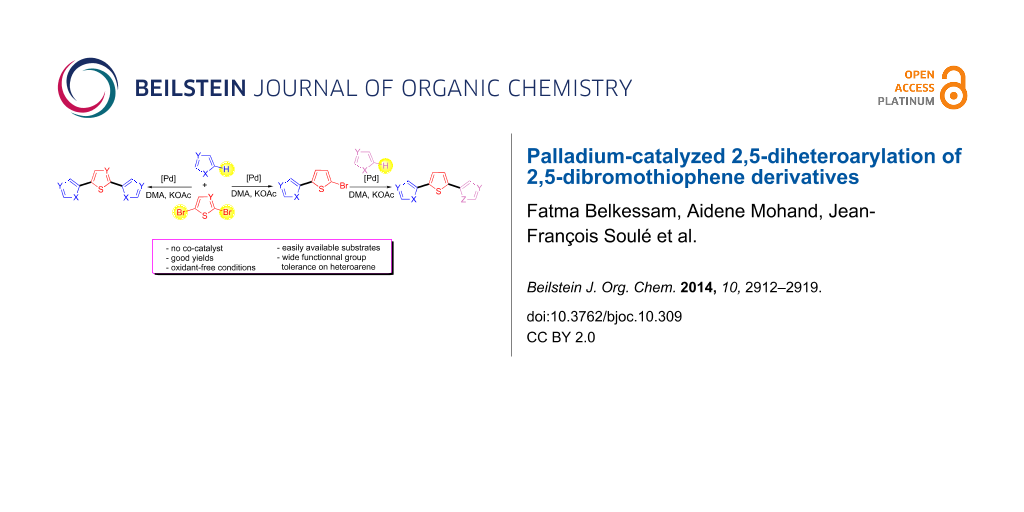
Abstract
Conditions allowing the one pot 2,5-diheteroarylation of 2,5-dibromothiophene derivatives in the presence of palladium catalysts are reported. Using KOAc as the base, DMA as the solvent and only 0.5–2 mol % palladium catalysts, the target 2,5-diheteroarylated thiophenes were obtained in moderate to good yields and with a wide variety of heteroarenes such as thiazoles, thiophenes, furans, pyrroles, pyrazoles or isoxazoles. Moreover, sequential heteroarylation reactions allow the access to 2,5-diheteroarylated thiophenes bearing two different heteroaryl units.
Graphical Abstract
Introduction
2,2':5',2"-Terthiophene (or 2,5-di(2-thienyl)thiophene) (Figure 1) and many of its derivatives are important structures due to their biological and/or physical properties. For example, 2,2':5',2"-terthiophene itself is a pigment of Tagetes minuta. Some 2,2':5',2"-terthiophene derivatives such as 5,5''-dichloro-α-terthiophene also occur naturally [1]. Moreover, terthiophenes are widely used as building blocks for the synthesis of semiconductors [2]. Due to these multiple uses, the discovery of a simpler access to terthiophene derivatives would be very useful.
Suzuki, Stille or Negishi Pd-catalyzed cross-coupling reactions represent some of the most efficient methods for the preparation of 2,5-diheteroarylated thiophenes [3-16]. However, an organometallic derivative must be prepared to perform such reactions. In 1990, Ohta and co-workers reported the Pd-catalyzed direct arylation of heteroaromatics using aryl halides as coupling partners via a C–H bond activation [17,18]. Since then Pd-catalyzed direct arylation of heteroaryls, especially with aryl halides as coupling partners, has been shown to be a very powerful method for an easier and greener access to a very broad range of arylated heterocycles [19-32]. This method is more attractive than other Pd-catalyzed cross-coupling reactions as it avoids the preparation of an organometallic derivative and also as the major byproducts of the reaction are not metallic salts but a base associated to HX.
The metal-catalyzed direct arylation of a wide variety of heteroarenes using aryl halides as coupling partners has been reported in recent years [19-36]. However, to our knowledge, only a few examples of Pd-catalyzed direct arylations at both C2 and C5 carbons of 2,5-dihalothiophene derivatives have been described. In 2006, Borgese et al. reported the Pd-catalyzed coupling of 2,5-dibromothiophene with 3-methoxythiophene to afford the corresponding terthiophene in 29% yield [37]. From 2,5-diiodothiophene and benzoxazole, using 5 mol % Pd(phen)2(PF6)2 catalyst, the 2,5-diheteroarylated thiophene was obtained in 89% yield by Murai et al. [38]. A fluorescent π-conjugate thiophene derivative bearing spiro[fluorene-9,4’-[4H]indeno[1,2-b]furan] substituents at C2 and C5 has been prepared in 46% yield by this reaction using Pd(OAc)2 (5 mol %) associated to PPh3 (10 mol %) as catalytic system [39]. A pyrrole derivative was coupled with 2,5-dibromothiophene in the presence of Pd(OAc)2 (5 mol %) and PCy3 (10 mol %) catalyst to afford the 2,5-di(pyrrolyl)thiophene in 59% yield [40]. Finally, an indolizine was also successfully coupled with 2,5-dibromothiophene in 47% yield in the presence of Pd(OAc)2 as catalyst [41]. To our knowledge, so far sequential Pd-catalyzed direct couplings using 2,5-dihalothiophene derivatives have not been described. Therefore, the discovery of effective general conditions, for the direct coupling of heteroarenes at both C2 and C5 positions of 2,5-dihalothiophene derivatives, would constitute a considerable advantage allowing a simpler access to terthiophene derivatives.
Here, we wish to report (i) that only 0.5–2 mol % of air-stable palladium catalysts associated to KOAc promote the direct access to 2,5-diheteroarylated thiophenes in one pot, (ii) on the reaction scope using a large set of heteroarenes, and (iii) conditions allowing the sequential diheteroarylation of 2,5-dibromothiophene.
Results and Discussion
Based on our previous results, DMA was initially chosen as the solvent and KOAc as the base for this study [42,43]. The reactions were conducted at 140 °C under inert conditions using PdCl(C3H5)(dppb) or Pd(OAc)2 catalysts. Using only 0.5 mol % Pd(OAc)2, the reaction of 1 equiv of 2,5-dibromothiophene with 2 equiv 2-ethyl-4-methylthiazole as coupling partners affords the mono- and diarylation products 1a and 1b in a 2:98 ratio and the desired product 1b was isolated in 79% yield (Scheme 1, Table 1, entry 1). The use of 3 equiv of 2-ethyl-4-methylthiazole afforded 1b in similar yield (Table 1, entry 2). Then, we examined the influence of the amount of catalyst and other parameters on the reaction. The use of 1 or 2 mol % PdCl(C3H5)(dppb) catalyst, which had been previously found to be very effective to promote the direct arylation of several hereroaromatics [42-44], also afforded 1b in high yields (Table 1, entries 3–5). Even at 100 °C, the desired product 1b was obtained in 78% yield (Table 1, entry 6). When CsOAc was employed as the base instead of KOAc, in the presence of 2 mol % PdCl(C3H5)(dppb) catalyst, 1b was isolated in 80% yield, whereas NaOAc led to target product 1b in only 68% yield and Cs2CO3 was ineffective (Table 1, entries 7–9). It should be noted that in the presence of an excess of 2,5-dibromothiophene (4 equiv) with 1 equiv of 2-ethyl-4-methylthiazole the products 1a and 1b were produced in a 72:28 ratio and 1a was isolated in 52% yield, without cleavage of the second C–Br bond on the thiophene ring allowing sequential arylations (Table 1, entry 10).
Scheme 1: Palladium-catalyzed direct arylation using 2,5-dibromothiophene and 2-ethyl-4-methylthiazole as coupling partners.
Scheme 1: Palladium-catalyzed direct arylation using 2,5-dibromothiophene and 2-ethyl-4-methylthiazole as cou...
Table 1: Influence of the reaction conditions for palladium-catalyzed direct arylation using 2,5-dibromothiophene and 2-ethyl-4-methylthiazole as coupling partners (Scheme 1).a
| Entry | Catalyst (mol %) | Base | 2-Ethyl-4-methylthiazole (equiv) | Temperature (°C) | Ratio 1a:1b | Yield in 1b (%) |
|---|---|---|---|---|---|---|
| 1 | Pd(OAc)2 (0.5) | KOAc | 2 | 140 | 2:98 | 79 |
| 2 | Pd(OAc)2 (0.5) | KOAc | 3 | 140 | 1:99 | 80 |
| 3 | PdCl(C3H5)(dppb) (2) | KOAc | 3 | 140 | 0:100 | 81 |
| 4 | PdCl(C3H5)(dppb) (1) | KOAc | 3 | 140 | 0:100 | 80 |
| 5 | PdCl(C3H5)(dppb) (2) | KOAc | 2.2 | 140 | 1:99 | 78 |
| 6 | PdCl(C3H5)(dppb) (2) | KOAc | 3 | 100 | 0:100 | 78 |
| 7 | PdCl(C3H5)(dppb) (2) | NaOAc | 3 | 140 | 7:93 | 68 |
| 8 | PdCl(C3H5)(dppb) (2) | CsOAc | 3 | 140 | 0:100 | 80 |
| 9 | PdCl(C3H5)(dppb) (2) | Cs2CO3 | 3 | 140 | nd | <5 |
| 10 | PdCl(C3H5)(dppb) (2) | KOAc | 3 | 140 | 72:28 | 52b |
aConditions: 2,5-dibromothiophene (1 equiv), base (3 equiv), DMA, 20 h, isolated yields. b2,5-Dibromothiophene (4 equiv), 2-ethyl-4-methylthiazole (1 equiv), yield in 1a.
Then, with the most effective reaction conditions in hand for diheteroarylation (DMA, KOAc, Pd(OAc)2 or PdCl(C3H5)(dppb), 100 or 140 °C, 20 h), we explored the scope of this reaction using a variety of heteroarenes as the coupling partner (Scheme 2).
Scheme 2: Reactivity of 2,5-dibromothiophene with different heteroarenes.
Scheme 2: Reactivity of 2,5-dibromothiophene with different heteroarenes.
First, we investigated the reaction of 2,5-dibromothiophene with 4-methylthiazole (Scheme 2). The reaction proceeded very smoothly to afford the product 2 in 82% yield. It should be noted that no arylation at C2 of this thiazole derivative was observed. Then, a set of thiophene derivatives was employed. Both, 2-methyl- and 2-chlorothiophenes afforded the desired products 3 and 4 in good yields in the presence of PdCl(C3H5)(dppb) as the catalyst. Yields of 62% and 73% of these two products were obtained using 0.5 mol % Pd(OAc)2 catalyst at 140 °C, whereas a reaction performed at 100 °C led to only a partial conversion of 2,5-dibromothiophene to afford 3 in 45% yield. This slightly lower reactivity of thiophene derivatives under these conditions was expected, as they are known to be less reactive than thiazole derivatives [44]. Moderate yields for 5 and 6 were obtained starting form thiophene-2-carbonitrile and ethyl thiophene-2-carboxylate, respectively in the presence of 2 mol % PdCl(C3H5)(dppb) catalyst due to the formation of unidentified degradation products. The use of 6 equiv of thiophene allowed the formation of 2,2':5',2"-terthiophene (7) in 85% yield. The reactivity of three furan derivatives was also studied using PdCl(C3H5)(dppb) as the catalyst. From 2-n-butylfuran, 8 was obtained in 79% yield, whereas 2-acetylfuran and methyl 2-methylfuran-3-carboxylate afforded 9 and 10 in 60% and 63% yield, respectively. The reaction of 1 equiv of 2,5-dibromothiophene with 5 equiv of 1-methylpyrrole gave 11 in 78% yield. No significant formation of other polyheterocycles was observed by GC–MS analysis of the crude mixture. Arylation at C4 of 3,5-dimethylisoxazole and 5-chloro-1,3-dimethylpyrazole afforded 12 and 13 in 80% and 83% yields, respectively. With 3,5-dimethylisoxazole, a reaction performed using only 0.5 mol% Pd(OAc)2 catalyst at 100 °C led to a partial conversion of 2,5-dibromothiophene.
As several terthiophene derivatives bearing alkyl substituents at C3 in their central unit have been employed in material chemistry [2], the reactivity of 2,5-dibromo-3-methylthiophene was also examined (Scheme 3). Similar results to those of 2,5-dibromothiophene were obtained. Both, 2-ethyl-4-methylthiazole and 4-methylthiazole reacted nicely to afford 14 and 15 in 83% and 80% yields, respectively. The four terthiophenes 16–19 were also obtained in satisfactory yields. Again a moderate yield in 20 was obtained in the presence of methyl 2-methylfuran-3-carboxylate due to the formation of degradation products, whereas the reaction with 1-methylpyrrole and 3,5-dimethylisoxazole resulted in good yields of 21 and 22, respectively.
Scheme 3: Reactivity of 2,5-dibromo-3-methylthiophene with different heteroarenes.
Scheme 3: Reactivity of 2,5-dibromo-3-methylthiophene with different heteroarenes.
To our knowledge, the sequential Pd-catalyzed direct diheteroarylation of 2,5-dibromothiophene has not yet been reported. A sequential heteroarylation would allow the synthesis of non-symmetrically 2,5-disubstituted thiophene derivatives. Our attempts to prepare these compounds are shown in Scheme 4. Eight heteroarenes were reacted with 1a to afford the 2,5-diheteroarylated thiophenes 23–30 in 41–89% yield. A high yield of 89% for 23 was obtained from 1a and 2-isobutylthiazole as the coupling partners. The reactions with 2-methylthiophene and thiophene-2-carbonitrile also afforded the desired products 24 and 25 in good yields. A decreased yield of 41% for 26 was obtained with thiophene as coupling partner, whereas, 1-methylpyrrole gave 27 in 74%. Coupling of 1a with methyl 2-methylfuran-3-carboxylate afforded 28 in 62% yield. The arylation at C4 of 3,5-dimethylisoxazole and 5-chloro-1,3-dimethylpyrazole also proceeded nicely to give 29 and 30 in 66% and 72% yield, respectively.
Scheme 4: Sequential diheteroarylation of 2,5-dibromothiophene.
Scheme 4: Sequential diheteroarylation of 2,5-dibromothiophene.
It should be noted that, for the synthesis of 24, the introduction of the thiazole unit in the first step (Scheme 1 and Scheme 4, 36% over 2 steps) led to a slightly higher yield than the introduction of 2-methylthiophene followed by the coupling with 2-ethyl-4-methylthiazole (Scheme 5, 32% yield over 2 steps).
Scheme 5: Sequential diheteroarylation of 2,5-dibromothiophene.
Scheme 5: Sequential diheteroarylation of 2,5-dibromothiophene.
We also compared the preparation of 2,2':5',2"-terthiophene (7) starting from either 2,5-dibromothiophene (Scheme 2) or from 2-bromothiophene (Scheme 6). The reaction of 1 equiv thiophene with 2 equiv of 2-bromothiophene resulted in a poor yield for 7 due to the formation of a mixture of bithiophene 32, terthiophene 7 and also a quaterthiophene (as was observed by GC–MS analysis of the crude mixture). On the other hand, the use of 6 equiv of thiophene in the presence of 1 equiv of 2-bromothiophene afforded 7 and 32 in a 30:70 ratio and only low amounts of a quaterthiophene were observed; compound 32 was isolated in 58% yield (Scheme 6, middle). The same reaction conditions allowed to prepare 1-methyl-2-(thiophen-2-yl)pyrrole (33) in 61% yield (Scheme 6, bottom).
Scheme 6: Heteroarylation of 2-bromothiophene.
Scheme 6: Heteroarylation of 2-bromothiophene.
Finally, as 4,7-diarylbenzothiadiazoles also display important physical properties [45], we applied our procedure to 4,7-dibromobenzothiadiazole which is commercially available (Scheme 7). In all cases, the desired 4,7-diarylbenzothiadiazoles 34–38 were obtained in high yields.
Scheme 7: Reactivity of 4,7-dibromobenzothiadiazole.
Scheme 7: Reactivity of 4,7-dibromobenzothiadiazole.
Conclusion
In summary we report here a simple one-pot catalytic method leading to 2,5-diheteroarylated thiophenes in good yields. We established that 2 mol % of air-stable PdCl(C3H5)(dppb) catalyst (and in some cases 0.5 mol % Pd(OAc)2 catalyst) in the presence of KOAc as the base promotes the 2,5-diheteroarylation of 2,5-dibromothiophene in the presence of a variety of heteroarenes such as thiophenes, furans, pyrroles, pyrazoles or isoxazoles as the coupling partners. The sequential diheteroarylation of 2,5-dibromothiophene was also found to be possible to afford 2,5-diheteroarylated thiophenes bearing two different heteroarene units. As both, 2,5-dibromothiophene and a wide variety of heteroarenes are commercially available, this method gives a convenient access to a large number of terthiophene derivatives.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data. | ||
| Format: PDF | Size: 345.8 KB | Download |
References
-
Liu, Y.; Ye, M.; Guo, H.-Z.; Zhao, Y.-Y.; Guo, D.-A. J. Asian Nat. Prod. Res. 2002, 4, 175–178. doi:10.1080/1028602021000000071
Return to citation in text: [1] -
Gohier, F.; Frère, P.; Roncali, J. J. Org. Chem. 2013, 78, 1497–1503. doi:10.1021/jo302571u
Return to citation in text: [1] [2] -
Li, J. J.; Gribble, G. W. Palladium in Heterocyclic Chemistry; Pergamon: Amsterdam, 2000.
Return to citation in text: [1] -
Negishi, E., Ed. Handbook of Organopalladium Chemistry for Organic Synthesis; Part III; Wiley-Interscience: New York, 2002; p 213.
Return to citation in text: [1] -
Schnürch, M.; Flasik, R.; Khan, A. F.; Spina, M.; Mihovilovic, M. D.; Stanetty, P. Eur. J. Org. Chem. 2006, 3283–3307. doi:10.1002/ejoc.200600089
Return to citation in text: [1] -
Tung, D. T.; Tuân, D. T.; Rasool, N.; Villinger, A.; Reinke, H.; Fischer, C.; Langer, P. Adv. Synth. Catal. 2009, 351, 1595–1609. doi:10.1002/adsc.200900044
Return to citation in text: [1] -
Pernites, R. B.; Ponnapati, R. R.; Advincula, R. C. Adv. Mater. 2011, 23, 3207–3213. doi:10.1002/adma.201100469
Return to citation in text: [1] -
Zhou, J.; Xie, S.; Amond, E. F.; Becker, M. L. Macromolecules 2013, 46, 3391–3394. doi:10.1021/ma400531v
Return to citation in text: [1] -
Qian, D.; Ma, W.; Li, Z.; Guo, X.; Zhang, S.; Ye, L.; Ade, H.; Tan, Z.; Hou, J. J. Am. Chem. Soc. 2013, 135, 8464–8467. doi:10.1021/ja402971d
Return to citation in text: [1] -
Tanaka, S.; Tanaka, D.; Tatsuta, G.; Murakami, K.; Tamba, S.; Sugie, A.; Mori, A. Chem. – Eur. J. 2013, 19, 1658–1665. doi:10.1002/chem.201203331
Return to citation in text: [1] -
Nagura, K.; Saito, S.; Yusa, H.; Yamawaki, H.; Fujihisa, H.; Sato, H.; Shimoikeda, Y.; Yamaguchi, S. J. Am. Chem. Soc. 2013, 135, 10322–10325. doi:10.1021/ja4055228
Return to citation in text: [1] -
Klingstedt, T.; Shirani, H.; Åslund, K. O. A.; Cairns, N. J.; Sigurdson, C. J.; Goedert, M.; Nilsson, K. P. R. Chem. – Eur. J. 2013, 19, 10179–10192. doi:10.1002/chem.201301463
Return to citation in text: [1] -
Mallet, C.; Didane, Y.; Watanabe, T.; Yoshimoto, N.; Allain, M.; Videlot-Ackermann, C.; Frère, P. ChemPlusChem 2013, 78, 459–466. doi:10.1002/cplu.201300037
Return to citation in text: [1] -
Bruns, C. J.; Herman, D. J.; Minuzzo, J. B.; Lehrman, J. A.; Stupp, S. I. Chem. Mater. 2013, 25, 4330–4339. doi:10.1021/cm402505p
Return to citation in text: [1] -
Salamoun, J.; Anderson, S.; Burnett, J. C.; Gussio, R.; Wipf, P. Org. Lett. 2014, 16, 2034–2037. doi:10.1021/ol500620m
Return to citation in text: [1] -
Ferrer Flegeau, E.; Popkin, M. E.; Greaney, M. F. J. Org. Chem. 2008, 73, 3303–3306. doi:10.1021/jo800121y
See for access to trisoxazoles.
Return to citation in text: [1] -
Ohta, A.; Akita, Y.; Ohkuwa, T.; Chiba, M.; Fukunaga, R.; Miyafuji, A.; Nakata, T.; Tani, N.; Aoyagi, Y. Heterocycles 1990, 31, 1951–1958. doi:10.3987/COM-90-5467
Return to citation in text: [1] -
Aoyagi, Y.; Inoue, A.; Koizumi, I.; Hashimoto, R.; Tokunaga, K.; Gohma, K.; Komatsu, J.; Sekine, K.; Miyafuji, A.; Kunoh, J.; Honma, R.; Akita, Y.; Ohta, A. Heterocycles 1992, 33, 257–272. doi:10.3987/COM-91-S29
Return to citation in text: [1] -
Alberico, D.; Scott, M. E.; Lautens, M. Chem. Rev. 2007, 107, 174–238. doi:10.1021/cr0509760
Return to citation in text: [1] [2] -
Satoh, T.; Miura, M. Chem. Lett. 2007, 36, 200–205. doi:10.1246/cl.2007.200
Return to citation in text: [1] [2] -
Li, B.-J.; Yang, S.-D.; Shi, Z.-J. Synlett 2008, 949–957. doi:10.1055/s-2008-1042907
Return to citation in text: [1] [2] -
Bellina, F.; Rossi, R. Tetrahedron 2009, 65, 10269–10310. doi:10.1016/j.tet.2009.10.015
Return to citation in text: [1] [2] -
Ackermann, L.; Vincente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996
Return to citation in text: [1] [2] -
Roger, J.; Gottumukkala, A. L.; Doucet, H. ChemCatChem 2010, 2, 20–40. doi:10.1002/cctc.200900074
Return to citation in text: [1] [2] -
Wu, X.-F.; Anbarasan, P.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2010, 49, 7316–7319. doi:10.1002/anie.201003895
Return to citation in text: [1] [2] -
Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 10236–10254. doi:10.1002/anie.201203269
Return to citation in text: [1] [2] -
Yamaguchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. doi:10.1002/anie.201201666
Return to citation in text: [1] [2] -
Wencel-Delord, J.; Glorius, F. Nat. Chem. 2013, 5, 369–375. doi:10.1038/nchem.1607
Return to citation in text: [1] [2] -
Kuzhushkov, S. I.; Potukuchi, H. K.; Ackermann, L. Catal. Sci. Technol. 2013, 3, 562–571. doi:10.1039/c2cy20505j
Return to citation in text: [1] [2] -
Yuan, K.; Doucet, H. ChemCatChem 2013, 5, 3495–3496. doi:10.1002/cctc.201300533
Return to citation in text: [1] [2] -
Rossi, R.; Bellina, F.; Lessi, M.; Manzini, C. Adv. Synth. Catal. 2014, 356, 17–117. doi:10.1002/adsc.201300922
Return to citation in text: [1] [2] -
He, M.; Soulé, J.-F.; Doucet, H. ChemCatChem 2014, 6, 1824–1859. doi:10.1002/cctc.201402020
Return to citation in text: [1] [2] -
Beydoun, K.; Zaarour, M.; Williams, J. A. G.; Doucet, H.; Guerchais, V. Chem. Commun. 2012, 48, 1260–1262. doi:10.1039/c2cc16327f
Return to citation in text: [1] -
Zhao, L.; Bruneau, C.; Doucet, H. Chem. Commun. 2013, 49, 5598–5600. doi:10.1039/c3cc42226g
Return to citation in text: [1] -
Xu, Y.; Zhao, L.; Li, Y.; Doucet, H. Adv. Synth. Catal. 2013, 355, 1423–1432. doi:10.1002/adsc.201300123
Return to citation in text: [1] -
Yuan, K.; Doucet, H. Chem. Sci. 2014, 5, 392–396. doi:10.1039/c3sc52420e
Return to citation in text: [1] -
Borghese, A.; Geldhof, G.; Antoine, L. Tetrahedron Lett. 2006, 47, 9249–9252. doi:10.1016/j.tetlet.2006.10.130
Return to citation in text: [1] -
Shibahara, F.; Yamaguchi, E.; Murai, T. Chem. Commun. 2010, 46, 2471–2473. doi:10.1039/b920794e
Return to citation in text: [1] -
Kowada, T.; Ohe, K. Bull. Korean Chem. Soc. 2010, 31, 577–581. doi:10.5012/bkcs.2010.31.03.577
Return to citation in text: [1] -
Murata, T.; Murai, M.; Ikeda, Y.; Miki, K.; Ohe, K. Org. Lett. 2012, 14, 2296–2299. doi:10.1021/ol300718x
Return to citation in text: [1] -
Koszarna, B.; Matczak, R.; Krzeszewski, M.; Vakuliuk, O.; Klajn, J.; Tasior, M.; Nowicki, J. T.; Gryko, D. T. Tetrahedron 2014, 70, 225–231. doi:10.1016/j.tet.2013.11.088
Return to citation in text: [1] -
Fu, H. Y.; Chen, L.; Doucet, H. J. Org. Chem. 2012, 77, 4473–4478. doi:10.1021/jo300528b
Return to citation in text: [1] [2] -
Zhao, L.; Bruneau, C.; Doucet, H. ChemCatChem 2013, 5, 255–262. doi:10.1002/cctc.201200521
Return to citation in text: [1] [2] -
Bensaid, S.; Doucet, H. ChemSusChem 2012, 5, 1559–1567. doi:10.1002/cssc.201100771
Return to citation in text: [1] [2] -
Cai, T.; Zhou, Y.; Wang, E.; Hellström, S.; Zhang, F.; Xu, S.; Inganäs, O.; Andersson, M. R. Sol. Energy Mater. Sol. Cells 2010, 94, 1275–1281. doi:10.1016/j.solmat.2010.03.024
Return to citation in text: [1]
| 1. | Liu, Y.; Ye, M.; Guo, H.-Z.; Zhao, Y.-Y.; Guo, D.-A. J. Asian Nat. Prod. Res. 2002, 4, 175–178. doi:10.1080/1028602021000000071 |
| 19. | Alberico, D.; Scott, M. E.; Lautens, M. Chem. Rev. 2007, 107, 174–238. doi:10.1021/cr0509760 |
| 20. | Satoh, T.; Miura, M. Chem. Lett. 2007, 36, 200–205. doi:10.1246/cl.2007.200 |
| 21. | Li, B.-J.; Yang, S.-D.; Shi, Z.-J. Synlett 2008, 949–957. doi:10.1055/s-2008-1042907 |
| 22. | Bellina, F.; Rossi, R. Tetrahedron 2009, 65, 10269–10310. doi:10.1016/j.tet.2009.10.015 |
| 23. | Ackermann, L.; Vincente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996 |
| 24. | Roger, J.; Gottumukkala, A. L.; Doucet, H. ChemCatChem 2010, 2, 20–40. doi:10.1002/cctc.200900074 |
| 25. | Wu, X.-F.; Anbarasan, P.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2010, 49, 7316–7319. doi:10.1002/anie.201003895 |
| 26. | Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 10236–10254. doi:10.1002/anie.201203269 |
| 27. | Yamaguchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. doi:10.1002/anie.201201666 |
| 28. | Wencel-Delord, J.; Glorius, F. Nat. Chem. 2013, 5, 369–375. doi:10.1038/nchem.1607 |
| 29. | Kuzhushkov, S. I.; Potukuchi, H. K.; Ackermann, L. Catal. Sci. Technol. 2013, 3, 562–571. doi:10.1039/c2cy20505j |
| 30. | Yuan, K.; Doucet, H. ChemCatChem 2013, 5, 3495–3496. doi:10.1002/cctc.201300533 |
| 31. | Rossi, R.; Bellina, F.; Lessi, M.; Manzini, C. Adv. Synth. Catal. 2014, 356, 17–117. doi:10.1002/adsc.201300922 |
| 32. | He, M.; Soulé, J.-F.; Doucet, H. ChemCatChem 2014, 6, 1824–1859. doi:10.1002/cctc.201402020 |
| 2. | Gohier, F.; Frère, P.; Roncali, J. J. Org. Chem. 2013, 78, 1497–1503. doi:10.1021/jo302571u |
| 17. | Ohta, A.; Akita, Y.; Ohkuwa, T.; Chiba, M.; Fukunaga, R.; Miyafuji, A.; Nakata, T.; Tani, N.; Aoyagi, Y. Heterocycles 1990, 31, 1951–1958. doi:10.3987/COM-90-5467 |
| 18. | Aoyagi, Y.; Inoue, A.; Koizumi, I.; Hashimoto, R.; Tokunaga, K.; Gohma, K.; Komatsu, J.; Sekine, K.; Miyafuji, A.; Kunoh, J.; Honma, R.; Akita, Y.; Ohta, A. Heterocycles 1992, 33, 257–272. doi:10.3987/COM-91-S29 |
| 45. | Cai, T.; Zhou, Y.; Wang, E.; Hellström, S.; Zhang, F.; Xu, S.; Inganäs, O.; Andersson, M. R. Sol. Energy Mater. Sol. Cells 2010, 94, 1275–1281. doi:10.1016/j.solmat.2010.03.024 |
| 3. | Li, J. J.; Gribble, G. W. Palladium in Heterocyclic Chemistry; Pergamon: Amsterdam, 2000. |
| 4. | Negishi, E., Ed. Handbook of Organopalladium Chemistry for Organic Synthesis; Part III; Wiley-Interscience: New York, 2002; p 213. |
| 5. | Schnürch, M.; Flasik, R.; Khan, A. F.; Spina, M.; Mihovilovic, M. D.; Stanetty, P. Eur. J. Org. Chem. 2006, 3283–3307. doi:10.1002/ejoc.200600089 |
| 6. | Tung, D. T.; Tuân, D. T.; Rasool, N.; Villinger, A.; Reinke, H.; Fischer, C.; Langer, P. Adv. Synth. Catal. 2009, 351, 1595–1609. doi:10.1002/adsc.200900044 |
| 7. | Pernites, R. B.; Ponnapati, R. R.; Advincula, R. C. Adv. Mater. 2011, 23, 3207–3213. doi:10.1002/adma.201100469 |
| 8. | Zhou, J.; Xie, S.; Amond, E. F.; Becker, M. L. Macromolecules 2013, 46, 3391–3394. doi:10.1021/ma400531v |
| 9. | Qian, D.; Ma, W.; Li, Z.; Guo, X.; Zhang, S.; Ye, L.; Ade, H.; Tan, Z.; Hou, J. J. Am. Chem. Soc. 2013, 135, 8464–8467. doi:10.1021/ja402971d |
| 10. | Tanaka, S.; Tanaka, D.; Tatsuta, G.; Murakami, K.; Tamba, S.; Sugie, A.; Mori, A. Chem. – Eur. J. 2013, 19, 1658–1665. doi:10.1002/chem.201203331 |
| 11. | Nagura, K.; Saito, S.; Yusa, H.; Yamawaki, H.; Fujihisa, H.; Sato, H.; Shimoikeda, Y.; Yamaguchi, S. J. Am. Chem. Soc. 2013, 135, 10322–10325. doi:10.1021/ja4055228 |
| 12. | Klingstedt, T.; Shirani, H.; Åslund, K. O. A.; Cairns, N. J.; Sigurdson, C. J.; Goedert, M.; Nilsson, K. P. R. Chem. – Eur. J. 2013, 19, 10179–10192. doi:10.1002/chem.201301463 |
| 13. | Mallet, C.; Didane, Y.; Watanabe, T.; Yoshimoto, N.; Allain, M.; Videlot-Ackermann, C.; Frère, P. ChemPlusChem 2013, 78, 459–466. doi:10.1002/cplu.201300037 |
| 14. | Bruns, C. J.; Herman, D. J.; Minuzzo, J. B.; Lehrman, J. A.; Stupp, S. I. Chem. Mater. 2013, 25, 4330–4339. doi:10.1021/cm402505p |
| 15. | Salamoun, J.; Anderson, S.; Burnett, J. C.; Gussio, R.; Wipf, P. Org. Lett. 2014, 16, 2034–2037. doi:10.1021/ol500620m |
| 16. |
Ferrer Flegeau, E.; Popkin, M. E.; Greaney, M. F. J. Org. Chem. 2008, 73, 3303–3306. doi:10.1021/jo800121y
See for access to trisoxazoles. |
| 42. | Fu, H. Y.; Chen, L.; Doucet, H. J. Org. Chem. 2012, 77, 4473–4478. doi:10.1021/jo300528b |
| 43. | Zhao, L.; Bruneau, C.; Doucet, H. ChemCatChem 2013, 5, 255–262. doi:10.1002/cctc.201200521 |
| 44. | Bensaid, S.; Doucet, H. ChemSusChem 2012, 5, 1559–1567. doi:10.1002/cssc.201100771 |
| 2. | Gohier, F.; Frère, P.; Roncali, J. J. Org. Chem. 2013, 78, 1497–1503. doi:10.1021/jo302571u |
| 44. | Bensaid, S.; Doucet, H. ChemSusChem 2012, 5, 1559–1567. doi:10.1002/cssc.201100771 |
| 39. | Kowada, T.; Ohe, K. Bull. Korean Chem. Soc. 2010, 31, 577–581. doi:10.5012/bkcs.2010.31.03.577 |
| 41. | Koszarna, B.; Matczak, R.; Krzeszewski, M.; Vakuliuk, O.; Klajn, J.; Tasior, M.; Nowicki, J. T.; Gryko, D. T. Tetrahedron 2014, 70, 225–231. doi:10.1016/j.tet.2013.11.088 |
| 38. | Shibahara, F.; Yamaguchi, E.; Murai, T. Chem. Commun. 2010, 46, 2471–2473. doi:10.1039/b920794e |
| 42. | Fu, H. Y.; Chen, L.; Doucet, H. J. Org. Chem. 2012, 77, 4473–4478. doi:10.1021/jo300528b |
| 43. | Zhao, L.; Bruneau, C.; Doucet, H. ChemCatChem 2013, 5, 255–262. doi:10.1002/cctc.201200521 |
| 37. | Borghese, A.; Geldhof, G.; Antoine, L. Tetrahedron Lett. 2006, 47, 9249–9252. doi:10.1016/j.tetlet.2006.10.130 |
| 19. | Alberico, D.; Scott, M. E.; Lautens, M. Chem. Rev. 2007, 107, 174–238. doi:10.1021/cr0509760 |
| 20. | Satoh, T.; Miura, M. Chem. Lett. 2007, 36, 200–205. doi:10.1246/cl.2007.200 |
| 21. | Li, B.-J.; Yang, S.-D.; Shi, Z.-J. Synlett 2008, 949–957. doi:10.1055/s-2008-1042907 |
| 22. | Bellina, F.; Rossi, R. Tetrahedron 2009, 65, 10269–10310. doi:10.1016/j.tet.2009.10.015 |
| 23. | Ackermann, L.; Vincente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996 |
| 24. | Roger, J.; Gottumukkala, A. L.; Doucet, H. ChemCatChem 2010, 2, 20–40. doi:10.1002/cctc.200900074 |
| 25. | Wu, X.-F.; Anbarasan, P.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2010, 49, 7316–7319. doi:10.1002/anie.201003895 |
| 26. | Kuhl, N.; Hopkinson, M. N.; Wencel-Delord, J.; Glorius, F. Angew. Chem., Int. Ed. 2012, 51, 10236–10254. doi:10.1002/anie.201203269 |
| 27. | Yamaguchi, J.; Yamaguchi, A. D.; Itami, K. Angew. Chem., Int. Ed. 2012, 51, 8960–9009. doi:10.1002/anie.201201666 |
| 28. | Wencel-Delord, J.; Glorius, F. Nat. Chem. 2013, 5, 369–375. doi:10.1038/nchem.1607 |
| 29. | Kuzhushkov, S. I.; Potukuchi, H. K.; Ackermann, L. Catal. Sci. Technol. 2013, 3, 562–571. doi:10.1039/c2cy20505j |
| 30. | Yuan, K.; Doucet, H. ChemCatChem 2013, 5, 3495–3496. doi:10.1002/cctc.201300533 |
| 31. | Rossi, R.; Bellina, F.; Lessi, M.; Manzini, C. Adv. Synth. Catal. 2014, 356, 17–117. doi:10.1002/adsc.201300922 |
| 32. | He, M.; Soulé, J.-F.; Doucet, H. ChemCatChem 2014, 6, 1824–1859. doi:10.1002/cctc.201402020 |
| 33. | Beydoun, K.; Zaarour, M.; Williams, J. A. G.; Doucet, H.; Guerchais, V. Chem. Commun. 2012, 48, 1260–1262. doi:10.1039/c2cc16327f |
| 34. | Zhao, L.; Bruneau, C.; Doucet, H. Chem. Commun. 2013, 49, 5598–5600. doi:10.1039/c3cc42226g |
| 35. | Xu, Y.; Zhao, L.; Li, Y.; Doucet, H. Adv. Synth. Catal. 2013, 355, 1423–1432. doi:10.1002/adsc.201300123 |
| 36. | Yuan, K.; Doucet, H. Chem. Sci. 2014, 5, 392–396. doi:10.1039/c3sc52420e |
| 40. | Murata, T.; Murai, M.; Ikeda, Y.; Miki, K.; Ohe, K. Org. Lett. 2012, 14, 2296–2299. doi:10.1021/ol300718x |
© 2014 Belkessam et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)