36. doi:10.3762/bjoc.6.36 |
| 16. | Al-Maharik, N.; O'Hagan, D. Aldrichimica Acta 2011, 44, 65–75. |
| 17. | Gouverneur, V.; Müller, K., Eds. Fluorine in Pharmaceutical and Medicinal Chemistry; World Scientific Imperial College Press: Singapore, 2012. doi:10.1142/p746 |
| 7. | Larionov, O. V.; Savel'eva, T. F.; Kochetkov, K. A.; Ikonnokov, N. S.; Kozhushkov, S. I.; Yufit, D. S.; Howard, J. A. K.; Khrustalev, V. N.; Belokon', Y. N.; de Meijere, A. Eur. J. Org. Chem. 2003, 869–877. doi:10.1002/ejoc.200390131 |
| 9. | Zlatopolskiy, B. D.; de Meijere, A. Chem. – Eur. J. 2004, 10, 4718–4727. doi:10.1002/chem.200400249 |
| 3. | Andres, N.; Wolf, H.; Zähner, H.; Rössner, E.; Zeeck, A.; König, W. A.; Sinnwell, V. Helv. Chim. Acta 1989, 72, 426–437. doi:10.1002/hlca.19890720303 |
| 4. |
Rössner, E.; Zeeck, A.; König, W. A. Angew. Chem. 1990, 102, 84–85. doi:10.1002/ange.19901020122
Angew. Chem., Int. Ed., Engl. 1990, 29, 64–65. doi:10.1002/anie.199000641 |
| 13. | Otoguro, K.; Ui, H.; Ishiyama, A.; Arai, N.; Kobayashi, M.; Takahashi, Y.; Masuma, R.; Shiomi, K.; Yamada, H.; Omura, S. J. Antibiot. 2003, 56, 322–324. doi:10.7164/antibiotics.56.322 |
| 21. | Csuk, R.; von Scholz, Y. Tetrahedron 1994, 50, 10431–10442. doi:10.1016/S0040-4020(01)89583-1 |
| 3. | Andres, N.; Wolf, H.; Zähner, H.; Rössner, E.; Zeeck, A.; König, W. A.; Sinnwell, V. Helv. Chim. Acta 1989, 72, 426–437. doi:10.1002/hlca.19890720303 |
| 4. |
Rössner, E.; Zeeck, A.; König, W. A. Angew. Chem. 1990, 102, 84–85. doi:10.1002/ange.19901020122
Angew. Chem., Int. Ed., Engl. 1990, 29, 64–65. doi:10.1002/anie.199000641 |
| 62. | Kobertz, W. R.; Essigmann, J. M. J. Am. Chem. Soc. 1996, 118, 7101–7107. doi:10.1021/ja960511e |
| 11. | Zlatopolskiy, B. D.; Razdom, M.; Zeeck, A.; de Meijere, A. Eur. J. Org. Chem. 2006, 1525–1534. doi:10.1002/ejoc.200500856 |
| 12. | Reinscheid, U. M.; Zlatopolskiy, B. D.; Griesinger, C.; Zeeck, A.; de Meijere, A. Chem. – Eur. J. 2005, 11, 2929–2945. doi:10.1002/chem.200400977 |
| 18. | Belokon', Y. N.; Kochetkov, K. A.; Ikonnikov, N. S.; Strelkova, T. V.; Harutyunyan, S. R.; Saghiyan, A. S. Tetrahedron: Asymmetry 2001, 12, 481–485. doi:10.1016/S0957-4166(01)00071-4 |
| 19. | Saghiyan, A. S.; Geolchanyan, A. V.; Djamgaryan, S. M.; Vardapetyan, S. M.; Tararov, V. I.; Kuz'mina, N. A.; Ikonnikov, N. S.; Belokon', Y. N.; North, M. Russ. Chem. Bull. 2000, 49, 1460–1463. doi:10.1007/BF02495097 |
| 20. | Belokon', Y. N. Janssen Chim. Acta 1992, 10, 4–12. |
| 58. | Orhan, I.; Şener, B.; Kaiser, M.; Brun, R.; Tasdemir, D. Mar. Drugs 2010, 8, 47–58. doi:10.3390/md8010047 |
| 7. | Larionov, O. V.; Savel'eva, T. F.; Kochetkov, K. A.; Ikonnokov, N. S.; Kozhushkov, S. I.; Yufit, D. S.; Howard, J. A. K.; Khrustalev, V. N.; Belokon', Y. N.; de Meijere, A. Eur. J. Org. Chem. 2003, 869–877. doi:10.1002/ejoc.200390131 |
| 26. | Hanack, M.; Meyer, H. Justus Liebigs Ann. Chem. 1968, 720, 81–97. doi:10.1002/jlac.19687200107 |
| 27. | Ratier, M.; Pereyre, M.; Davies, A. G.; Sutcliffe, R. J. Chem. Soc., Perkin Trans. 2 1984, 1907–1915. doi:10.1039/p29840001907 |
| 26. | Hanack, M.; Meyer, H. Justus Liebigs Ann. Chem. 1968, 720, 81–97. doi:10.1002/jlac.19687200107 |
| 27. | Ratier, M.; Pereyre, M.; Davies, A. G.; Sutcliffe, R. J. Chem. Soc., Perkin Trans. 2 1984, 1907–1915. doi:10.1039/p29840001907 |
| 34. | Belokon', Y. N.; Popkov, A. N.; Chernoglazova, N. I.; Saporovskaya, M. B.; Bakhmutov, V. I.; Belikov, V. M. J. Chem. Soc., Chem. Commun. 1988, 1336–1338. doi:10.1039/C39880001336 |
| 35. | Belokon', Y. N.; Bulychev, A. G.; Vitt, S. V.; Struchkov, Y. T.; Batsanov, A. S.; Timofeeva, T. V.; Tsyryapkin, V. A.; Ryzhov, M. G.; Lysova, L. A.; Bakhmutov, V. I.; Belikov, V. M. J. Am. Chem. Soc. 1985, 107, 4252–4259. doi:10.1021/ja00300a030 |
| 32. | Belokon', Y. N.; Zel'tzer, I. E.; Ryzhov, M. G.; Saporovskaya, M. B.; Bakhmutov, V. I.; Belikov, V. M. J. Chem. Soc., Chem. Commun. 1982, 180–181. doi:10.1039/C39820000180 |
| 33. | Belokon', Y. N.; Maleyev, V. I.; Vitt, S. V.; Ryzhov, M. G.; Kondrashov, Y. D.; Golubev, S. N.; Vauchskii, Y. P.; Kazika, A. I.; Novikova, M. I.; Krasutskii, P. A.; Yurchenko, A. G.; Dubchak, I. L.; Shklover, V. E.; Struchkov, Y. T.; Bakhmutov, V. I.; Belikov, V. M. J. Chem. Soc., Dalton Trans. 1985, 17–26. doi:10.1039/dt9850000017 |
| 29. | Andersson, P. G.; Guijarro, D.; Tanner, D. J. Org. Chem. 1997, 62, 7364–7375. doi:10.1021/jo970918h |
| 30. | Belokon', Y. N.; Bakhmutov, V. I.; Chernoglazova, N. I.; Kochetkov, K. A.; Vitt, S. V.; Garbalinskaya, N. S.; Belikov, V. M. J. Chem. Soc., Perkin Trans. 1 1988, 305–312. doi:10.1039/p19880000305 |
| 31. | Belokon', Y. N.; Bulychev, A. G.; Pavlov, V. A.; Fedorova, E. B.; Tsyryapkin, V. A.; Bakhmutov, V. A.; Belikov, V. M. J. Chem. Soc., Perkin Trans. 1 1988, 2075–2083. doi:10.1039/P19880002075 |
| 7. | Larionov, O. V.; Savel'eva, T. F.; Kochetkov, K. A.; Ikonnokov, N. S.; Kozhushkov, S. I.; Yufit, D. S.; Howard, J. A. K.; Khrustalev, V. N.; Belokon', Y. N.; de Meijere, A. Eur. J. Org. Chem. 2003, 869–877. doi:10.1002/ejoc.200390131 |
| 28. | CCDC-991863 [(2S,1'R,2'S)-26a], -991864 [2S,1'R,2'S)-26b], -991865 [(2R,1'R,2'S)-26b], -991862 [(R,R,R)-28], -991866 [(2S,3S)-32], -1018305 [(2S,3R)-32-m-Cl] and -1018304 [(2S,3R)-32-p-F] contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB21EZ, UK; fax: (+44)1223-336-033; or deposit@ccdc.cam.ac.uk). |
© 2014 de Meijere et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)