Abstract
Reaction of benzyl and ethyl allenoates with TMSX (X = I, Br, Cl) and with NH4SCN were investigated in MeCN, DMF, and in imidazolium ionic liquids [BMIM][NTf2] and [BMIM][PF6] as solvent, in the presence and absence of Selectfluor. Comparative product analysis studies demonstrate that the ability of Selectflour to promote oxidative/electrophilic dihalogenation/dithiocyanation with TMSX/NH4SCN (as observed previously for 1-arylallenes) is diminished in allenoates, most significantly in reactions with TMSCl, and essentially disappearing in reactions with NH4SCN, in favor of nucleophilic/conjugate addition. The study underscores the contrasting reactivity patterns in 1-arylallenes and allenoates toward electrophilic and nucleophilic additions in halofunctionalization with TMSX/Selectfluor and thiocyanation reactions with NH4SCN/Selectfluor. These competing pathways are influenced by the nature of the anion, allene structure, and the choice of solvent.
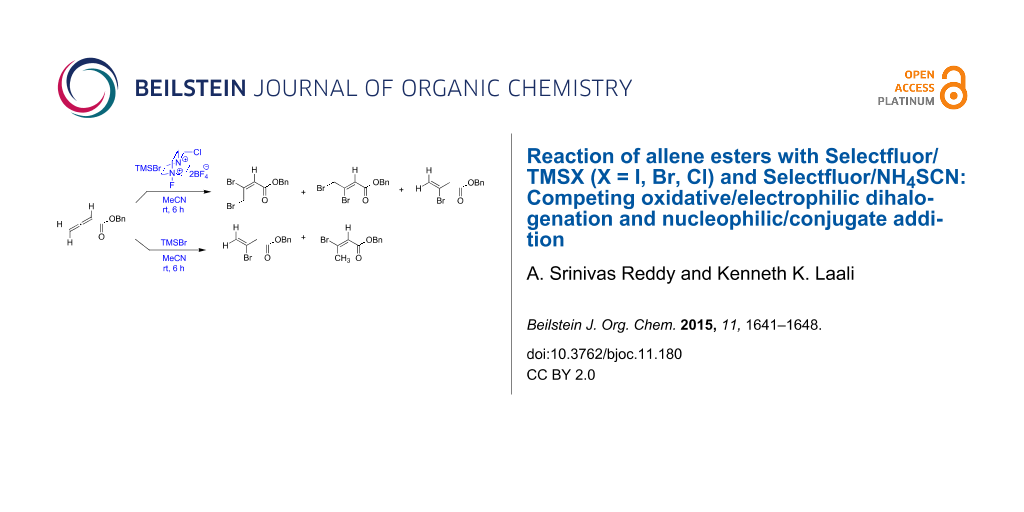
Graphical Abstract
Introduction
Whereas the synthetic potential of SelectfluorTM (F-TEDA-BF4) as an efficient, mild, and selective reagent for fluoro-functionalization of organic compounds is widely recognized and exploited [1-10], its ability to act as mediator or catalyst for oxidative functionalization is comparatively less explored [11]. Notable examples of oxidative functionalization by Selectfluor include in situ generation of electrophile equivalents Cl+, Br+, SCN+ and NO2+ and their reactions with aromatics [12], the bromination of representative alkenes with Selectfluor/KBr [13], and the thiocyanation of representative heteroarenes and ketones with NH4SCN [14,15]. Oxidative transformations such as amide to imide mediated by Selectfluor in combination with CuBr have also been shown [16,17].
In an earlier study, we reported on the potential of Selectfluor to act as mediator and oxidant in the reaction of 1-arylallenes with TMSX (X = Cl, Br, I, NCS) and with NH4SCN to bring about dihalogenation and dithiocyanation [18]. The predominant formation of dihaloalkenes and dithiocyanoalkenes observed in these reactions were rationalized by an electrophilic attack of “X+” or “SCN+” at the central carbon of the allenyl moiety to form incipient allyl cations which on subsequent quenching with X− or SCN− furnished the 2,3-adducts as major products. The 1,2-addition products were only observed with TMSCl. The reactions were carried out in MeCN and in imidazolium ionic liquids (ILs) as solvent in which Selectfluor is soluble.
Previous studies have shown that the major products arising from the reaction of 2,3-allenoates with MX/HX are hydrohalogenated compounds [19-21]. Similarly, reactions with NuH lead to Michael-type nucleophilic additions, but in the presence of phosphane catalysts an umpolung addition takes place, whereby the nucleophilic addition occurs inversely at the beta-gamma double bond [22,23]. Vinyl azides have been prepared by hydroazidation of allenyl esters through a Michael-type addition with high regio- and stereoselectivity [24].
Inspired by these results we focused our attention in the present study on the reaction of allenoates with TMSX with the aim to determine the extent by which Selectfluor could influence the electrophilic versus nucleophilic addition manifolds.
Results and Discussion
The reaction of benzyl allenoate (1) with TMSBr (2 equivalents) and Selectfluor (1 equivalent) (Scheme 1) in MeCN as solvent gave the regioisomeric 2,3-dibromoalkenoates 1a, 1b as major products along with the hydrobromination product 1c (compounds 1b and 1c were inseparable by chromatography and were isolated together). In the absence of Selectfluor, dibromination products were not observed and the major product was 1c along with tiny amounts of isomeric 1d.
Scheme 1: Reaction of benzyl allenoate (1) with TMSBr with and without Selectfluor (E/Z designations, as illustrated, were made by NMR; see Supporting Information File 1).
Scheme 1: Reaction of benzyl allenoate (1) with TMSBr with and without Selectfluor (E/Z designations, as illu...
Switching to DMF as solvent and in the presence of Selectfluor, the 2,3-dibromoalkenoate 1a and hydrobromination product 1c were obtained, with 1c isolated as a minor component, whereas in the absence of Selectfluor 1c became the predominant product, and minor amounts of the isomeric 1d was also isolated (Scheme 1).
Switching to imidazolium ILs as solvent (Scheme 2), from the reaction of 1 with TMSBr/Selectfluor in [BMIM][NTf2] the dibromoalkenoate 1a and the hydrobromination product 1d were isolated in comparable amounts along with a trace of 1c. Surprisingly no dihalogenation products were isolated when [BMIM][PF6] was employed as solvent. In this case 1c was isolated as a major product along with minor amounts of 1d. In an effort to enhance the oxidative power of Selectfluor and to promote dihalogenation, CuBr was used as an additive [16,17], but the outcome remained unchanged. In the absence of Selectfluor the same hydrohalogenation products 1c and 1d were isolated but in different ratios (Scheme 2).
Scheme 2: Reaction of benzyl allenoate (1) with TMSBr with and without Selectfluor in IL solvents (E/Z designations, as illustrated, were made by NMR; see Supporting Information File 1).
Scheme 2: Reaction of benzyl allenoate (1) with TMSBr with and without Selectfluor in IL solvents (E/Z design...
The reaction of benzyl allenoate (1) with TMSI in MeCN (Scheme 3) produced four products, namely the regioisomeric diiodoalkenoates 1e, 1g and the isomeric HI addition products 1f and 1h (compounds 1e/1f and 1g/1h were chromatographically inseparable and were isolated in pairs). Overall, the proportion of the oxidative dihalogenation products was notably larger than the hydroiodination products.
Scheme 3: Reaction of benzyl allenoate (1) with TMSI and TMSCl, with and without Selectfluor (E/Z designations, as illustrated, were made by NMR; see Supporting Information File 1).
Scheme 3: Reaction of benzyl allenoate (1) with TMSI and TMSCl, with and without Selectfluor (E/Z designation...
The reaction of allenoate 1 with TMSCl/Selectfluor in MeCN (Scheme 3) gave only the hydrochlorination products 1i (major) and 1j (minor). The same products were isolated in the absence of Selectfluor but with higher proportion of 1i. Interestingly, repeating the reaction in [BMIM][NTf2] as the solvent (Scheme 3) resulted in the formation of the dichloroalkenoate 1k as a major component, along with isomeric 1j and 1i (1k and 1j were inseparable by chromatography and were isolated together).
Focusing on thiocyanation, the reaction of benzyl allenoate (1) was studied with NH4SCN/Selectfluor in MeCN, DMF, as well as in [BMIM][PF6] and [BMIM][NTf2]. The isomeric conjugate addition products 1l and 1m were isolated, with 1l as the major isomer. Unlike previous findings with 1-arylallenes [18], no dithiocyanation products were found irrespective of the choice of solvent (Scheme 4).
Scheme 4: Reaction of benzyl allenoate (1) with NH4SCN/Selectfluor in different solvents (E/Z designations, as illustrated, were made by NMR; see Supporting Information File 1).
Scheme 4: Reaction of benzyl allenoate (1) with NH4SCN/Selectfluor in different solvents (E/Z designations, a...
In order to examine a possible influence of the structure of the allenoate on the product distribution, allene esters 2–6 were synthesized (Figure 1).
Figure 1: Allene esters synthesized for this study.
Figure 1: Allene esters synthesized for this study.
The reactions of ethyl allenoate (2) with TMSBr, TMSI and with NH4SCN were studied in the presence of Selectfluor in MeCN and DMF (Scheme 5). With TMSBr and TMSI the dibromo- and the diiodoalkenoates 2a and 2b were isolated as the main products, respectively, along with minor amounts of 2c (accompanied by unidentified side products). With NH4SCN however only the conjugate addition products 2d and 2e were observed.
Scheme 5: Reaction of ethyl allenoate (2) with TMSX/Selectfluor and NH4SCN/Selectfluor (E/Z designations, as illustrated, were made by NMR; see Supporting Information File 1).
Scheme 5: Reaction of ethyl allenoate (2) with TMSX/Selectfluor and NH4SCN/Selectfluor (E/Z designations, as ...
The reactions listed in Scheme 5 were then repeated with ethyl allenoate 3 in MeCN as the solvent and the results are sketched in Scheme 6. The diiodo- and dibromoalkenoates 3a and 3b were obtained in good isolated yields. With NH4SCN/Selectfluor, on the other hand, only the isomeric conjugate addition (hydrothiocyantion) products 3c and 3d were isolated.
Scheme 6: Reaction of ethyl allenoate 3 with TMSX/Selectfluor and NH4SCN/Selectfluor (E/Z designations, as illustrated, were made by NMR; see Supporting Information File 1).
Scheme 6: Reaction of ethyl allenoate 3 with TMSX/Selectfluor and NH4SCN/Selectfluor (E/Z designations, as il...
The reaction of benzyl allenoate 4 was examined with TMSX/Selectfluor (X = Br and Cl) and with NH4SCN/Selectfluor in MeCN as the solvent (Scheme 7). The dibromoalkenoate 4c was isolated as a minor component in the reaction with TMSBr/Selectfluor, along with regioisomeric hydrobromination products 4a, 4b (in a 1:1 ratio by NMR; this fraction also contained traces of unreacted 4). With TMSCl/Selectfluor and NH4SCN/Selectfluor only the corresponding regioisomeric conjugate addition products 4d, 4e and 4f, 4g were isolated.
Scheme 7: Reaction of benzyl allenoate 4 with TMSX (X = Br, and Cl)/Selectfluor and NH4SCN/Selectfluor (E/Z designations, as illustrated, were made by NMR; see Supporting Information File 1).
Scheme 7: Reaction of benzyl allenoate 4 with TMSX (X = Br, and Cl)/Selectfluor and NH4SCN/Selectfluor (E/Z d...
The diiodoalkenoate 5a was obtained in good isolated yield from the reaction of ethyl allenoate 5 with TMSI/Selectfluor in DMF and in MeCN and the conjugate addition products were not observed (Scheme 8).
Scheme 8: Reaction of ethyl allenoate 5 with TMSI/Selecfluor in DMF and MeCN as the solvents (E/Z designations, as illustrated, were made by NMR; see Supporting Information File 1).
Scheme 8: Reaction of ethyl allenoate 5 with TMSI/Selecfluor in DMF and MeCN as the solvents (E/Z designation...
Finally, the reaction of benzyl allenoate 6 with NH4SCN with and without Selectfluor was studied in MeCN as the solvent. Consistent with earlier cases examined, the conjugate addition product 6a was isolated in good yield, and products resulting from oxidative dithiocyanation were not observed (Scheme 9).
Scheme 9: Reaction of benzyl allenoate 6 with NH4SCN in presence and absence of Selectfluor.
Scheme 9: Reaction of benzyl allenoate 6 with NH4SCN in presence and absence of Selectfluor.
Comparative discussion
Collectively, the comparative product analysis studies described herein demonstrate that the efficacy of Selectfluor in promoting oxidative/electrophilic dihalogenation (with TMSX) and dithiocyanation (with NH4SCN), which were previously studied in reactions with 1-arylallenes [18], is notably diminished toward electron-deficient allenoates, whereby the nucleophilic conjugate addition effectively competes, resulting in mixtures of both types of products.
The electrophilic dihalogenation competes most effectively in the case of the TMSI/Selectfluor system leading to 2,3-diiodoalkenoates as the major products. By contrast, conjugate addition products are predominantly formed with TMSCl/Selectfluor and exclusively with NH4SCN/Selectfluor. These competing pathways are influenced by the nature of the anion, reflecting the ease of X− → “X+” oxidative transformation, and the choice of the solvent [25]. The increased chemoselectivity (and yields) toward formation of the 2,3-dihalogenation products observed with the methyl-substituted allenoates 3 and 5, especially in reactions with TMSI/Selectfluor (see Scheme 10), appears consistent with stabilization of the incipient allenyl cation in the oxidative/electrophilic pathway.
Scheme 10: Influence of allenoate structure.
Scheme 10: Influence of allenoate structure.
References
-
Nyffeler, P. T.; Durón, S. G.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648
Return to citation in text: [1] -
Singh, R. P.; Shreeve, J. M. Acc. Chem. Res. 2004, 37, 31–44. doi:10.1021/ar030043v
Return to citation in text: [1] -
Taylor, S. D.; Kotoris, C. C.; Hum, G. Tetrahedron 1999, 55, 12431–12477. doi:10.1016/S0040-4020(99)00748-6
Return to citation in text: [1] -
Banks, R. E. J. Fluorine Chem. 1998, 87, 1–17. doi:10.1016/S0022-1139(97)00127-9
Return to citation in text: [1] -
Stavber, S.; Zupan, M. N-Fluoro-1,4-Diazoniabicyclo[2.2.2]octane Dication Salts; Efficient Fluorinating Agents and Functionalization Mediators for Organic Compounds. In Advances in Organic Synthesis: Modern Organofluorine Chemistry-Synthetic Aspects; Atta-ur-Rahman, Ed.; Bentham: Hilversum, The Netherlands, 2006; Vol. 2, pp 213–268. doi:10.2174/978160805198410602010213
Return to citation in text: [1] -
Pavlinac, J.; Zupan, M.; Stavber, S. Molecules 2009, 14, 2394–2409. doi:10.3390/molecules14072394
Return to citation in text: [1] -
Laali, K. K.; Borodkin, G. I. J. Chem. Soc., Perkin Trans. 2 2002, 953–957. doi:10.1039/b111725d
Return to citation in text: [1] -
Baudequin, C.; Plaquevent, J.-C.; Audouard, C.; Cahard, D. Green Chem. 2002, 4, 584–586. doi:10.1039/b208817g
Return to citation in text: [1] -
Baudoux, J.; Salit, S.-F.; Cahard, D.; Plaquevent, J.-C. Tetrahedron Lett. 2002, 43, 6573–6574. doi:10.1016/S0040-4039(02)01417-X
Return to citation in text: [1] -
Heravi, M. R. P. J. Fluorine Chem. 2008, 129, 217–221. doi:10.1016/j.jfluchem.2007.11.006
Return to citation in text: [1] -
Stavber, S. Molecules 2011, 16, 6432–6464. doi:10.3390/molecules16086432
Return to citation in text: [1] -
Syvret, R. G.; Butt, K. M.; Nguyen, T. P.; Bulleck, V. L.; Rieth, R. D. J. Org. Chem. 2002, 67, 4487–4493. doi:10.1021/jo020053u
Return to citation in text: [1] -
Ye, C.; Shreeve, J. M. J. Org. Chem. 2004, 69, 8561–8563. doi:10.1021/jo048383x
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Reddy, Y. J. Chem. Lett. 2008, 37, 652–653. doi:10.1246/cl.2008.652
Return to citation in text: [1] -
Wu, D.; Yang, X.; Wu, L. J. Chem. Sci. 2012, 124, 901–905. doi:10.1007/s12039-012-0270-0
Return to citation in text: [1] -
Jin, Z.; Xu, B.; Hammond, G. B. Tetrahedron Lett. 2011, 52, 1956–1959. doi:10.1016/j.tetlet.2011.02.059
Return to citation in text: [1] [2] -
Jin, Z.; Xu, B.; DiMagno, S. G.; Hammond, G. B. J. Fluorine Chem. 2012, 143, 226–230. doi:10.1016/j.jfluchem.2012.05.010
Return to citation in text: [1] [2] -
Laali, K. K.; Nandi, G. C.; Bunge, S. D. Tetrahedron Lett. 2014, 55, 2401–2405. doi:10.1016/j.tetlet.2014.02.110
Return to citation in text: [1] [2] [3] -
Ma, S.; Xie, H.; Wang, G.; Zhang, J.; Shi, Z. Synthesis 2001, 713–730. doi:10.1055/s-2001-12759
Return to citation in text: [1] -
Ma, S.; Li, L. Synlett 2001, 1206–1213. doi:10.1055/s-2001-16032
Return to citation in text: [1] -
Ma, S.-M.; Wang, G.-W. Chin. J. Chem. 1999, 17, 545–549. doi:10.1002/cjoc.19990170517
Return to citation in text: [1] -
Martin, T. J.; Vakhshori, V. G.; Tran, Y. S.; Kwon, O. Org. Lett. 2011, 13, 2586–2589. doi:10.1021/ol200697m
Return to citation in text: [1] -
Guan, X.-Y.; Wei, Y.; Shi, M. Eur. J. Org. Chem. 2011, 2673–2677. doi:10.1002/ejoc.201100095
and related references cited there.
Return to citation in text: [1] -
Huang, X.; Shen, R.; Zhang, T. J. Org. Chem. 2007, 72, 1534–1537. doi:10.1021/jo062376m
Return to citation in text: [1] -
Reagents employed in previous studies of nucleophilic addition reactions of electron-deficient allenes were typically MX and protic solvents (but also MX/THF) (see [19-21]), as well as phenols and thiols (in [22,23]). Hydroazidation was affected with NaN3 in t-BuOH/H2O (in [23]). In the present study adventitious moisture or traces of water in MeCN or DMF, or in the ionic liquids can either directly or via hydrolysis of TMSX produce the needed H+ to complete the conjugate addition reactions.
Return to citation in text: [1]
| 23. |
Guan, X.-Y.; Wei, Y.; Shi, M. Eur. J. Org. Chem. 2011, 2673–2677. doi:10.1002/ejoc.201100095
and related references cited there. |
| 1. | Nyffeler, P. T.; Durón, S. G.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648 |
| 2. | Singh, R. P.; Shreeve, J. M. Acc. Chem. Res. 2004, 37, 31–44. doi:10.1021/ar030043v |
| 3. | Taylor, S. D.; Kotoris, C. C.; Hum, G. Tetrahedron 1999, 55, 12431–12477. doi:10.1016/S0040-4020(99)00748-6 |
| 4. | Banks, R. E. J. Fluorine Chem. 1998, 87, 1–17. doi:10.1016/S0022-1139(97)00127-9 |
| 5. | Stavber, S.; Zupan, M. N-Fluoro-1,4-Diazoniabicyclo[2.2.2]octane Dication Salts; Efficient Fluorinating Agents and Functionalization Mediators for Organic Compounds. In Advances in Organic Synthesis: Modern Organofluorine Chemistry-Synthetic Aspects; Atta-ur-Rahman, Ed.; Bentham: Hilversum, The Netherlands, 2006; Vol. 2, pp 213–268. doi:10.2174/978160805198410602010213 |
| 6. | Pavlinac, J.; Zupan, M.; Stavber, S. Molecules 2009, 14, 2394–2409. doi:10.3390/molecules14072394 |
| 7. | Laali, K. K.; Borodkin, G. I. J. Chem. Soc., Perkin Trans. 2 2002, 953–957. doi:10.1039/b111725d |
| 8. | Baudequin, C.; Plaquevent, J.-C.; Audouard, C.; Cahard, D. Green Chem. 2002, 4, 584–586. doi:10.1039/b208817g |
| 9. | Baudoux, J.; Salit, S.-F.; Cahard, D.; Plaquevent, J.-C. Tetrahedron Lett. 2002, 43, 6573–6574. doi:10.1016/S0040-4039(02)01417-X |
| 10. | Heravi, M. R. P. J. Fluorine Chem. 2008, 129, 217–221. doi:10.1016/j.jfluchem.2007.11.006 |
| 14. | Yadav, J. S.; Reddy, B. V. S.; Reddy, Y. J. Chem. Lett. 2008, 37, 652–653. doi:10.1246/cl.2008.652 |
| 15. | Wu, D.; Yang, X.; Wu, L. J. Chem. Sci. 2012, 124, 901–905. doi:10.1007/s12039-012-0270-0 |
| 19. | Ma, S.; Xie, H.; Wang, G.; Zhang, J.; Shi, Z. Synthesis 2001, 713–730. doi:10.1055/s-2001-12759 |
| 20. | Ma, S.; Li, L. Synlett 2001, 1206–1213. doi:10.1055/s-2001-16032 |
| 21. | Ma, S.-M.; Wang, G.-W. Chin. J. Chem. 1999, 17, 545–549. doi:10.1002/cjoc.19990170517 |
| 13. | Ye, C.; Shreeve, J. M. J. Org. Chem. 2004, 69, 8561–8563. doi:10.1021/jo048383x |
| 22. | Martin, T. J.; Vakhshori, V. G.; Tran, Y. S.; Kwon, O. Org. Lett. 2011, 13, 2586–2589. doi:10.1021/ol200697m |
| 23. |
Guan, X.-Y.; Wei, Y.; Shi, M. Eur. J. Org. Chem. 2011, 2673–2677. doi:10.1002/ejoc.201100095
and related references cited there. |
| 12. | Syvret, R. G.; Butt, K. M.; Nguyen, T. P.; Bulleck, V. L.; Rieth, R. D. J. Org. Chem. 2002, 67, 4487–4493. doi:10.1021/jo020053u |
| 18. | Laali, K. K.; Nandi, G. C.; Bunge, S. D. Tetrahedron Lett. 2014, 55, 2401–2405. doi:10.1016/j.tetlet.2014.02.110 |
| 25. | Reagents employed in previous studies of nucleophilic addition reactions of electron-deficient allenes were typically MX and protic solvents (but also MX/THF) (see [19-21]), as well as phenols and thiols (in [22,23]). Hydroazidation was affected with NaN3 in t-BuOH/H2O (in [23]). In the present study adventitious moisture or traces of water in MeCN or DMF, or in the ionic liquids can either directly or via hydrolysis of TMSX produce the needed H+ to complete the conjugate addition reactions. |
| 22. | Martin, T. J.; Vakhshori, V. G.; Tran, Y. S.; Kwon, O. Org. Lett. 2011, 13, 2586–2589. doi:10.1021/ol200697m |
| 23. |
Guan, X.-Y.; Wei, Y.; Shi, M. Eur. J. Org. Chem. 2011, 2673–2677. doi:10.1002/ejoc.201100095
and related references cited there. |
| 16. | Jin, Z.; Xu, B.; Hammond, G. B. Tetrahedron Lett. 2011, 52, 1956–1959. doi:10.1016/j.tetlet.2011.02.059 |
| 17. | Jin, Z.; Xu, B.; DiMagno, S. G.; Hammond, G. B. J. Fluorine Chem. 2012, 143, 226–230. doi:10.1016/j.jfluchem.2012.05.010 |
| 19. | Ma, S.; Xie, H.; Wang, G.; Zhang, J.; Shi, Z. Synthesis 2001, 713–730. doi:10.1055/s-2001-12759 |
| 20. | Ma, S.; Li, L. Synlett 2001, 1206–1213. doi:10.1055/s-2001-16032 |
| 21. | Ma, S.-M.; Wang, G.-W. Chin. J. Chem. 1999, 17, 545–549. doi:10.1002/cjoc.19990170517 |
| 18. | Laali, K. K.; Nandi, G. C.; Bunge, S. D. Tetrahedron Lett. 2014, 55, 2401–2405. doi:10.1016/j.tetlet.2014.02.110 |
| 18. | Laali, K. K.; Nandi, G. C.; Bunge, S. D. Tetrahedron Lett. 2014, 55, 2401–2405. doi:10.1016/j.tetlet.2014.02.110 |
| 16. | Jin, Z.; Xu, B.; Hammond, G. B. Tetrahedron Lett. 2011, 52, 1956–1959. doi:10.1016/j.tetlet.2011.02.059 |
| 17. | Jin, Z.; Xu, B.; DiMagno, S. G.; Hammond, G. B. J. Fluorine Chem. 2012, 143, 226–230. doi:10.1016/j.jfluchem.2012.05.010 |
| 24. | Huang, X.; Shen, R.; Zhang, T. J. Org. Chem. 2007, 72, 1534–1537. doi:10.1021/jo062376m |
© 2015 Reddy and Laali; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)