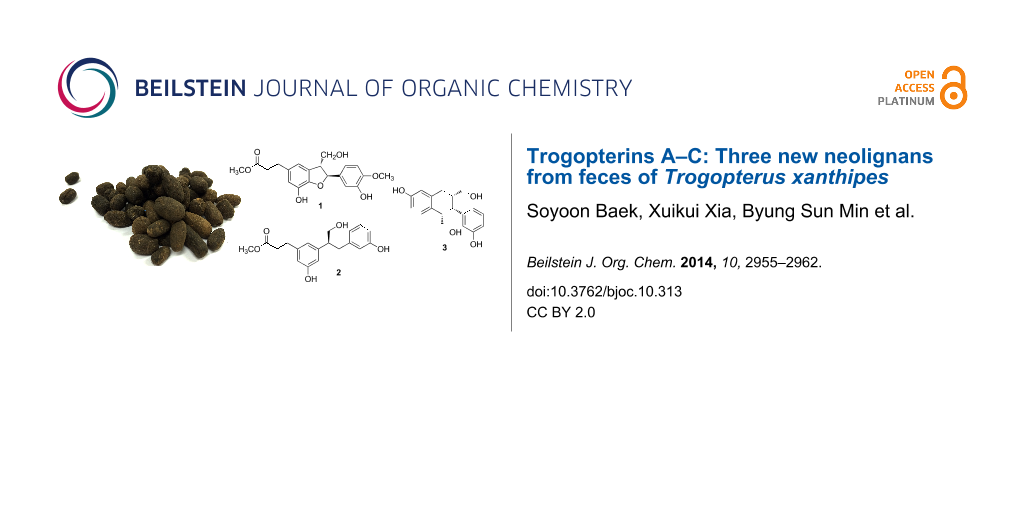
Abstract
Seven compounds, including three neolignans 1–3, a norlignan 4, and three diterpenoids 5–7, were isolated from the feces of Trogopterus xanthipes. Structures of these compounds were identified by 1D and 2D NMR as well as MS. The absolute configurations of compounds 1, 2, and 4 were determined by comparing CD spectra and optical rotations. Among the isolated compounds, 1–3 were novel and subsequently named trogopterins A, B, and C, respectively. Likewise, compound 4 was isolated from nature for the first time. Cytotoxic activities of compounds 1–4 were evaluated. Compounds 1–3 exhibited moderate cytotoxic activities against HL-60 cells with IC50 values of 34.77–45.68 μM.
Graphical Abstract
Introduction
Chemical studies of natural products including ones derived from plants and microorganisms have led to the isolation of numerous novel metabolites with biological activities [1,2]. As a continuation of these investigations, feces from Trogopterus were selected since organic extracts from this material were found to exhibit potent cytotoxic activities in our preliminary study. Trogopterus feces, called “Wulingzhi”, are dried excrements of Trogopterus (T.) xanthipes Milne-Edwars (Petauristidae) that are known as complex-toothed flying squirrels to eat branches, leaves, and fruits of pine trees [3]. Trogopterus feces have been reported to promote blood circulation and resolve stasis. Thus, this material has been used as traditional medicine for treating amenorrhea, dysmenorrheal, menstrual pain, and retained lochia due to stasis [4].
Recent studies have indicated that Trogopterus feces mainly consist of terpenoids [5-9], phenolic acids, sterols, aliphatics, fatty acids, and flavonoids [10,11]. They have been reported to possess various pharmacological properties such as controlling of antithrombin levels, inhibition of platelet aggregation, cytotoxic activity, immunity enhancement, and anti-inflammatory activities. Isolation of compounds from the methanol extract of Trogopterus feces was presented before by our group [12]. In the present investigation, chemical evaluation of fecal extracts led to the isolation of seven compounds including three new neolignans named trogopterins A–C (1–3). Here we describe the isolation, structure, and cytotoxic activities of compounds 1–3.
Results and Discussion
Seven compounds, including three neolignans (1–3), a phenolic compound 4, and three diterpenoids (5–7), were isolated from the methanolic extract of T. xanthipes feces. Their chemical names are methyl 3-((2S,3R)-7-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-3-(hydroxymethyl)-2,3-dihydrobenzofuran-5-yl)propanoate (1), (S)-methyl 3-(3-hydroxy-5-(1-hydroxy-3-(3-hydroxyphenyl)propan-2-yl)phenyl)propanoate (2), ((1RS,2RS,3RS)-6-hydroxy-2-(3-hydroxyphenyl)-1,2,3,4-tetrahydronaphthalene-1,3-diyl)dimethanol (3), ((R)-3,3'-(3-hydroxypropane-1,2-diyl)diphenol (4) [13], 8β-hydroxy-3-oxopimara-15-ene (5) [14], 9β-hydroxy-9(11),13-abietadien-12-one (6) [15], and ent-pimar-15-en-9α,19-diol (7) [16] based on spectral data (Figure 1). Compounds 1–3 were identified to be new and compound 4 was isolated from nature for the first time. In addition, compounds 6 and 7 were reported from this species in this study for the first time.
Figure 1: Structures of compounds 1–7 isolated from feces of Trogopterus.
Figure 1: Structures of compounds 1–7 isolated from feces of Trogopterus.
Compound 1 was isolated as colorless oil. The molecular formula of compound 1 was determined to be C20H22O7 (ten unsaturations) based on 1H, 13C, and HMQC data, and verified by HREIMS (Table 1 and Table 2). Nineteen protons were bound to carbons, so three exchangeable hydrogens were present. Detailed analysis of the 1H, 13C, and HMQC spectra of compound 1 revealed the presence of two methoxy groups at δH 3.63 (δC 52.0) and δH 3.80 (δC 56.4), an oxygenated methine group at δH 5.47 (δC 88.8), an oxygenated methylene group at δH 3.80 and 3.73 (δC 65.1), an sp3 methine group at δH 3.43 (δC 55.7), two sp3 methylene groups at δH 2.78 (δC 31.7) and δH 2.56 (δC 37.1); and five sp2 methine groups at δH 6.95 (δC 110.5), δH 6.82 (δC 119.7), δH 6.74 (δC 116.1), δH 6.59 (δC 116.5), and δH 6.55 (δC 116.9). In addition, seven sp2 non-hydrogenated carbon signals appeared at δC 149.1, 147.4, 146.9, 142.0, 135.2, 135.0, and 130.0 along with an ester at 175.3. Based on 1H and 13C NMR findings (Table 1 and Table 2), compound 1 was hypothesized to have a lignan skeleton formed through oxidative coupling of two phenylpropanoids units.
Table 1: 1H NMR data for compounds 1–4 (δ, ppm, and coupling constant J in Hz).
| Position | 1a | 2b | 3a | 4a |
|---|---|---|---|---|
| 1 | ||||
| 2 | 6.59 (s) | 6.88 (br s) | 6.61 (t, 3.0) | |
| 3 | 6.51 (d, 8.4) | |||
| 4 | 7.19 (br s) | 6.43 (dd, 8.4, 2.4) | 6.58 (ddd, 7.8, 3.0, 0.6) | |
| 5 | 7.05 (t, 7.8) | |||
| 6 | 6.55 (s) | 6.98 (br s) | 6.54 (br s) | 6.63 (d, 7.8) |
| 7 | 2.78 (t, 7.8) | 2.93 (t, 8.4) | 2.76 (d, 7.8) | 2.92 (m) |
| 8a | 2.56 (t, 7.8) | 2.64 (t, 8.4) | 1.98 (m) | 3.68 (dd, 6.6, 1.2) |
| 8b | 3.64 (m) | |||
| 9a | 3.66 (dd, 11, 3.6) | 2.71 (dd, 13, 8.4) | ||
| 9b | 3.61 (dd, 11, 6.0) | 3.00 (dd, 13,6.6) | ||
| 9-OCH3 | 3.63 (s) | 3.57 (s) | ||
| 1' | ||||
| 2' | 6.95 (s) | 7.20 (d, 1.8) | 6.55 (br s) | 6.51 (s) |
| 3' | ||||
| 4' | 7.01 (dd, 7.8, 1.8) | 6.62 (dd, 7.8, 2.4) | 6.52 (d, 7.2) | |
| 5' | 6.74 (d, 8.4) | 7.20 (t, 7.8) | 7.09 (t, 7.8) | 6.97 (t, 7.2) |
| 6' | 6.82 (br d, 8.4) | 6.90 (d, 7.2) | 6.64 (br d, 7.8) | 6.53 (d, 7.2) |
| 7'a | 5.47 (d, 6.0) | 3.47 (dd, 13, 6.0) | 3.84 (d, 10) | |
| 7'b | 3.12 (dd, 13, 8.4) | |||
| 8' | 3.43 (m) | 3.40 (m) | 1.79 (m) | |
| 9'a | 3.80 (m) | 4.14 (m) | 3.39 (dd, 10, 4.2) | |
| 9'b | 3.73 (dd, 11, 7.2) | 3.64 (dd, 10, 3.6) | ||
| 4'-OCH3 | 3.80 (s) | |||
aRecorded in CD3OD at 600 MHz. bRecorded in pyridine-d5 at 600 MHz.
Table 2: 13C–NMR data for compounds 1–4.
| Position | 1a | 2b | 3a | 4a |
|---|---|---|---|---|
| 1 | 135.2 | 142.8 | 139.1 | 145.0 |
| 2 | 116.5 | 120.2 | 132.4 | 116.1 |
| 3 | 130.0 | 146.4 | 131.7 | 158.3 |
| 4 | 146.9 | 114.8 | 114.2 | 114.3 |
| 5 | 142.0 | 159.4 | 156.2 | 130.2 |
| 6 | 116.9 | 114.6 | 115.3 | 120.6 |
| 7 | 31.7 | 31.7 | 34.1 | 51.8 |
| 8 | 37.1 | 36.3 | 39.9 | 67.1 |
| 9 | 175.3 | 173.7 | 66.0 | 39.9 |
| 9-OCH3 | 52.0 | 51.7 | ||
| 1' | 135.0 | 143.7 | 149.0 | 143.3 |
| 2' | 110.5 | 117.7 | 117.2 | 117.0 |
| 3' | 147.4 | 159.2 | 158.5 | 158.1 |
| 4' | 149.1 | 114.1 | 114.1 | 113.7 |
| 5' | 116.1 | 130.0 | 130.2 | 130.0 |
| 6' | 119.7 | 120.9 | 121.9 | 121.5 |
| 7' | 88.8 | 39.5 | 48.0 | |
| 8' | 55.7 | 51.7 | 48.1 | |
| 9' | 65.1 | 66.7 | 62.5 | |
| 4'-OCH3 | 56.4 | |||
aRecorded in CD3OD at 150 MHz. bRecorded in pyridine-d5 at 150 MHz.
This compound was also presumed to have three rings in its structure based on the unsaturation degree since it contained two aromatic moieties and an ester group. 1H,1H COSY spectrum of compound 1 revealed the presence of two isolated proton spin systems of CH–CH–CH2 corresponding to C7′–C8′–C9′ and CH2–CH2 corresponding to C7–C8. In addition, coupling constants as well as the 1H,1H COSY data indicated the presence of a 1,3,4-trisubstituted benzene moiety at δ 6.82 (br d, J = 8.4 Hz, H-6′), 6.74 (d, J = 8.4 Hz, H-5′), and 6.95 (br s, H-2′). The extension of the spin systems and attachments of functional groups were confirmed by HMBC correlations (Figure 2).
Figure 2: Key HMBC correlations for compounds 1–3.
Figure 2: Key HMBC correlations for compounds 1–3.
HMBC correlations of a methoxy proton at δH 3.63 and H2-7 at δH 2.78 (t, J = 7.8 Hz) with the ester carbonyl carbon at δC 175.3 indicated the presence of a methyl propanoate. HMBC correlations of H-2–H-7 with C-1 at δC 135.2, C-2 at δC 116.5, and C-6 at δC 116.9 demonstrated that the methyl propanoate was attached to the C-1 position of one phenylpropanoid moiety in the lignan skeleton. Together with HMBC correlations, two singlet aromatic protons for H-2 and H-6 indicated that the first phenylpropanoid moiety had a 1,3,4,5-tetrasubstituted benzene ring, which was attached to the C-1 position of the methyl propanoate. H-7' at δH 5.47 (d, J = 6.0 Hz) of the CH–CH–CH2 spin system had HMBC correlations with C-1', C-2', and C-6' of the 1,3,4-trisubstituted benzene ring, indicating that the CH–CH–CH2 spin system was linked to C-1' of the 1,3,4-trisubstituted benzene ring. In addition, HMBC correlations of H-8' with C-3 and C-4 suggested that C-8' was linked to the C-3 position of the first phenylpropanoid moiety. HMBC correlations of H-7' with C-4 allowed linkage of the oxygenated sp3 methine carbon C-7' to the oxygenated sp2 carbon C-4 through oxygen to form a dihydrofuran ring. The presence of a dihydrofuran moiety in compound 1 was also demonstrated by chemical shifts of C-4 (δ 146.9) and C-7' (δ 88.8) together with the unsaturation requirement. Thus, compound 1 was proved to be a neolignan containing a dihydrobenzofuran skeleton. HMBC correlations of the methoxy proton at δH 3.80 with C-4' (δ 149.1) showed that the methoxy group was attached to C-4' of the 1,3,4-trisubstituted benzene ring, and a hydroxy group was presumed to be attached to C-3' based on the chemical shifts. Ultimately, the chemical structure of compound 1 was identified as shown in Figure 1 and named trogopterin A. Detailed 1H and 13C NMR data are presented in Table 1 and Table 2.
The relative stereochemistry of compound 1 was established by interpreting the NOESY data. A strong NOESY correlation between H-7' and H-9' indicated that the 1,3,4-trisubstututed benzene ring and hydroxymethylene group were on opposite faces of the molecule. The absolute configurations of C-7' and C-8' in compound 1 were established by comparing the circular dichroism (CD) data to those of previously reported neoligans containing a dihydrobenzofuran skeleton [17]. The CD of compound 1 showed positive cotton effects at 255 and 327 nm along with a negative cotton effect at 234 nm. These features were very similar to those of neolignans [17], indicating that C-7' and C-8' in compound 1 have S and R configurations, respectively. Thus, compound 1 was determined to be methyl 3-((2S,3R)-7-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-3-(hydroxymethyl)-2,3-dihydrobenzofuran-5-yl)propanoate.
Compound 2 was isolated as a light brown oil. The molecular formula of compound 2 was determined to be C19H22O5 based on HREIMS, which gave a molecular ion peak at m/z 330.1462 (calcd 330.1467 for C19H22O5) indicating the presence of nine unsaturations in the structure. The 1H NMR spectrum of compound 2 (Table 1) was similar to that of compound 1 and contained one methoxy group (δ 3.57), a coupled sp3 CH2–CH2 (δ 2.93 and 2.64), and seven sp2 methine protons (δ 7.21–6.88). 13C NMR data for compound 2 demonstrated the presence of 12 sp2 carbons and five sp3 carbons together with an ester carbon, which suggested that compound 2 has a lignan skeleton similar to compound 1. Based on the unsaturation degrees, compound 2 does not contain a ring except for two aromatic moieties. The 1H,1H COSY spectrum of compound 2 revealed the presence of two isolated proton spin systems of CH2–CH–CH2 corresponding to C7′–C8′–C9′ and CH2–CH2 corresponding to C7–C8. HMBC correlations of a methoxy proton at δH 3.57 with the ester carbon at δC 173.7 as well as H2-8 at δH 2.64 with C-1 at δC 142.8 indicated that a methyl propanoate group was attached to the C-1 position of one phenylpropanoid moiety of the lignan skeleton (Figure 2). HMBC correlations of the methine proton H′-8 in the CH2–CH–CH2 spin system with C-2 at δC 120.2, C-3 at δC 146.4, C-4 at δC 114.8, and C-1′ at δC 143.7 suggested that C-8′ of the second phenylpropanoid was attached to the C-3 position of the first phenylpropanoid moiety. In addition, HMBC correlation of H-7′ with C-2′ together with the coupling constant of H-2′ (1.8 Hz) supported that the second phenylpropanoid moiety has a 1,3-disubstituted benzene ring. Based on these findings, compound 2 was identified as methyl (S)-methyl 3-(3-hydroxy-5-(1-hydroxy-3-(3-hydroxyphenyl)propan-2-yl)phenyl)propanoate and named trogopterin B. Compound 2 was different in that it did not contain a methoxy group at the C-4′ position nor an ether linkage between C-4 and C-7′ to form a dihydrofuran ring. The absolute configuration of compound 2 was determined by comparing its optical rotation with those of (R)-2,3-diphenyl-1-propanol and (S)-2,3-diphenyl-1-propanol [18,19]. Compound 2 had a positive optical rotation value of [a]D25 +20.2, which was similar to that of (S)-2,3-diphenyl-1-propanol (+107°). Thus, C-8′ was determined to have S configuration.
Compound 3 was obtained as colorless oil. Its molecular formula was found to be C18H20O3 based on HREIMS (m/z 300.1360), requiring nine degrees of unsaturation. The 1H NMR spectrum (Table 2) indicated the presence of seven aromatic protons at δ 7.09–6.43, two oxygenated methylene protons at δ 3.66, 3.64, 3.61, and 3.39, and three sp3 methine protons at δ 3.84, 1.98, and 1.79. The 13C NMR spectrum of compound 1 indicated the presence of 12 sp2 carbons and six sp3 carbons, suggesting that compound 3 might also have a lignan skeleton similar to compounds 1 and 2. In order to meet the requirements of unsaturation, this compound was presumed to have one more ring in addition to two aromatic moieties. The 1H,1H COSY spectrum of compound 3 revealed the presence of two isolated proton spin systems of CH2–CH–CH2 corresponding to C7–C8–C9 and CH–CH–CH2 corresponding to C7′–C8′–C9′. In addition, coupling constants as well as the 1H,1H COSY data [δ 6.54 (br s, H-6), 6.43 (dd, J = 8.4, 2.4 Hz, H-4), and 6.51 (d, J = 8.4 Hz, H-3)] indicated the presence of a 1,3,4-trisubstituted benzene ring. Extension of the spin systems was confirmed by HMBC correlations (Figure 2). HMBC correlations of the sp3 methylene protons H2-7 (δ 2.76) with C-1 (δ 139.1), C-2 (δ 132.4), C-6 (δ 115.3), and C-7′ (δ 48.0) indicated that the first spin system was connected to C-1 (δ 139.1) of the 1,3,4-trisubstituted benzene ring while C-8 was connected to C-7′ of the second spin system. HMBC correlations of H-7′ with C-6 (δ 115.3) together with the unsaturation requirement allowed the connection of C-8′ with C-2 to form a cyclohexene moiety. Furthermore, splitting patterns of the protons in the other aromatic moiety [6.55 (br s), 6.62 (dd, 7.8, 2.4), 7.09 (t, 7.8), and 6.64 (d, 7.8)] and downfield-shifted chemical shift of C-3′ (δ 158.5) indicated that the 3-hydroxy-1,3-disubstituted benzene ring was attached to the C-7′ carbon. In this way, the planar structure of compound 3 was elucidated as shown in Figure 1.
The relative stereochemistry of compound 3 was deduced based on NOESY data and comparison with interproton distances calculated by MM2 with ChemDraw. A strong NOESY correlation between H-7′ and H-8′ indicated that the phenyl group at C-7′ and the hydroxymethylene group at C-8′ are on the same face of the molecule (Figure 3). Additionally, a strong NOESY correlation between H-8 and H-8′ indicated that the hydroxymethylene group at C-8 and the hydroxymethylene group at C-8′ are on the same face of the molecule (Figure 3). A structure model of this compound was created using MM2 with ChemDraw 3.0 (Figure 3).
![[1860-5397-10-313-3]](/bjoc/content/figures/1860-5397-10-313-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Key NOESY correlations (1H↔1H) for trogopterin C (compound 3) identified in the ChemDraw 3D MM2-minimized model.
Figure 3: Key NOESY correlations (1H↔1H) for trogopterin C (compound 3) identified in the ChemDraw 3D MM2-min...
The resulting calculated interproton distances were in close agreement with the NOESY data (Table 3).
A careful modeling study considering all the possible conformers of compound 3 exhibited that interproton distances between H-7, H-8, and H-8′ are within 3 Å when two hydroxymethylene groups and a hydroxyphenyl group in compound 3 are on the same face of the molecule. Thus, the structure of compound 3 was determined to be ((1RS,2RS,3RS)-6-hydroxy-2-(3-hydroxyphenyl)-1,2,3,4-tetrahydronaphthalene-1,3-diyl)dimethanol and named trogopterin C.
Compound 4 was isolated as light brown oil. The 1H and 13C NMR spectra contained signals similar to those of compound 2 except for those corresponding to the methyl propanoate moiety in compound 2. Based on the 1H, 13C, HMQC, and HMBC data, compound 4 had an aromatic proton instead of the methyl propanoate found in compound 2. Thus, compound 4 was determined to be ((R)-3,3'-(3-hydroxypropane-1,2-diyl)diphenol as previously reported [13]. Even though this compound has been described in the literature, the absolute stereochemistry was not elucidated. Similar to compound 2, the optical rotation of compound 4 was compared to those of (R)-2,3-diphenyl-1-propanol and (S)-2,3-diphenyl-1-propanol [18,19]. Compound 4 had a negative optical rotation of [a]D25 −15.4 similar to (R)-2,3-diphenyl-1-propanol [19]. Thus, the structure of 4 was identified as (R)-3,3'-(3-hydroxypropane-1,2-diyl)diphenol.
The in vitro cytotoxic activities of compounds 1–4 against HL-60 (human leukemia), HeLa (human cervical carcinoma), and MCF-7 (human breast cancer) cells were evaluated using an MTT assay. As shown in Table 4, compounds 1–3 exerted moderate cytotoxic effects against HL-60 cells with IC50 values of 34.77–45.68 μM using adriamycin as a positive control (IC50 = 0.18 μM). Additionally, compound 1 showed very weak cytotoxic activity against MCF-7 cells with an IC50 of 94.69 μM. None of the compounds affected the HeLa cells.
Conclusion
In summary, two novel neoligans (trogopterins A and B) and a new phenolic compound (trogopterin C) were isolated from the crude methanol extract of Trogopterus feces for the first time. The absolute configurations of compounds 1, 2, and 4 were determined by comparing CD spectra and optical rotations. The levels of cytotoxicity against three tumor cell lines (HL-60, HeLa, and MCF-7) were evaluated. Compounds 1–3 had moderate cytotoxic effects against HL-60 cells even though the activities were not significant. Thus, Trogopterus feces could be a potential source of lignans with cytotoxic activity.
Experimental
General Methods
Optical rotations were measured with a Jasco P1000 digital polarimeter. UV spectra were recorded by a Hewlett Packard 8453 UV–vis spectrometer. CD spectra were obtained with a Jasco J-715 circular dichroism spectrophotometer. 1D and 2D NMR were performed on a VNS 600 MHz spectrometer operating at 600 MHz for protons and 150 MHz for carbon. Chemical shifts are expressed in ppm and referenced relative to the residual solvent signals. Mass spectra were recorded with a Micromass LCT mass spectrometer, and the lock mass calibration was applied to accurately measure masses. Semi-preparative HPLC was performed with an Agilent system consisting of a vacuum degasser, quaternary pump, diode array detector (DAD), and Luna 5u C18 (2) 100 Å column (250 × 10.00 mm; Phenomenex). Column chromatography (CC) was carried out on either silica gel (Merck KGaA, 70–230 mesh) or SephadexTM LH-20 (GE Healthcare Sweden). TLC was performed with precoated silica gel 60 F254 plates (Merck KGaA) and spots were visualized under UV light (254 and 365 nm) or by spraying with 20% H2SO4 and heating.
Material
Dried T. xanthipes feces were provided by the oriental hospital of Dongguk University (Seoul, South Korea). A voucher specimen (No. YU-BT-2013-03) was deposited in the Natural Products Chemistry Laboratory of the School of Biotechnology, Yeungnam University (Gyeongsan, South Korea).
Extraction and isolation
The air-dried Trogopterus feces (1 kg) were subjected to extraction three times (3 h per cycle) with refluxing methanol. The solvent was evaporated under reduced pressure to recover the methanolic extracts (98 g) that were partitioned successively between H2O and CHCl3 and EtOAc. The EtOAc extract (6.5 g) was subjected to silica gel CC (5 × 60 cm column) using a gradient of methylene chloride (MC) and acetonitrile (ACN) as eluents to acquire 27 fractions (Fr. 1–27). Fr. 16 (74 mg) was further separated with SephadexTM LH-20 and methanol to recover eight subfractions (SFr. 16-1–16-8). SFr. 16-4 was further subjected to semi-preparative reverse-phase HPLC (Luna 5u Phenomenex column; 250 × 10.00 mm; flow rate, 2 mL/min; 20–40% ACN in H2O for 20 min, 40–50% for 15 min, 50–100% for 10 min; UV detection at 254 nm) to collect compound 1 (3.5 mg, tR = 24.7 min). Fr. 17 (27.2 mg) underwent semi-preparative reversed-phase HPLC using the same method as that performed to recover compound 1; this yielded compounds 2 (15.0 mg, tR = 22.1 min) and 4 (1.7 mg, tR = 19.3 min). Fr. 23 (184.7 mg) was also separated by silica gel CC to collect 15 subfractions (SFr. 23-1–23-15). Compound 3 (3.2 mg, tR = 15.1 min) was obtained from SFr. 23-10 (12 mg) by semi-preparative reversed-phase HPLC (Luna 5u Phenomenex column; 250 × 10.00 mm; flow rate, 2 mL/min; 20–50% ACN in H2O for 20 min, 50–65% for 20 min, 65–100% for 10 min; UV detection at 254 nm). Fr. 3 (58 mg) was subjected to semi-preparative reversed-phase HPLC (Luna 5u C18 column; 250 × 10.00 mm; flow rate, 2 mL/min; 15% ACN in H2O for 20 min, 15–65% for 15 min, 65–100% for 5 min; UV detection at 254 nm) to afford compounds 5 (4.5 mg, tR = 7.5 min) and 6 (2.3 mg, tR = 12.3 min). The CHCl3-soluble layer (4.5 g) was subjected to silica gel CC (5 × 60 cm column) using a gradient of n-hexane and ethyl acetate (EtOAc) as eluents to recover 12 fractions (HF. 1–12). Fraction 3 (86 mg) was further separated by silica gel CC (5 × 60 cm column) using a gradient of n-hexane, EtOAc, and methanol to collect compound 7. In addition, HF. 7 was further subjected to semi-preparative reverse-phase HPLC (Luna 5u Phenomenex column; 250 × 10.00 mm; flow rate, 2 mL/min; 40–80% ACN in H2O for 35 min, 80–100% for 10 min; UV detection at 220 nm) to recover compound 7.
Characterization
Trogopterin A (1): light brown oil; [α]D25 +33.62 (c 1.0 × 10−3 g/mL, EtOH); CD (MeOH) 234 (∆ε −2.53), 255 (∆ε +0.92), 327 (∆ε +2.12); 1H and 13C NMR (600 and 150 MHz,CD3OD), see Table 1. HMBC correlations (CD3OD, H-#→C-#) H-2→C-4, C-6, C-7, and C-8'; H-6→C-2, C-4, C-5, and C-7; H-7→C-1, C-2, C-8, and C-9; H-8→C-1, C-7, and C-9; H-2'→C-1', C-3', C-4', C-6', and C-7'; H-5'→C-1', C-3', and C-4'; H-6'→C-2', C-4', and C-7'; H-7'→C-3, C-4, C-1', C-2', C-6', C-8', and C-9'; H-8'→C-3, C-4, C-1', C-7', and C-9'; H-9'a→C-3, C-7', and C-8'; H-9'b→C-3, C-7', and C-8'; 9-OCH3→C-9; 4'-OCH3→C-4'; 1H,1H COSY correlations (CD3OD, H-#↔H-#) H-7↔H-8, H-5'↔H-6', H-7'↔H-8', H-8'↔H-9'a and H-9'b, H-9'a↔H-8' and H-9'b; NOESY correlations (CD3OD, H-#↔H-#) H-2↔H-8' and H-9', H-6'↔H-5', H-7', and H-8'; H-7'↔H-2', H-8', and H-9'; H-8'↔H-2' and H-9'; HREIMS (m/z): calcd for C20H22O7, 374.1365; found, 374.1364.
Trogopterin B (2): light brown oil; [α]D25 +20.2 (c 1.0 × 10−3 g/mL, EtOH); 1H and 13C NMR (600 and 150 MHz, pyridine-d5) spectroscopic analysis, see Table 1. HMBC correlations (pyridine-d5, H-#→C-#) H-2→C-4, C-6, and C-7; H-4→C-2, C-6, and C-8'; H-6→C-2, C-4, and C-7; H-7→C-1, C-2, C-6, and C-9; H-8→C-1 and C-9, H-2'→C-4' and C-6', H-4'→C-2' and C-6', H-5'→C-1', C-3', C-4', and C-6'; H-6'→C-2' and C-4', H-7'a→C-5, C-1', C-2', and C-6'; H-7'b→C-5, C-1', C-2', and C-6'; H-8'→C-2, C-3, C-4, and C-1'; H-9'→C-3, C-7', and C-8'; 9-OCH3→C-9; 1H,1H COSY correlations (pyridine, H-#↔H-#) H-7↔H-8, H-4'↔H-5', H-5'↔H-6', H-7'a↔H-7'b and H-8', H-7'b↔H-8', H-8'↔H-9'; HREIMS (m/z): calcd for C19H22O5, 330.1467; found, 330.1469.
Trogopterin C (3): light brown oil; [α]D25 −14.8 (c 1.0 × 10−3 g/mL, EtOH); 1H and 13C NMR (600 and 150 MHz, CD3OD) spectroscopic analysis, see Table 2. HMBC correlations (CD3OD, H-#→C-#): H-1→C-5 and C-9, H-3→C-1 and C-5, H-4→C-2, C-7, and C-10; H-6→C-12, H-7→C-4, C-5, C-6, C-10, C-12, C-1', C-2', and C-6'; H-8→C-6 and C-7, H-9→C-7, C-8, and C-11; H-11a→C-7, C-8, and C-9; H-11b→C-7, C-8, and C-9; H-12a→C-6, C-7, and C-8; H-12b→C-6, C-7, and C-8; H-2'→C-6, C-3', C-4', and C-6'; H-4'→C-2' and C-6', H-5'→C-1' and C-3', H-6'→C-6, C-2', and C-4'; 1H-1H COSY correlations (CD3OD, H-#↔H-#) H-6↔H-7, H-12a, and H-12b; H-8↔H-9, H-11a, and H-11b; H-4'↔H-5', H-5'↔H-6'; NOESY correlations (CD3OD, H-#↔H-#): H-7↔H-8, H-8', and H-9'a; H-8↔ H-7', H-8', H-9'a, and H-9'b; H-5'↔H-4' and H-6'; H-6'↔H-5', H-7', and H-8'; H-7'↔H-8' and H-9'a; H-8'↔H-9'a and H-9'b; H-9'a↔H-9'b; HREIMS (m/z): calcd for C18H20O3, 300.1362; found, 300.1360.
3-(1-hydroxy-3-(3-hydroxyphenyl)propan-2-yl)phenol (4): light brown oil; [α]D25 −15.4 (c 1.0 × 10−3 g/mL, EtOH); 1H and 13C NMR (600 and 150 MHz, pyridine-d5) spectroscopic analysis, see Table 2. HMBC correlations (CD3OD, H-#→C-#) H-2→C-3, C-6, and C-7; H-4→C-2, C-3, and C-6; H-5→C-1 and C-3; H-6→C-2, C-4, and C-7; H-7→C-1, C-2, C-6, C-8, C-9, and C-1'; H-8→C-1, C-7, and C-9; H-9a→C-1, C-7, C-8, C-1', C-2', and C-6'; H-9b→C-1, C-7, C-8, C-1', C-2', and C-6'; H-2'→C-9, C-3', C-4', and C-6'; H-4'→C-2', and C-6'; H-5'→C-1' and C-3', H-6'→C-9, C-2', and C-4'; HREIMS (m/z): calcd for C15H16O3, 244.1099; found, 244.1105.
Assessment of cytotoxicity
Different types of cancer cells (HL-60, HeLa, and MCF-7) were maintained in RPMI 1640 medium supplemented with L-glutamine, 10% fetal bovine serum (FBS), and 2% penicillin–streptomycin. The cells were cultured at 37 °C in a 5% CO2 incubator. Cytotoxic activity was measured using a modified MTT assay [20]. Viable cells were seeded with the growth medium (100 µL) in 96-well microtiter plates (1 × 104 cells per well) and incubated at 37 °C in a 5% CO2 incubator. The test sample was dissolved in DMSO for the final sample concentrations to be adjusted from 5.0 to 150 µM by diluting with the growth medium. Each sample was prepared in triplicate. The final DMSO concentration was adjusted to be below 0.1%. After standing for 24 h, 10 µL of the test sample was added to each well. The same volume of DMSO alone was added to the control wells. The medium was removed after 48 h of incubation with the test samples, and 10 µL of MTT were then added to the each well (final concentration, 5 mg/mL). After an additional 4 h of incubation, the resulting formazan crystals were dissolved in 150 mL of DMSO and the optical density (O.D.) was measured at 570 nm. The IC50 value was defined as the concentration of sample that reduced absorbance by 50% relative to the vehicle-treated control. Adriamycin was used as a positive control.
Supporting Information
| Supporting Information File 1: NMR and MS spectra of compounds. | ||
| Format: PDF | Size: 2.0 MB | Download |
References
-
Lee, H.; Kim, Y.; Choi, I.; Min, B. S.; Shim, S. H. Bioorg. Med. Chem. Lett. 2010, 20, 288–290. doi:10.1016/j.bmcl.2009.10.116
Return to citation in text: [1] -
Shim, S. H.; Baltrusaitis, J.; Gloer, J. B.; Wicklow, D. T. J. Nat. Prod. 2011, 74, 395–401. doi:10.1021/np100791b
Return to citation in text: [1] -
Tang, X. G.; Huang, W. Q. J. Emerg. Tradi. Chin. Med. 2008, 17, 101–102.
Return to citation in text: [1] -
Yang, N.-Y.; Tao, W.-W.; Duan, J.-A. J. Asian Nat. Prod. Res. 2009, 11, 1032–1039. doi:10.1080/10286020903352518
Return to citation in text: [1] -
Yang, D. M.; Su, S. W.; Li, X.; Zhu, R. Acta Pharm. Sin. 1987, 22, 756–760.
Return to citation in text: [1] -
Numata, A.; Yang, P.; Takahashi, C.; Fujiki, R.; Nabae, M.; Fujita, E. Chem. Pharm. Bull. 1989, 37, 648–651. doi:10.1248/cpb.37.648
Return to citation in text: [1] -
Numata, A.; Takahashi, C.; Miyamoto, T.; Yoneda, M.; Yang, P. M. Chem. Pharm. Bull. 1990, 38, 942–944. doi:10.1248/cpb.38.942
Return to citation in text: [1] -
Yang, N.-Y.; Tao, W.-W.; Zhu, M.; Duan, J.-A.; Jiang, J.-G. Fitoterapia 2010, 81, 381–384. doi:10.1016/j.fitote.2009.11.006
Return to citation in text: [1] -
Zhao, J.; Zhu, H.-J.; Zhou, X.-J.; Yang, T.-H.; Wang, Y.-Y.; Su, J.; Li, Y.; Cheng, Y.-X. J. Nat. Prod. 2010, 73, 865–869. doi:10.1021/np900814s
Return to citation in text: [1] -
Jiao, Y.; Li, D.; Liu, X. Q.; Shi. C. Zhongyaocai 2009, 32, 1039–1041.
Return to citation in text: [1] -
Yang, N.-Y.; Tao, W.-W.; Duan, J.-A. Nat. Prod. Res. 2010, 24, 1843–1849. doi:10.1080/14786419.2010.482057
Return to citation in text: [1] -
Baek, S. Y.; Shim, S. H. Planta Med. 2012, 78, PI323. doi:10.1055/s-0032-1321010
Return to citation in text: [1] -
Mei, W.; Liu, Z.; Li, X.; Dai, H. Redai Yaredai Zhiwu Xuebao 2010, 18, 573–576.
Return to citation in text: [1] [2] -
Yang, H. O.; Suh, D.-Y.; Han, B. H. Planta Med. 1995, 61, 37–40. doi:10.1055/s-2006-957995
Return to citation in text: [1] -
Guerrero, I. C.; Andrés, L. S.; León, L. G.; Machin, R. P.; Padrón, J. M.; Luis, J. G.; Delgadillo, J. J. Nat. Prod. 2006, 69, 1803–1805. doi:10.1021/np060279i
Return to citation in text: [1] -
Chamy, M. C.; Piovano, M.; Garbarino, J. A.; Miranda, C.; Gambaro, V. Phytochemistry 1990, 29, 2943–2946. doi:10.1016/0031-9422(90)87111-7
Return to citation in text: [1] -
Yuen, M. S. M.; Xue, F.; Mak, T. C. W.; Wong, H. N. C. Tetrahedron 1998, 54, 12429–12444. doi:10.1016/S0040-4020(98)00725-X
Return to citation in text: [1] [2] -
Buchan, R.; Watson, M. B. J. Chem. Soc. C 1968, 2465–2467. doi:10.1039/j39680002465
Return to citation in text: [1] [2] -
Podestá, J. C.; Chopa, A. B.; Koll, L. C.; Mandolesi, S. D. J. Organomet. Chem. 1992, 434, 269–285. doi:10.1016/0022-328X(92)83365-O
Return to citation in text: [1] [2] [3] -
Kim, D. C.; Kim, J. A.; Min, B. S.; Jang, T.-S.; Na, M.; Lee, S. H. Helv. Chim. Acta 2010, 93, 692–697. doi:10.1002/hlca.200900278
Return to citation in text: [1]
| 20. | Kim, D. C.; Kim, J. A.; Min, B. S.; Jang, T.-S.; Na, M.; Lee, S. H. Helv. Chim. Acta 2010, 93, 692–697. doi:10.1002/hlca.200900278 |
| 1. | Lee, H.; Kim, Y.; Choi, I.; Min, B. S.; Shim, S. H. Bioorg. Med. Chem. Lett. 2010, 20, 288–290. doi:10.1016/j.bmcl.2009.10.116 |
| 2. | Shim, S. H.; Baltrusaitis, J.; Gloer, J. B.; Wicklow, D. T. J. Nat. Prod. 2011, 74, 395–401. doi:10.1021/np100791b |
| 10. | Jiao, Y.; Li, D.; Liu, X. Q.; Shi. C. Zhongyaocai 2009, 32, 1039–1041. |
| 11. | Yang, N.-Y.; Tao, W.-W.; Duan, J.-A. Nat. Prod. Res. 2010, 24, 1843–1849. doi:10.1080/14786419.2010.482057 |
| 18. | Buchan, R.; Watson, M. B. J. Chem. Soc. C 1968, 2465–2467. doi:10.1039/j39680002465 |
| 19. | Podestá, J. C.; Chopa, A. B.; Koll, L. C.; Mandolesi, S. D. J. Organomet. Chem. 1992, 434, 269–285. doi:10.1016/0022-328X(92)83365-O |
| 5. | Yang, D. M.; Su, S. W.; Li, X.; Zhu, R. Acta Pharm. Sin. 1987, 22, 756–760. |
| 6. | Numata, A.; Yang, P.; Takahashi, C.; Fujiki, R.; Nabae, M.; Fujita, E. Chem. Pharm. Bull. 1989, 37, 648–651. doi:10.1248/cpb.37.648 |
| 7. | Numata, A.; Takahashi, C.; Miyamoto, T.; Yoneda, M.; Yang, P. M. Chem. Pharm. Bull. 1990, 38, 942–944. doi:10.1248/cpb.38.942 |
| 8. | Yang, N.-Y.; Tao, W.-W.; Zhu, M.; Duan, J.-A.; Jiang, J.-G. Fitoterapia 2010, 81, 381–384. doi:10.1016/j.fitote.2009.11.006 |
| 9. | Zhao, J.; Zhu, H.-J.; Zhou, X.-J.; Yang, T.-H.; Wang, Y.-Y.; Su, J.; Li, Y.; Cheng, Y.-X. J. Nat. Prod. 2010, 73, 865–869. doi:10.1021/np900814s |
| 19. | Podestá, J. C.; Chopa, A. B.; Koll, L. C.; Mandolesi, S. D. J. Organomet. Chem. 1992, 434, 269–285. doi:10.1016/0022-328X(92)83365-O |
| 4. | Yang, N.-Y.; Tao, W.-W.; Duan, J.-A. J. Asian Nat. Prod. Res. 2009, 11, 1032–1039. doi:10.1080/10286020903352518 |
| 18. | Buchan, R.; Watson, M. B. J. Chem. Soc. C 1968, 2465–2467. doi:10.1039/j39680002465 |
| 19. | Podestá, J. C.; Chopa, A. B.; Koll, L. C.; Mandolesi, S. D. J. Organomet. Chem. 1992, 434, 269–285. doi:10.1016/0022-328X(92)83365-O |
| 13. | Mei, W.; Liu, Z.; Li, X.; Dai, H. Redai Yaredai Zhiwu Xuebao 2010, 18, 573–576. |
| 15. | Guerrero, I. C.; Andrés, L. S.; León, L. G.; Machin, R. P.; Padrón, J. M.; Luis, J. G.; Delgadillo, J. J. Nat. Prod. 2006, 69, 1803–1805. doi:10.1021/np060279i |
| 17. | Yuen, M. S. M.; Xue, F.; Mak, T. C. W.; Wong, H. N. C. Tetrahedron 1998, 54, 12429–12444. doi:10.1016/S0040-4020(98)00725-X |
| 14. | Yang, H. O.; Suh, D.-Y.; Han, B. H. Planta Med. 1995, 61, 37–40. doi:10.1055/s-2006-957995 |
| 17. | Yuen, M. S. M.; Xue, F.; Mak, T. C. W.; Wong, H. N. C. Tetrahedron 1998, 54, 12429–12444. doi:10.1016/S0040-4020(98)00725-X |
| 13. | Mei, W.; Liu, Z.; Li, X.; Dai, H. Redai Yaredai Zhiwu Xuebao 2010, 18, 573–576. |
| 12. | Baek, S. Y.; Shim, S. H. Planta Med. 2012, 78, PI323. doi:10.1055/s-0032-1321010 |
| 16. | Chamy, M. C.; Piovano, M.; Garbarino, J. A.; Miranda, C.; Gambaro, V. Phytochemistry 1990, 29, 2943–2946. doi:10.1016/0031-9422(90)87111-7 |
© 2014 Baek et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)