Abstract
An efficient copper-promoted hydration reaction and its application in the synthesis of benzo[b]furan and benzo[b]thiophene derivatives is presented starting from readily available 2-fluorophenylacetylene derivatives. The key annulation step involves the hydration of the C–F bond of 2-fluorophenylacetylene derivatives followed by an intramolecular annulation to afford benzo[b]furan and benzo[b]thiophene derivatives. Moreover, structurally important 2,2'-bisbenzofuran scaffolds are provided in good yields.
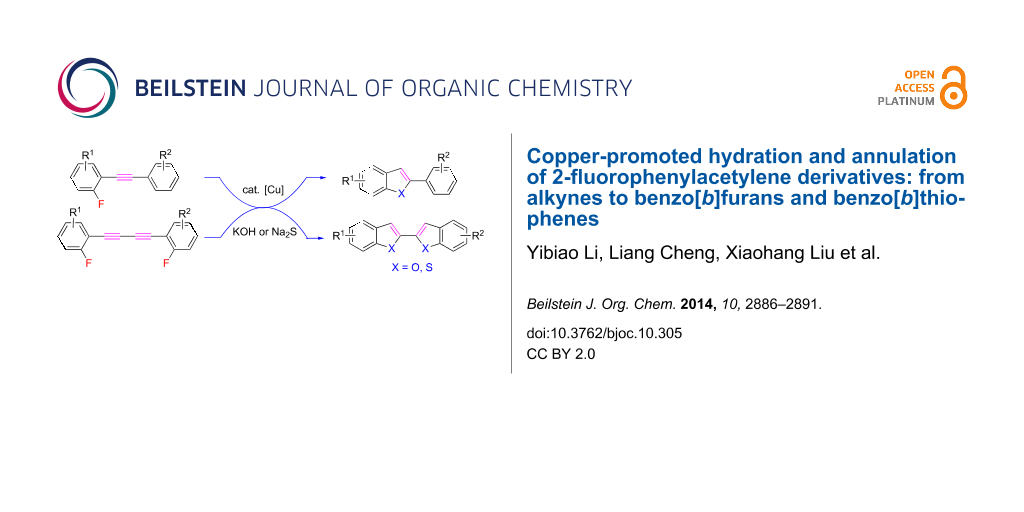
Graphical Abstract
Introduction
The development of general and efficient methodologies for the synthesis of complex heterocycle skeletons has received much attention in the past decades. Among the most ubiquitous heterocyclic moieties in natural and bioactive products are the benzo[b]furan and benzo[b]thiophene units [1-8]. Despite the existence of established methods for the synthesis of benzo[b]furan and benzo[b]thiophene derivatives, the development of more convenient methods is of significant importance [9-14]. Commonly, the preparation of 2-substituted benzo[b]furans involves the usage of 2-halophenols as reaction precursors (Scheme 1a) [15-18], which can be cumbersome due to the precursors’ instability and the protecting and deprotecting steps necessary to synthesize the precursors [19-23]. Ackermann et al. utilized bromo- and iodo-substituted phenylacetylene in their TiCl4-catalyzed intramolecular nucleophilic annulation process (Scheme 1b) [24]. But this method involves a two-step process and the usage of two different metal salts may complicate further processing. The direct design of a Pd or Cu-catalyzed one-pot synthesis of benzo[b]thiophenes from 2-bromoalkynylbenzenes and a thiol derivative has eliminated these problems to a large extent [25-29]. Nevertheless, the direct synthesis of benzo[b]furans from 2-haloalkynylbenzenes and the usage of 2-fluorophenylacetylene derivatives as substrates continues to represent a challenge. Indeed, Tsuji and co-workers have developed a transition metal-free process for the synthesis of benzo[b]furans from 2-fluorophenylacetylene derivatives. But the reaction requires conditions with a high reaction temperature for satisfactory yields. Unfortunately, only benzo[b]furans were obtained in this reaction [30].
Scheme 1: Synthetic approaches to benzo[b]furans from 2-alkynylphenols, ketones and 2-fluorophenylacetylene derivatives.
Scheme 1: Synthetic approaches to benzo[b]furans from 2-alkynylphenols, ketones and 2-fluorophenylacetylene d...
Typically, the aryl halides used in the annulation reactions are iodides and bromides. It is rare to employ aryl fluorides because of their low reactivity [31-33]. To extend the application of our strategy of the copper-catalyzed synthesis of heterocycles, we report herein a one-pot process for the synthesis of benzo[b]furans and benzo[b]thiophenes with 2-fluorophenylacetylene derivatives as precursors (Scheme 1c).
Results and Discussion
We report an efficient synthesis of functionalized benzo[b]furans from commercially available alkynes by a copper-catalyzed, intramolecular annulation process. Initially, our investigation commenced with the annulation of (2-(2-fluorophenyl)ethynyl)benzene (1a) to give the corresponding product 2-phenylbenzofuran (2a) by using 2 equiv KOH as a base under various conditions. In the presence of the Pd(PPh3)4 catalyst the reaction of (2-(2-fluorophenyl)ethynyl)benzene in CH3CN does not give any corresponding product (Table 1, entry 1). The usage of CuCl and 1,10-phenanthroline (1,10-phen) as a ligand in CH3CN at 80 °C showed that 2a could be isolated in 35% yield (Table 1, entry 2). The screening of the various solvents revealed that the solvent played an important role in this hydration and annulation process. Compared with the other solvents, DMSO is more suitable for the annulation process (Table 1, entries 2–4). These investigations revealed that the usage of CuI instead of CuCl as a catalyst resulted in the isolation of 2a in a satisfactory 88% yield after 4 hour (Table 1, entry 5). To our delight, the use of 0.2 equiv of KI as an additive afforded 2a in a satisfactory 95% yield (Table 1, entry 6). The base loading had a strong influence on the yield with 2 equiv KOH being the optimal amount (Table 1, entries 7 and 12). Further screening of bases did not lead to better yields and confirmed that the reaction did not proceed in the presence of CsCO3 (Table 1, entry 8). Other catalytic systems, such as Cu(OAc)2, Cu(OTf)2 and Cu(acac)2, were less effective for this annulation process (Table 1, entries 9–11). A decrease in the temperature lowered the yield of the reaction (Table 1, entry 13). The importance of water was confirmed by a lower yield under dry conditions (Table 1, entry 14). In the absence of CuI, we found that the reaction of (2-(2-fluorophenyl)ethynyl)benzene with KOH in DMSO at 80 °C for 4 h gave 55% yield of the annulation product (Table 1, entry 15).
Table 1: Optimization of the reaction conditions.a
|
|
||||
| Entry | Catalyst | Solvent | Additive | Yield [%]b |
|---|---|---|---|---|
| 1 | Pd(PPh3)4 | CH3CN | – | – |
| 2 | CuCl | CH3CN | 1,10-phen | 35 |
| 3 | CuCl | DMF | 1,10-phen | 71 |
| 4 | CuCl | DMSO | 1,10-phen | 75 |
| 5 | CuI | DMSO | 1,10-phen | 88 |
| 6 | CuI | DMSO | KI | 95 |
| 7c | CuI | DMSO | KI | 68 |
| 8d | CuI | DMSO | KI | <5 |
| 9 | Cu(OAc)2 | DMSO | KI | 65 |
| 10 | Cu(OTf)2 | DMSO | KI | 68 |
| 11 | Cu(acac)2 | DMSO | KI | 54 |
| 12e | CuI | DMSO | KI | <5 |
| 13f | CuI | DMSO | KI | 25 |
| 14g | CuI | DMSO | KI | 38 |
| 15 | – | DMSO | KI | 55 |
aReaction conditions: alkyne 1a (1.0 mmol), catalyst (10 mol %), base (2.0 mmol), H2O (1.5 mmol) and additives (0.2 mmol) in 3 mL of solvent at 80 °C for 4 h; byields are given for isolated products; c1 equiv KOH was used; dCsCO3 instead of KOH; eomitting KOH and starting material recovered; freaction was carried out at 30 °C. gomitting H2O (dry conditions).
Next, we explored the scope and generality of the process by using the conditions for Tabe 1, entry 6. As shown in Scheme 2, substrates with either electron-donating or electron-withdrawing substituents on the benzene ring can undergo the reaction smoothly, and the corresponding benzo[b]furan products were obtained in good to excellent yields. The reaction tolerated a variety of substituents including -Cl, -Br, -F, -OMe, -NMe2 and thiophenyl groups. The use of 2-fluorophenylacetylene derivatives with electron-withdrawing substituents as R2 afforded benzo[b]furan products in higher yields. It is noteworthy that the 2-(2-(2-fluorophenyl)ethynyl)thiophene was also successfully converted to 2-(thiophen-2-yl)benzofuran (2j) in good yields. Subsequently, the R1 substituent of the 2-fluorophenylacetylene derivatives was varied from hydrogen to other functional groups. Substituents at the ortho position of the benzyl group did not have an impact on the reaction yield. The presence of an additional electron-donating substituent marginally decreased the conversion of 2-fluorophenylacetylene derivatives resulting in products in moderate yields (Scheme 2, 2k and 2o). Interestingly, the p-fluoro atom was kept intact during the reaction and fluoro-substituted benzofuran was obtained (Scheme 2, 2q) [34-37]. This shows the good selectivity of the current reaction system. It should be emphasized that the 1,3-bis(2-(2-fluorophenyl)ethynyl)benzene was also successfully converted to benzo[b]furan 2r in good yield. Unfortunately, when aliphatic alkynes were employed, the desired annulation products were formed in low yields.
Scheme 2: Copper-promoted reaction of 2-fluorophenylacetylene derivatives to yield benzo[b]furans. Reaction conditions: Alkyne 1a (1.0 mmol), catalyst (10 mol %), KOH (2.0 mmol), H2O (1.5 mmol) and KI (0.2 mmol) in 3 mL of DMSO at 80 °C for 4–8 h; yields are given for isolated products.
Scheme 2: Copper-promoted reaction of 2-fluorophenylacetylene derivatives to yield benzo[b]furans. Reaction c...
The above studies dealt only with 1,2-diphenylethyne derivatives as a reactive group in the substrates. Inspired by the results of the nucleophilic annulation process, we wondered whether we could further explore the annulation of 1,3-diynes, which have great synthetic potential in medicine and materials sciences [38-40]. For extensions, we used 1,4-bis(2-fluorophenyl)buta-1,3-diyne as a substrate to investigate the possibility of this transformation. Similar to (2-(2-fluorophenyl)ethynyl)benzene, 1,4-bis(2-fluorophenyl)buta-1,3-diyne was able to offer the corresponding annulation products 2s in 78% yield (Scheme 3).
Scheme 3: Copper-promoted synthesis of 2,2'-bisbenzofuran derivatives.
Scheme 3: Copper-promoted synthesis of 2,2'-bisbenzofuran derivatives.
To gain a deeper mechanistic understanding of the present catalytic process, the direct intramolecular annulation of 1-bromo-2-(2-(2-fluorophenyl)ethynyl)benzene and 1-chloro-2-(2-(2-fluorophenyl)ethynyl)benzene were performed, as shown in Scheme 4. In F/Br-substituted 1-bromo-2-(2-(2-fluorophenyl)ethynyl)benzene, the fluoro moiety served as leaving group and gave 2-(2-bromophenyl)benzofuran (2t) as a major product. In F/Cl-substituted 1-chloro-2-(2-(2-fluorophenyl)ethynyl)benzene was able to offer the chloro-substituted product 2v as the only product. A reactivity order of F > Br > Cl can be derived from these data.
Scheme 4: Intramolecular competition experiments.
Scheme 4: Intramolecular competition experiments.
Reactions with Na2S·9H2O as a nucleophile were successful, and the corresponding benzo[b]thiophene products were obtained in high yields (Scheme 5). We obtained the best results with DMSO as the solvent and a reaction temperature of 60 °C. Using the optimized reaction conditions, 3-chloro and 4-chloro substituted 2-fluoroalkynylbenzenes were reacted with Na2S·9H2O to yield benzo[b]thiophenes in good yields.
Scheme 5: Copper-promoted synthesis of benzo[b]thiophenes.
Scheme 5: Copper-promoted synthesis of benzo[b]thiophenes.
The postulated reaction mechanism is depicted in Scheme 6 [25-29]. The catalytic cycle is initiated by the nucleophilic substitution of 2-fluorophenylacetylene derivative 1 with OH−. This might provide unstable 2-alkynylphenol A, which could then form the corresponding potassium phenolate intermediate B. The coordination of CuI with B may provide intermediate C, and the subsequent addition to the C–C triple bond gives the copper complex D. Protonolysis of intermediate D generates benzo[b]furan 2 and regenerates the active catalyst species.
Scheme 6: Proposed mechanism for the annulation reaction.
Scheme 6: Proposed mechanism for the annulation reaction.
Conclusion
In summary, we have developed a new protocol for the synthesis of benzo[b]furan and benzo[b]thiophene derivatives starting from 2-fluorophenylacetylene derivatives. The hydration and annulation is catalyzed by CuI with KOH or Na2S·9H2O as a base at 60–80 °C to give the corresponding products in moderate to good yields. Various functional groups are accepted resulting in a wide range of substituted benzo[b]furans and benzo[b]thiophenes. Further studies, which are focused on the extension of the scope and the application of the reaction to the synthesis of bioactive products, are currently ongoing in our laboratory.
Supporting Information
| Supporting Information File 1: Full experimental details and copies of NMR spectral data. | ||
| Format: PDF | Size: 2.9 MB | Download |
Acknowledgements
We thank the National Natural Science Foundation of China (21302146), the Guangdong Natural Science Foundation (S2013040012354), the Foundation for Distinguished Young Talents in Higher Education of Guangdong (2013LYM_0094), and the Science Foundation for Young Teachers of Wuyi University (201210291040098) for financial support.
References
-
Wu, X.-F.; Neumann, H.; Beller, M. Chem. Rev. 2013, 113, 1–35. doi:10.1021/cr300100s
Return to citation in text: [1] -
Zeni, G.; Larock, R. C. Chem. Rev. 2006, 106, 4644–4680. doi:10.1021/cr0683966
Return to citation in text: [1] -
Lipshutz, B. H. Chem. Rev. 1986, 86, 795–819. doi:10.1021/cr00075a005
Return to citation in text: [1] -
Lu, H.; Liu, G.-T. Planta Med. 1992, 58, 311–313. doi:10.1055/s-2006-961473
Return to citation in text: [1] -
Navarro, E.; Alonso, S. J.; Trujillo, J.; Jorge, E.; Pérez, C. J. Nat. Prod. 2001, 64, 134–135. doi:10.1021/np9904861
Return to citation in text: [1] -
Cacchi, S.; Fabrizi, G.; Goggiamani, A. Org. Biomol. Chem. 2011, 9, 641–652. doi:10.1039/c0ob00501k
Return to citation in text: [1] -
Flynn, B. L.; Hamel, E.; Jung, M. K. J. Med. Chem. 2002, 45, 2670–2673. doi:10.1021/jm020077t
Return to citation in text: [1] -
Palkowitz, A. D.; Glasebrook, A. L.; Thrasher, K. J.; Hauser, K. L.; Short, L. L.; Philips, D. L.; Muehl, B. S.; Sato, M.; Shetler, P. K.; Cullinan, G. J.; Pell, T. R.; Bryant, H. U. J. Med. Chem. 1997, 40, 1407–1416. doi:10.1021/jm970167b
Return to citation in text: [1] -
Wang, X.; Liu, M.; Xu, L.; Wang, Q.; Chen, J.; Ding, J.; Wu, H. J. Org. Chem. 2013, 78, 5273–5281. doi:10.1021/jo400433m
Return to citation in text: [1] -
Kraus, G. A.; Schroeder, J. D. Synlett 2005, 2504–2506. doi:10.1055/s-2005-872690
Return to citation in text: [1] -
Katritzky, A. R.; Ji, Y.; Fang, Y.; Prakash, I. J. Org. Chem. 2001, 66, 5613–5615. doi:10.1021/jo010278p
Return to citation in text: [1] -
Siddiqui, I. R.; Waseem, M. A.; Shamim, S.; Shireen; Srivastava, A.; Srivastava, A. Tetrahedron Lett. 2013, 54, 4154–4158. doi:10.1016/j.tetlet.2013.05.150
Return to citation in text: [1] -
Eidamshaus, C.; Burch, J. D. Org. Lett. 2008, 10, 4211–4214. doi:10.1021/ol801510n
Return to citation in text: [1] -
Liang, Z.; Hou, W.; Du, Y.; Zhang, Y.; Pan, Y.; Mao, D.; Zhao, K. Org. Lett. 2009, 11, 4978–4981. doi:10.1021/ol902157c
Return to citation in text: [1] -
Zeni, G.; Larock, R. C. Chem. Rev. 2004, 104, 2285–2310. doi:10.1021/cr020085h
Return to citation in text: [1] -
Cho, C.-H.; Neuenswander, B.; Lushington, G. H.; Larock, R. C. J. Comb. Chem. 2008, 10, 941–947. doi:10.1021/cc800120y
Return to citation in text: [1] -
Cano, R.; Yus, M.; Ramón, D. J. Tetrahedron 2012, 68, 1393–1400. doi:10.1016/j.tet.2011.12.042
Return to citation in text: [1] -
Liang, Y.; Tang, S.; Zhang, X.-D.; Mao, L.-Q.; Xie, Y.-X.; Li, J.-H. Org. Lett. 2006, 8, 3017–3020. doi:10.1021/ol060908f
Return to citation in text: [1] -
Arcadi, A.; Cacchi, S.; Di Giuseppe, S.; Fabrizi, G.; Marinelli, F. Org. Lett. 2002, 4, 2409–2412. doi:10.1021/ol0261581
Return to citation in text: [1] -
Okitsu, T.; Nakazawa, D.; Taniguchi, R.; Wada, A. Org. Lett. 2008, 10, 4967–4970. doi:10.1021/ol8020463
Return to citation in text: [1] -
Yue, D.; Yao, T.; Larock, R. C. J. Org. Chem. 2005, 70, 10292–10296. doi:10.1021/jo051299c
Return to citation in text: [1] -
Arcadi, A.; Cacchi, S.; Fabrizi, G.; Marinelli, F.; Moro, L. Synlett 1999, 1432–1434. doi:10.1055/s-1999-2839
Return to citation in text: [1] -
Colobert, F.; Castanet, A.-S.; Abillard, O. Eur. J. Org. Chem. 2005, 3334–3341. doi:10.1002/ejoc.200500166
Return to citation in text: [1] -
Ackermann, L.; Kaspar, L. T. J. Org. Chem. 2007, 72, 6149–6153. doi:10.1021/jo070887i
Return to citation in text: [1] -
Sun, L.-L.; Deng, C.-L.; Tang, R.-Y.; Zhang, X.-G. J. Org. Chem. 2011, 76, 7546–7550. doi:10.1021/jo201081v
Return to citation in text: [1] [2] -
Ma, D.; Xie, S.; Xue, P.; Zhang, X.; Dong, J.; Jiang, Y. Angew. Chem., Int. Ed. 2009, 48, 4222–4225. doi:10.1002/anie.200900486
Return to citation in text: [1] [2] -
Kuhn, M.; Falk, F. C.; Paradies, J. Org. Lett. 2011, 13, 4100–4103. doi:10.1021/ol2016093
Return to citation in text: [1] [2] -
Guilarte, V.; Fernández-Rodríguez, M. A.; García-García, P.; Hernando, E.; Sanz, R. Org. Lett. 2011, 13, 5100–5103. doi:10.1021/ol201970m
Return to citation in text: [1] [2] -
Prasad, D. J. C.; Sekar, G. Org. Biomol. Chem. 2013, 11, 1659–1665. doi:10.1039/c3ob26915a
Return to citation in text: [1] [2] -
Tsuji, H.; Cantagrel, G.; Ueda, Y.; Chen, T.; Wan, L.-J.; Nakamura, E. Chem. – Asian J. 2013, 8, 2377–2382. doi:10.1002/asia.201300106
Return to citation in text: [1] -
Amii, H.; Uneyama, K. Chem. Rev. 2009, 109, 2119–2183. doi:10.1021/cr800388c
Return to citation in text: [1] -
Grecian, S. A.; Hadida, S.; Warren, S. D. Tetrahedron Lett. 2005, 46, 4683–4685. doi:10.1016/j.tetlet.2005.04.111
Return to citation in text: [1] -
He, C.-Y.; Fan, S.; Zhang, X. J. Am. Chem. Soc. 2010, 132, 12850–12852. doi:10.1021/ja106046p
Return to citation in text: [1] -
Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455–529. doi:10.1021/cr100166a
Return to citation in text: [1] -
O’Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a
Return to citation in text: [1] -
Lectard, S.; Hamashima, Y.; Sodeoka, M. Adv. Synth. Catal. 2010, 352, 2708–2732. doi:10.1002/adsc.201000624
Return to citation in text: [1] -
Cho, E. J.; Senecal, T. D.; Kinzel, T.; Zhang, Y.; Watson, D. A.; Buchwald, S. L. Science 2010, 328, 1679–1681. doi:10.1126/science.1190524
Return to citation in text: [1] -
Matsuda, S.; Takahashi, M.; Monguchi, D.; Mori, A. Synlett 2009, 1941–1944. doi:10.1055/s-0029-1217537
Return to citation in text: [1] -
Jacubert, M.; Provot, O.; Peyrat, J.-F.; Hamze, A.; Brion, J.-D.; Alami, M. Tetrahedron 2010, 66, 3775–3787. doi:10.1016/j.tet.2010.03.055
Return to citation in text: [1] -
Pan, W.-B.; Chen, C.-C.; Wei, L.-L.; Wei, L.-M.; Wu, M.-J. Tetrahedron Lett. 2013, 54, 2655–2657. doi:10.1016/j.tetlet.2013.03.040
Return to citation in text: [1]
| 1. | Wu, X.-F.; Neumann, H.; Beller, M. Chem. Rev. 2013, 113, 1–35. doi:10.1021/cr300100s |
| 2. | Zeni, G.; Larock, R. C. Chem. Rev. 2006, 106, 4644–4680. doi:10.1021/cr0683966 |
| 3. | Lipshutz, B. H. Chem. Rev. 1986, 86, 795–819. doi:10.1021/cr00075a005 |
| 4. | Lu, H.; Liu, G.-T. Planta Med. 1992, 58, 311–313. doi:10.1055/s-2006-961473 |
| 5. | Navarro, E.; Alonso, S. J.; Trujillo, J.; Jorge, E.; Pérez, C. J. Nat. Prod. 2001, 64, 134–135. doi:10.1021/np9904861 |
| 6. | Cacchi, S.; Fabrizi, G.; Goggiamani, A. Org. Biomol. Chem. 2011, 9, 641–652. doi:10.1039/c0ob00501k |
| 7. | Flynn, B. L.; Hamel, E.; Jung, M. K. J. Med. Chem. 2002, 45, 2670–2673. doi:10.1021/jm020077t |
| 8. | Palkowitz, A. D.; Glasebrook, A. L.; Thrasher, K. J.; Hauser, K. L.; Short, L. L.; Philips, D. L.; Muehl, B. S.; Sato, M.; Shetler, P. K.; Cullinan, G. J.; Pell, T. R.; Bryant, H. U. J. Med. Chem. 1997, 40, 1407–1416. doi:10.1021/jm970167b |
| 24. | Ackermann, L.; Kaspar, L. T. J. Org. Chem. 2007, 72, 6149–6153. doi:10.1021/jo070887i |
| 19. | Arcadi, A.; Cacchi, S.; Di Giuseppe, S.; Fabrizi, G.; Marinelli, F. Org. Lett. 2002, 4, 2409–2412. doi:10.1021/ol0261581 |
| 20. | Okitsu, T.; Nakazawa, D.; Taniguchi, R.; Wada, A. Org. Lett. 2008, 10, 4967–4970. doi:10.1021/ol8020463 |
| 21. | Yue, D.; Yao, T.; Larock, R. C. J. Org. Chem. 2005, 70, 10292–10296. doi:10.1021/jo051299c |
| 22. | Arcadi, A.; Cacchi, S.; Fabrizi, G.; Marinelli, F.; Moro, L. Synlett 1999, 1432–1434. doi:10.1055/s-1999-2839 |
| 23. | Colobert, F.; Castanet, A.-S.; Abillard, O. Eur. J. Org. Chem. 2005, 3334–3341. doi:10.1002/ejoc.200500166 |
| 15. | Zeni, G.; Larock, R. C. Chem. Rev. 2004, 104, 2285–2310. doi:10.1021/cr020085h |
| 16. | Cho, C.-H.; Neuenswander, B.; Lushington, G. H.; Larock, R. C. J. Comb. Chem. 2008, 10, 941–947. doi:10.1021/cc800120y |
| 17. | Cano, R.; Yus, M.; Ramón, D. J. Tetrahedron 2012, 68, 1393–1400. doi:10.1016/j.tet.2011.12.042 |
| 18. | Liang, Y.; Tang, S.; Zhang, X.-D.; Mao, L.-Q.; Xie, Y.-X.; Li, J.-H. Org. Lett. 2006, 8, 3017–3020. doi:10.1021/ol060908f |
| 9. | Wang, X.; Liu, M.; Xu, L.; Wang, Q.; Chen, J.; Ding, J.; Wu, H. J. Org. Chem. 2013, 78, 5273–5281. doi:10.1021/jo400433m |
| 10. | Kraus, G. A.; Schroeder, J. D. Synlett 2005, 2504–2506. doi:10.1055/s-2005-872690 |
| 11. | Katritzky, A. R.; Ji, Y.; Fang, Y.; Prakash, I. J. Org. Chem. 2001, 66, 5613–5615. doi:10.1021/jo010278p |
| 12. | Siddiqui, I. R.; Waseem, M. A.; Shamim, S.; Shireen; Srivastava, A.; Srivastava, A. Tetrahedron Lett. 2013, 54, 4154–4158. doi:10.1016/j.tetlet.2013.05.150 |
| 13. | Eidamshaus, C.; Burch, J. D. Org. Lett. 2008, 10, 4211–4214. doi:10.1021/ol801510n |
| 14. | Liang, Z.; Hou, W.; Du, Y.; Zhang, Y.; Pan, Y.; Mao, D.; Zhao, K. Org. Lett. 2009, 11, 4978–4981. doi:10.1021/ol902157c |
| 34. | Nie, J.; Guo, H.-C.; Cahard, D.; Ma, J.-A. Chem. Rev. 2011, 111, 455–529. doi:10.1021/cr100166a |
| 35. | O’Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a |
| 36. | Lectard, S.; Hamashima, Y.; Sodeoka, M. Adv. Synth. Catal. 2010, 352, 2708–2732. doi:10.1002/adsc.201000624 |
| 37. | Cho, E. J.; Senecal, T. D.; Kinzel, T.; Zhang, Y.; Watson, D. A.; Buchwald, S. L. Science 2010, 328, 1679–1681. doi:10.1126/science.1190524 |
| 25. | Sun, L.-L.; Deng, C.-L.; Tang, R.-Y.; Zhang, X.-G. J. Org. Chem. 2011, 76, 7546–7550. doi:10.1021/jo201081v |
| 26. | Ma, D.; Xie, S.; Xue, P.; Zhang, X.; Dong, J.; Jiang, Y. Angew. Chem., Int. Ed. 2009, 48, 4222–4225. doi:10.1002/anie.200900486 |
| 27. | Kuhn, M.; Falk, F. C.; Paradies, J. Org. Lett. 2011, 13, 4100–4103. doi:10.1021/ol2016093 |
| 28. | Guilarte, V.; Fernández-Rodríguez, M. A.; García-García, P.; Hernando, E.; Sanz, R. Org. Lett. 2011, 13, 5100–5103. doi:10.1021/ol201970m |
| 29. | Prasad, D. J. C.; Sekar, G. Org. Biomol. Chem. 2013, 11, 1659–1665. doi:10.1039/c3ob26915a |
| 31. | Amii, H.; Uneyama, K. Chem. Rev. 2009, 109, 2119–2183. doi:10.1021/cr800388c |
| 32. | Grecian, S. A.; Hadida, S.; Warren, S. D. Tetrahedron Lett. 2005, 46, 4683–4685. doi:10.1016/j.tetlet.2005.04.111 |
| 33. | He, C.-Y.; Fan, S.; Zhang, X. J. Am. Chem. Soc. 2010, 132, 12850–12852. doi:10.1021/ja106046p |
| 30. | Tsuji, H.; Cantagrel, G.; Ueda, Y.; Chen, T.; Wan, L.-J.; Nakamura, E. Chem. – Asian J. 2013, 8, 2377–2382. doi:10.1002/asia.201300106 |
| 25. | Sun, L.-L.; Deng, C.-L.; Tang, R.-Y.; Zhang, X.-G. J. Org. Chem. 2011, 76, 7546–7550. doi:10.1021/jo201081v |
| 26. | Ma, D.; Xie, S.; Xue, P.; Zhang, X.; Dong, J.; Jiang, Y. Angew. Chem., Int. Ed. 2009, 48, 4222–4225. doi:10.1002/anie.200900486 |
| 27. | Kuhn, M.; Falk, F. C.; Paradies, J. Org. Lett. 2011, 13, 4100–4103. doi:10.1021/ol2016093 |
| 28. | Guilarte, V.; Fernández-Rodríguez, M. A.; García-García, P.; Hernando, E.; Sanz, R. Org. Lett. 2011, 13, 5100–5103. doi:10.1021/ol201970m |
| 29. | Prasad, D. J. C.; Sekar, G. Org. Biomol. Chem. 2013, 11, 1659–1665. doi:10.1039/c3ob26915a |
| 38. | Matsuda, S.; Takahashi, M.; Monguchi, D.; Mori, A. Synlett 2009, 1941–1944. doi:10.1055/s-0029-1217537 |
| 39. | Jacubert, M.; Provot, O.; Peyrat, J.-F.; Hamze, A.; Brion, J.-D.; Alami, M. Tetrahedron 2010, 66, 3775–3787. doi:10.1016/j.tet.2010.03.055 |
| 40. | Pan, W.-B.; Chen, C.-C.; Wei, L.-L.; Wei, L.-M.; Wu, M.-J. Tetrahedron Lett. 2013, 54, 2655–2657. doi:10.1016/j.tetlet.2013.03.040 |
© 2014 Li et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)