Abstract
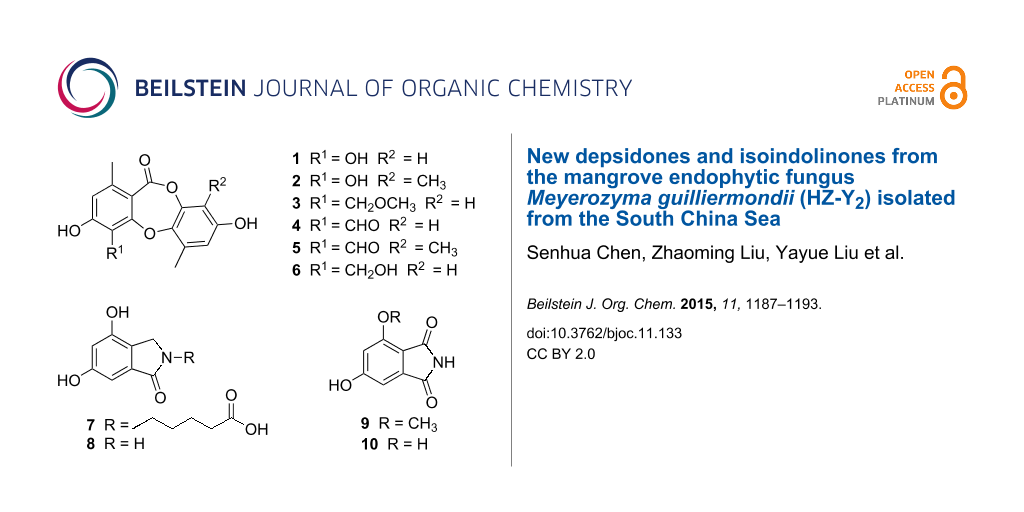
Three new depsidones, botryorhodines E–G (1–3), and two new isoindolinones, meyeroguillines A and B (7 and 9), along with five known compounds were isolated from an endophytic fungus Meyerozyma guilliermondii, derived from the mangrove plant Kandelia obovata. Their structures were elucidated by 1D and 2D NMR spectroscopy and high resolution mass spectrometry (HREIMS). Compounds 1–6 exhibited strong α-glucosidase inhibitory activity with IC50 values ranging from 2.1 to 13.3 μM. Moreover, kinetic studies of compounds 2 and 6 showed that both of them were noncompetitive inhibitors of α-glucosidase.
Graphical Abstract
Introduction
Depsidones are characterized by the presence of cyclic diaryl ethers with an ester link joining the two aromatic rings [1,2]. They have been isolated mainly from various lichens [3-5] and other fungi [6-11]. These metabolites displayed a wide range of bioactivities including antibacterial [10], antifungal [6], antiviral [12], antioxidant [5], and cytotoxic [9].
In the last decade, our research group has been focused on isolating novel compounds from endophytic fungi growing on mangroves in the South China Sea and investigating their bioactivity [13-17]. Recently, a chemical investigation of the endophytic fungal strain Meyerozyma guilliermondii (HZ-Y2), obtained from the roots of the mangrove plant Kandelia obovata, was carried out. The EtOAc extract of a fermentation broth of the fungus showed α-glucosidase inhibitory activity. Bioassay-guided fractionation of the bioactive extract led to the isolation of three new depsidones belonging to the analogues of the botryorhodines A–D [6], botryorhodines E–G (1–3), and two new isoindolinones, meyeroguillines A and B (7 and 9), together with five known compounds (4–6, 8 and 10) (Figure 1). In the bioactivity assay, all depsidones showed strong α-glucosidase inhibitory activity with IC50 values ranging from 2.1 to 13.3 μM. Details of the isolation, structure elucidation, and biological activity of these compounds are reported herein.
Results and Discussion
Botryorhodine E (1) was obtained as a white, amorphous powder. The molecular formula was assigned to be C15H12O6, with ten degrees of unsaturation, based on its HREIMS (m/z 288.0627, calcd for C15H12O6, 288.0628). The 1H NMR spectrum exhibited three aromatic protons (δH 6.55, 6.45, 6.42), and two aromatic methyl groups (δH 2.31, 2.47). The 13C NMR and DEPT spectra revealed the presence of 15 carbons (Table 1), of which 12 aromatic carbons for two phenyl units, one quaternary carbon belonging to a carbonyl carbon, and the other two sp3 carbons corresponding to two methyls. The HMBC correlations from H-5 to C-8, C-7, C-4, C-3, C-1 and H-8 to C-7, C-6, C-5, C-1 indicated the presence of a 1,2-disubstitited 6-methyl-3,4-dihdroxyphenyl ring (I) (Figure 2). The second phenyl group was substituted with two meta coupled protons at δH 6.45 (1H, d, J = 2.8, H-3′) and δH 6.42 (1H, d, J = 2.8, H-1′) as well as a methyl group at δH 2.47 (H-7′) whose protons correlated to C-6′, C-5′ and C-1′ in the HMBC. The HMBC correlations from H-3′ to C-5′, C-4′ and H-1′ to C-5′ further established the second aromatic ring (III). The remaining carbon at δC 165.9 (C-7) indicated a carbonyl ester as a result of the observation of a strong absorption at νmax 1680 cm−1 in the IR spectrum. It could be adjacent to C-1 due to contrary to the downfield shift observed for the oxygen bonded carbons C-2 (δC 152.4), C-5′ (δC 144.2), C-4′ (δC 146.3) and an upfield shift observed for C-1 (δC 113.7). The required degrees of unsaturation suggested that the aromatic rings (I) and (III) should be linked by ether and an ester bridge, revealing a depsidone skeleton with a seven-membered ring (II). Thus, the structure of 1 was revealed as a new depsidone belonging to the same family compound of the botryorhodines A–D [6], named botryorhodine E.
Table 1: 1H and 13C NMR spectroscopic data for compounds 1–3.
| Position | 1a | 2a | 3b | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 113.7, qC | 113.7, qC | 114.0, qC | |||
| 2 | 152.4, qC | 152.5, qC | 162.3, qC | |||
| 3 | 135.0, qC | 135.1, qC | 116.3, qC | |||
| 4 | 152.4, qC | 152.4, qC | 161.6, qC | |||
| 5 | 115.7, CH | 6.55, s | 115.7, CH | 6.54, s | 116.3, CH | 6.69, s |
| 6 | 135.0, qC | 135.0, qC | 145.7, qC | |||
| 7 | 165.9, qC | 166.1, qC | 163.7, qC | |||
| 8 | 20.6, CH3 | 2.31, s | 20.6, CH3 | 2.32, s | 21.3, CH3 | 2.38, s |
| 9 | 58.5, CH2 | 4.78, s | ||||
| 1′ | 114.9, CH | 6.42, d (2.8) | 113.8, CH | 6.43, s | 114.8, CH | 6.53, s |
| 2′ | 155.7, qC | 153.7, qC | 155.5, qC | |||
| 3′ | 105.7, CH | 6.45, d (2.8) | 115.0, qC | 105.9, CH | 6.53, s | |
| 4′ | 146.3, qC | 144.9, qC | 145.5, qC | |||
| 5′ | 144.2, qC | 144.6, qC | 143.2, qC | |||
| 6′ | 133.2, qC | 128.9, qC | 132.6, qC | |||
| 7′ | 16.6, CH3 | 2.47, s | 16.3, CH3 | 2.43, s | 16.7, CH3 | 2.43, s |
| 8′ | 9.2, CH3 | 2.12, s | ||||
| 9-OCH3 | 64.5, CH3 | 3.42, s | ||||
aSpectra were recorded at 500 MHz for 1H and 125 MHz for 13C in CD3OD. bSpectra were recorded at 400 MHz for 1H and 100 MHz for 13C in acetone-d6.
Figure 2: Selected HMBC (arrow) correlations of 1–3.
Figure 2: Selected HMBC (arrow) correlations of 1–3.
Botryorhodine F (2), a minor product from the fraction of compound 1, was obtained as an amorphous powder, deduced the molecular formula C16H14O6 from its HREIMS (m/z 302.0787, calcd for C16H14O6, 302.0785). The 1H and 13C NMR spectra of compound 2 were similar to those of compound 1, except for the absence of an aromatic proton at δH 6.45 and the presence of a methyl group at δH 3.42 (δC 9.2). This suggested that compound 2 was a homologue of compound 1 with the replacement of an aromatic proton by a methyl group, which was supported by the HMBC correlations from H-8′ (δH 2.12) to C-4′, C-3′ and C-2′ (Figure 2). Detailed analysis of the 2D NMR spectroscopic data, the structure of 2 was established as a 3′-methylated analogue of 1.
Botryorhodine G (3) was isolated as a white powder. Its molecular formula was determined as C16H14O6 by HRESIMS. The 1H and 13C NMR spectral data (Table 1) was greatly similar to those of compound 1 suggesting that both compounds have the same basic framework. The main difference between the two compounds was a hydroxy group at δC 135.0 (C-3) in 1 replaced by a methoxymethyl group in 3, which was supported by HMBC correlations of 9-OCH3 to C-9 and H-9 to C-4, C-3, C-1. Therefore, the structure of compound 3 was elucidated as shown.
Meyeroguilline A (7) was obtained as an amorphous powder. The molecular formula was established by analysis of the HREIMS (m/z 265.0946 calcd for C13H15O5N, 265.0945) in combination with 1H and 13C NMR data, indicating seven degrees of unsaturation. The UV spectral data at 242 (4.30), 291 (4.02), and 326 (3.86) nm indicated the existence of a benzoyl group. The 1H NMR spectrum (Table 2) along with HSQC spectrum showed signals due to the presence of five methylene protons (δH 1.44−4.19), two phenolic hydroxy groups (δH 9.89 and 9.55), and two aromatic protons (δH 6.43 and 6.48), showing a typical pattern of meta-coupling (J = 1.8 Hz) consistent with a 1,2,3,5-tetrasubstituted benzene moiety. Moreover, two carbonyl groups (δC 167.8 and 174.4) were clearly seen in the 13C NMR spectrum. Taking into account the required degrees of unsaturation, compound 7 contained a bicyclic aromatic lactam fragment. Further detailed analysis of the 1H and 13C NMR spectra suggested that 7 is an isoindolinone derivative [18]. Analysis of the 1H,1H COSY spectrum suggested the presence of one spin system, which included H-8/H-9/H-10/H-11 (Figure 3). In the HMBC spectrum (Figure 3), the correlations of H-10 and H-11 to C-12 (carbonyl), H-8 to C-10, and H-11 to C-9, established a valeric acid moiety. The linkage of valeric acid moiety to N-2 of the isoindolinone was assigned by HMBC correlations from H-8 to C-1 and C-3. The two aromatic hydroxy groups were accommodated at C-4 and C-6, based on the HMBC corrections from H-3 to C-4 (δC 153.2) and H-7 to C-6 (δC 158.8), respectively. To the best of our knowledge, compound 7 possessed a valeric acid moiety was the first reported example of a natural isoindolinone.
Table 2: 1H and 13C NMR spectroscopic data for compounds 7 and 9 in DMSO-d6.
| Position | 7a | 9a | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 167.8, qC | 169.2, qC | ||
| 3 | 46.9, CH2 | 4.19, s | 167.7, qC | |
| 3a | 118.4, qC | 109.2, qC | ||
| 4 | 153.2, qC | 158.5, qC | ||
| 5 | 105.4, CH | 6.43, d (1.75) | 104.3, CH | 6.68, br s |
| 6 | 158.8, qC | 165.6, qC | ||
| 7 | 100.1, CH | 6.48, d (1.75) | 102.8, CH | 6.70, br s |
| 7a | 134.9, qC | 137.3, qC | ||
| 8 | 41.2, CH2 | 3.46, t (6.74) | ||
| 9 | 27.2, CH2 | 1.58, m | ||
| 10 | 21.8, CH2 | 1.44, m | ||
| 11 | 33.2, CH2 | 2.24, t (7.25) | ||
| 12 | 174.4, qC | |||
| 4-OCH3 | 56.3 | 3.86 | ||
| 2-NH | 10.83 | |||
aSpectra were recorded at 400 MHz for 1H and 100 MHz for 13C.
Figure 3: 1H,1H COSY (bold) and selected HMBC (arrow) correlations of 7 and 9.
Figure 3: 1H,1H COSY (bold) and selected HMBC (arrow) correlations of 7 and 9.
Meyeroguilline B (9) was isolated as a pale yellow amorphous powder, the molecular formula was assigned as C9H7O4N based on HREIMS (m/z 193.0370). Nine signals in the 13C NMR were classified by the DEPT spectra, including a methyl, two methine, and six quaternary carbons (Table 2). The 1H NMR and HSQC spectra revealed a methoxy group (δH 3.86), two benzene protons (δH 6.68 and 6.70). The NMR signals of 9 were principally similar to those of 10 [18], except that the chelated hydroxy group (4-OH in 10) was replaced by a methoxy group (Figure 3). The HMBC correlations from 4-OCH3 to C-4 also indicated that the attachment of the methoxy group to C-4. The structure of 9 was thus established as 6-hydroxy-4-methoxyisoindoline-1,3-dione.
In addition, the structures of the known compounds were identified as botryorhodine A (4) [6], botryorhodine B (5) [6], botryorhodine D (6) [6], 4,6-dihydroxy-2,3-dihydro-1H-isoindol-1-one (8) [18], 4,6-dihydroxy-1H-isoindole-1,3(2H)-dione (10) [18], by comparison of their spectroscopic data with those reported in the literature.
The isolated compounds were tested for α-glucosidase inhibitory activity using acarbose as positive control. All depsidones 1–6 exhibited strong α-glucosidase inhibitory activity with IC50 (μM) values ranging 2.1–15.4 (Table 3). Compound 6 showed the best inhibitory activity (IC50 2.1 μM) among the depsidones. Comparison with those depsidones, the structural differences mainly occur in the functional group of C-3 and C-3′, which indicated that the hydroxymethyl group at C-3 could enhance the α-glucosidase inhibitory activity, while the methyl group at C-3′ did not affect that activity. Moreover, kinetic studies of compounds 2 and 6 showed that both of them were noncompetitive inhibitors of α-glucosidase (Figure 4). Compound 7 showed the promising α-glucosidase inhibitory activity, whereas compound 8 and 10 inhibited α-glucosidase with moderate to weak activities (Table 3).
Table 3: α-Glucosidase inhibitory activitiesa.
| Compounds | IC50 (μM) |
|---|---|
| 1 | 15.4 ± 0.4 |
| 2 | 9.8 ± 0.3 |
| 3 | 12.4 ± 0.4 |
| 4 | 13.3 ± 0.3 |
| 5 | 11.7 ± 0.4 |
| 6 | 2.1 ± 0.2 |
| 7 | 50.3 ± 1.3 |
| 8 | 180.1 ± 1.8 |
| 10 | 251.3 ± 2.5 |
| Acarboseb | 553.7 ± 2.4 |
aIC50 values are shown as mean ± SD from three independent experiments. The inhibitory activity of compound 9 were not tested due to the limited amount; bpositive control.
![[1860-5397-11-133-4]](/bjoc/content/figures/1860-5397-11-133-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Kinetic analysis of the inhibition of α-glucosidase by 2 (left) and 6 (right).
Figure 4: Kinetic analysis of the inhibition of α-glucosidase by 2 (left) and 6 (right).
All isolated compounds were also evaluated for their inhibitory activity against Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB), of which were inactive (IC50 > 200 μM).
Experimental
General experimental procedures. The NMR spectra were performed on Bruker Avance 400 spectrometer (1H 400 MHz, 13C 100 MHz) and Bruker Avance 500 spectrometer (1H 500 MHz, 13C 125 MHz). All chemical shifts (δ) are given in ppm with reference to the solvent signal (δC 49.0/δH 3.31 for CD3OD, δC 39.5/δH 2.50 for DMSO and δC 29.8/δH 2.05 for acetone-d6), and coupling constants (J) are given in Hz. UV data were measured on a PERSEE TU-1900 spectrophotometer. IR spectra were measured on a Nicolet Nexus 670 spectrophotometer, in KBr discs. EIMS on a DSQ EI-mass spectrometer (Thermo, Shanghai, China) and HREIMS data were measured on a DMAT95XP high-resolution mass spectrometer. ESIMS spectra were recorded on a Finnigan LCQ-DECA mass spectrometer and HRESIMS spectra were recorded on a Shimadzu LCMS-IT-TOF mass spectrometer. Column chromatography (CC) was performed on silica gel (200–300 mesh, Qingdao Marine Chemical Factory) and Sephadex LH-20 (Amersham Pharmacia, Piscataway). Precoated silica gel plates (Qingdao Huang Hai Chemical Group Co., G60, F-254) were used for thin-layer chromatography. Semipreparative HPLC was performed on a Waters Breeze HPLC system using a Phenomenex Luna (Phenomenex, Torrance) C18 column (250 × 10 mm, 5 μm).
Fungal material. The fungus used in this study was isolated from healthy roots of Kandelia obovata, which were collected in April 2012 from Huizhou Mangrove Nature Reserve in Guangdong Province, China. It was obtained using the standard protocol for the isolation. Fungal identification was carried out using a molecular biological protocol by DNA amplification and sequencing of the ITS region. The sequence data obtained from the fungal strain have been deposited at GenBank with accession no. KP975418. A BLAST search result showed that the sequence was the most similar (99%) to the sequence of Meyerozyma guilliermondii (compared to KF710038.1 FJ662408.1). A voucher strain was deposited in School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou, China.
Fermentation, extraction and isolation. The fungus was grown on autoclaved rice solid-substrate medium (thirty 500 mL Erlenmeyer flasks, each containing 50 g rice and 50 mL distilled water) at room temperature under static conditions and daylight for 28 days. Following incubation, the mycelia and solid rice medium were extracted with EtOAc. The extract was evaporated under reduced pressure to yield 21 g of residue. The residue was then divided into 36 fractions (Fr. 1–Fr. 36) by column chromatography on silica gel eluted by a gradient of petroleum ether/EtOAc from 1:0 to 0:1. Fr. 6 (230 mg) was applied to silica gel CC, eluted with petroleum ether/EtOAc (3:1), to obtain compounds 4 (10 mg) and 5 (6 mg). Fr. 8 (134 mg) was subsequently separated by Sephadex LH-20 CC eluted with MeOH to give subfraction Fr. 11.8, which was purified on silica gel (petroleum ether/EtOAc v/v, 7:3) to yield 3 (3.7 mg) and 6 (2.5 mg). Fr. 15 was chromatographed on Sephadex LH-20 CC (CHCl3/MeOH v/v, 1:1) to give subfraction Fr. 15.5, which was purified using semipreparative reversed-phase HPLC (MeOH/H2O, 80:20; 2.0 mL/min) to yield 1 (3.3 mg, tR = 18 min) and 2 (2.1 mg, tR = 20 min). Fr. 20.11 was applied to Sephadex LH-20 column eluted with MeOH to obtain 10 (15 mg). Fr. 21 (304 mg) was rechromatographed on silica gel (gradient of CHCl3/MeOH from 1:0 to 1:1) to give subfraction Fr. 22.8, which was purified by Sephadex LH-20 CC (CHCl3/MeOH v/v, 1:1) to yield 8 (1.3 mg). Fr. 25 (80 mg) was subsequently separated by Sephadex LH-20 CC eluted with MeOH to obtain 7 (2.4 mg) and 9 (3.5 mg), respectively.
Botryorhodine E (1): white amorphous powder; UV (MeOH) λmax (log ε): 262 (4.25), 364 (3.56) nm; IR (KBr) νmax: 3426, 2922, 2853, 1680, 1620, 1506, 1458, 1383, 1265, 1193, 1074, 908, 877, 809 cm−1; EIMS (m/z): 288; HREIMS (m/z): 288.0627 (calcd for C15H12O6, 288.0628); 1H NMR (CD3OD, 500 MHz) and 13C NMR (CD3OD, 125 MHz), see Table 1.
Botryorhodine F (2): white amorphous powder; UV (MeOH) λmax (log ε): 262 (4.25), 364 (3.56) nm; IR (KBr) νmax: 3426, 2922, 2853, 1686, 1620, 1587, 1458, 1357, 1284, 1193, 1155, 1074, 915, 875, 812 cm−1; EIMS (m/z): 302; HREIMS (m/z): 302.0787 (calcd for C16H14O6, 302.0785); 1H NMR (CD3OD, 500 MHz) and 13C NMR (CD3OD, 125 MHz), see Table 1.
Botryorhodine G (3): white amorphous powder; UV (MeOH) λmax (log ε): 262 (4.25), 364 (3.56) nm; IR (KBr) νmax: 3342, 3076, 2931, 2723, 1703, 1606, 1502, 1456, 1284, 1203, 1146, 1080, 995, 945, 850 cm−1; ESIMS (m/z): [M − H]− 315; HRESIMS (m/z): [M − H]− 315.0869 (calcd for C17H15O6, 315.0863); 1H NMR (acetone-d6, 400 MHz) and 13C NMR (acetone-d6, 100 MHz), see Table 1.
Meyeroguilline A (7): amorphous powder; UV (MeOH) λmax (log ε): 242 (4.30), 291 (4.02), 326 (3.86) nm; IR (KBr) νmax: 3448, 2927, 2853, 1644, 1610, 1460, 1315, 1166, 1013, 816 cm−1; EIMS (m/z): 265; HREIMS (m/z): 265.0946 (calcd for C13H15O5N, 265.0945); 1H NMR (DMSO, 400 MHz) and 13C NMR (DMSO, 100 MHz), see Table 2.
Meyeroguilline B (9): amorphous powder; UV (MeOH) λmax (log ε): 219 (4.25), 240 (3.94), 336 (3.56) nm; IR (KBr) νmax: 3438, 2926, 2853, 1648, 1609, 1459, 1264, 1165, 1015, 809 cm−1; EIMS (m/z): 193; HREIMS (m/z): 193.0370 (calcd for C9H7O4N, 193.0370); 1H NMR (DMSO, 400 MHz) and 13C NMR (DMSO, 100 MHz), see Table 2.
Biological assays. The assays for α-glucosidase [19] and MptpB inhibition inhibitory activities [13] were carried out as described previously.
Supporting Information
| Supporting Information File 1: 1H, 13C, 1H,1H COSY, HSQC and HMBC NMR spectra of the new compounds. | ||
| Format: PDF | Size: 740.6 KB | Download |
Acknowledgements
We thank the National Natural Science Foundation of China (21472251, 41276146), the Science & Technology Plan Project of Guangdong Province of China (2013B021100011), Special Financial Fund of Innovative Development of Marine Economic Demonstration Project (GD2012-D01-001), China’s Marine Commonwealth Research Project (201305017) for generous support.
References
-
Bedos-Belval, F.; Rouch, A.; Vanucci-Bacqué, C.; Baltas, M. Med. Chem. Commun. 2012, 3, 1356–1372. doi:10.1039/c2md20199b
Return to citation in text: [1] -
Armaleo, D.; Sun, X.; Culberson, C. Mycologia 2011, 103, 741–754. doi:10.3852/10-335
Return to citation in text: [1] -
Kumar, S.; Kaushik, N. Phytochem. Rev. 2012, 11, 507–522. doi:10.1007/s11101-013-9271-y
Return to citation in text: [1] -
Shukla, V.; Joshi, G. P.; Rawat, M. S. M. Phytochem. Rev. 2010, 9, 303–314. doi:10.1007/s11101-010-9189-6
Return to citation in text: [1] -
Lohézic-Le Dévéhat, F.; Tomasi, S.; Elix, J. A.; Bernard, A.; Rouaud, I.; Uriac, P.; Boustie, J. J. Nat. Prod. 2007, 70, 1218–1220. doi:10.1021/np070145k
Return to citation in text: [1] [2] -
Abdou, R.; Scherlach, K.; Dahse, H.-M.; Sattler, I.; Hertweck, C. Phytochemistry 2010, 71, 110–116. doi:10.1016/j.phytochem.2009.09.024
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Chomcheon, P.; Wiyakrutta, S.; Sriubolmas, N.; Ngamrojanavanich, N.; Kengtong, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Phytochemistry 2009, 70, 407–413. doi:10.1016/j.phytochem.2009.01.007
Return to citation in text: [1] -
Lang, G.; Cole, A. L. J.; Blunt, J. W.; Robinson, W. T.; Munro, M. H. G. J. Nat. Prod. 2007, 70, 310–311. doi:10.1021/np060202u
Return to citation in text: [1] -
Pittayakhajonwut, P.; Dramae, A.; Madla, S.; Lartpornmatulee, N.; Boonyuen, N.; Tanticharoen, M. J. Nat. Prod. 2006, 69, 1361–1363. doi:10.1021/np060190c
Return to citation in text: [1] [2] -
Poch, G. K.; Gloer, J. B. J. Nat. Prod. 1991, 54, 213–217. doi:10.1021/np50073a021
Return to citation in text: [1] [2] -
Niu, S.; Liu, D.; Hu, X.; Proksch, P.; Shao, Z.; Lin, W. J. Nat. Prod. 2014, 77, 1021–1030. doi:10.1021/np5000457
Return to citation in text: [1] -
Nicklaus, M. C.; Neamati, N.; Hong, H.; Mazumder, A.; Sunder, S.; Chen, J.; Milne, G. W. A.; Pommier, Y. J. Med. Chem. 1997, 40, 920–929. doi:10.1021/jm960596u
Return to citation in text: [1] -
Li, H.; Jiang, J.; Liu, Z.; Lin, S.; Xia, G.; Xia, X.; Ding, B.; He, L.; Lu, Y.; She, Z. J. Nat. Prod. 2014, 77, 800–806. doi:10.1021/np400880w
Return to citation in text: [1] [2] -
Huang, X.; Huang, H.; Li, H.; Sun, X.; Huang, H.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Org. Lett. 2013, 15, 721–723. doi:10.1021/ol303549c
Return to citation in text: [1] -
Xiao, Z.; Huang, H.; Shao, C.; Xia, X.; Ma, L.; Huang, X.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Org. Lett. 2013, 15, 2522–2525. doi:10.1021/ol401005j
Return to citation in text: [1] -
Wen, L.; Cai, X.; Xu, F.; She, Z.; Chan, W. L.; Vrijmoed, L. L. P.; Jones, E. B. G.; Lin, Y. J. Org. Chem. 2009, 74, 1093–1098. doi:10.1021/jo802096q
Return to citation in text: [1] -
Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Luo, J.; Zhou, S.; Vrijmoed, L. L. P.; Jones, E. B. G.; Krohn, K.; Steingröver, K.; Zsila, F. J. Org. Chem. 2001, 66, 6252–6256. doi:10.1021/jo015522r
Return to citation in text: [1] -
Lü, W.-W.; Gao, Y.-J.; Su, M.-Z.; Luo, Z.; Zhang, W.; Shi, G.-B.; Zhao, Q.-C. Helv. Chim. Acta 2013, 96, 109–113. doi:10.1002/hlca.201200279
Return to citation in text: [1] [2] [3] [4] -
Liu, Z.; Xia, G.; Chen, S.; Liu, Y.; Li, H.; She, Z. Mar. Drugs 2014, 12, 3669–3680. doi:10.3390/md12063669
Return to citation in text: [1]
| 19. | Liu, Z.; Xia, G.; Chen, S.; Liu, Y.; Li, H.; She, Z. Mar. Drugs 2014, 12, 3669–3680. doi:10.3390/md12063669 |
| 18. | Lü, W.-W.; Gao, Y.-J.; Su, M.-Z.; Luo, Z.; Zhang, W.; Shi, G.-B.; Zhao, Q.-C. Helv. Chim. Acta 2013, 96, 109–113. doi:10.1002/hlca.201200279 |
| 18. | Lü, W.-W.; Gao, Y.-J.; Su, M.-Z.; Luo, Z.; Zhang, W.; Shi, G.-B.; Zhao, Q.-C. Helv. Chim. Acta 2013, 96, 109–113. doi:10.1002/hlca.201200279 |
| 1. | Bedos-Belval, F.; Rouch, A.; Vanucci-Bacqué, C.; Baltas, M. Med. Chem. Commun. 2012, 3, 1356–1372. doi:10.1039/c2md20199b |
| 2. | Armaleo, D.; Sun, X.; Culberson, C. Mycologia 2011, 103, 741–754. doi:10.3852/10-335 |
| 6. | Abdou, R.; Scherlach, K.; Dahse, H.-M.; Sattler, I.; Hertweck, C. Phytochemistry 2010, 71, 110–116. doi:10.1016/j.phytochem.2009.09.024 |
| 6. | Abdou, R.; Scherlach, K.; Dahse, H.-M.; Sattler, I.; Hertweck, C. Phytochemistry 2010, 71, 110–116. doi:10.1016/j.phytochem.2009.09.024 |
| 10. | Poch, G. K.; Gloer, J. B. J. Nat. Prod. 1991, 54, 213–217. doi:10.1021/np50073a021 |
| 6. | Abdou, R.; Scherlach, K.; Dahse, H.-M.; Sattler, I.; Hertweck, C. Phytochemistry 2010, 71, 110–116. doi:10.1016/j.phytochem.2009.09.024 |
| 6. | Abdou, R.; Scherlach, K.; Dahse, H.-M.; Sattler, I.; Hertweck, C. Phytochemistry 2010, 71, 110–116. doi:10.1016/j.phytochem.2009.09.024 |
| 7. | Chomcheon, P.; Wiyakrutta, S.; Sriubolmas, N.; Ngamrojanavanich, N.; Kengtong, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Phytochemistry 2009, 70, 407–413. doi:10.1016/j.phytochem.2009.01.007 |
| 8. | Lang, G.; Cole, A. L. J.; Blunt, J. W.; Robinson, W. T.; Munro, M. H. G. J. Nat. Prod. 2007, 70, 310–311. doi:10.1021/np060202u |
| 9. | Pittayakhajonwut, P.; Dramae, A.; Madla, S.; Lartpornmatulee, N.; Boonyuen, N.; Tanticharoen, M. J. Nat. Prod. 2006, 69, 1361–1363. doi:10.1021/np060190c |
| 10. | Poch, G. K.; Gloer, J. B. J. Nat. Prod. 1991, 54, 213–217. doi:10.1021/np50073a021 |
| 11. | Niu, S.; Liu, D.; Hu, X.; Proksch, P.; Shao, Z.; Lin, W. J. Nat. Prod. 2014, 77, 1021–1030. doi:10.1021/np5000457 |
| 18. | Lü, W.-W.; Gao, Y.-J.; Su, M.-Z.; Luo, Z.; Zhang, W.; Shi, G.-B.; Zhao, Q.-C. Helv. Chim. Acta 2013, 96, 109–113. doi:10.1002/hlca.201200279 |
| 3. | Kumar, S.; Kaushik, N. Phytochem. Rev. 2012, 11, 507–522. doi:10.1007/s11101-013-9271-y |
| 4. | Shukla, V.; Joshi, G. P.; Rawat, M. S. M. Phytochem. Rev. 2010, 9, 303–314. doi:10.1007/s11101-010-9189-6 |
| 5. | Lohézic-Le Dévéhat, F.; Tomasi, S.; Elix, J. A.; Bernard, A.; Rouaud, I.; Uriac, P.; Boustie, J. J. Nat. Prod. 2007, 70, 1218–1220. doi:10.1021/np070145k |
| 6. | Abdou, R.; Scherlach, K.; Dahse, H.-M.; Sattler, I.; Hertweck, C. Phytochemistry 2010, 71, 110–116. doi:10.1016/j.phytochem.2009.09.024 |
| 13. | Li, H.; Jiang, J.; Liu, Z.; Lin, S.; Xia, G.; Xia, X.; Ding, B.; He, L.; Lu, Y.; She, Z. J. Nat. Prod. 2014, 77, 800–806. doi:10.1021/np400880w |
| 14. | Huang, X.; Huang, H.; Li, H.; Sun, X.; Huang, H.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Org. Lett. 2013, 15, 721–723. doi:10.1021/ol303549c |
| 15. | Xiao, Z.; Huang, H.; Shao, C.; Xia, X.; Ma, L.; Huang, X.; Lu, Y.; Lin, Y.; Long, Y.; She, Z. Org. Lett. 2013, 15, 2522–2525. doi:10.1021/ol401005j |
| 16. | Wen, L.; Cai, X.; Xu, F.; She, Z.; Chan, W. L.; Vrijmoed, L. L. P.; Jones, E. B. G.; Lin, Y. J. Org. Chem. 2009, 74, 1093–1098. doi:10.1021/jo802096q |
| 17. | Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Luo, J.; Zhou, S.; Vrijmoed, L. L. P.; Jones, E. B. G.; Krohn, K.; Steingröver, K.; Zsila, F. J. Org. Chem. 2001, 66, 6252–6256. doi:10.1021/jo015522r |
| 6. | Abdou, R.; Scherlach, K.; Dahse, H.-M.; Sattler, I.; Hertweck, C. Phytochemistry 2010, 71, 110–116. doi:10.1016/j.phytochem.2009.09.024 |
| 9. | Pittayakhajonwut, P.; Dramae, A.; Madla, S.; Lartpornmatulee, N.; Boonyuen, N.; Tanticharoen, M. J. Nat. Prod. 2006, 69, 1361–1363. doi:10.1021/np060190c |
| 18. | Lü, W.-W.; Gao, Y.-J.; Su, M.-Z.; Luo, Z.; Zhang, W.; Shi, G.-B.; Zhao, Q.-C. Helv. Chim. Acta 2013, 96, 109–113. doi:10.1002/hlca.201200279 |
| 5. | Lohézic-Le Dévéhat, F.; Tomasi, S.; Elix, J. A.; Bernard, A.; Rouaud, I.; Uriac, P.; Boustie, J. J. Nat. Prod. 2007, 70, 1218–1220. doi:10.1021/np070145k |
| 13. | Li, H.; Jiang, J.; Liu, Z.; Lin, S.; Xia, G.; Xia, X.; Ding, B.; He, L.; Lu, Y.; She, Z. J. Nat. Prod. 2014, 77, 800–806. doi:10.1021/np400880w |
| 12. | Nicklaus, M. C.; Neamati, N.; Hong, H.; Mazumder, A.; Sunder, S.; Chen, J.; Milne, G. W. A.; Pommier, Y. J. Med. Chem. 1997, 40, 920–929. doi:10.1021/jm960596u |
| 6. | Abdou, R.; Scherlach, K.; Dahse, H.-M.; Sattler, I.; Hertweck, C. Phytochemistry 2010, 71, 110–116. doi:10.1016/j.phytochem.2009.09.024 |
© 2015 Chen et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)