Abstract
A new palladium-catalyzed picolinamide (PA)-directed ortho-iodination reaction of ε-C(sp2)−H bonds of γ-arylpropylamine substrates is reported. This reaction proceeds selectively with a variety of γ-arylpropylamines bearing strongly electron-donating or withdrawing substituents, complementing our previously reported PA-directed electrophilic aromatic substitution approach to this transformation. As demonstrated herein, a three step sequence of Pd-catalyzed γ-C(sp3)−H arylation, Pd-catalyzed ε-C(sp2)−H iodination, and Cu-catalyzed C−N cyclization enables a streamlined synthesis of tetrahydroquinolines bearing diverse substitution patterns.
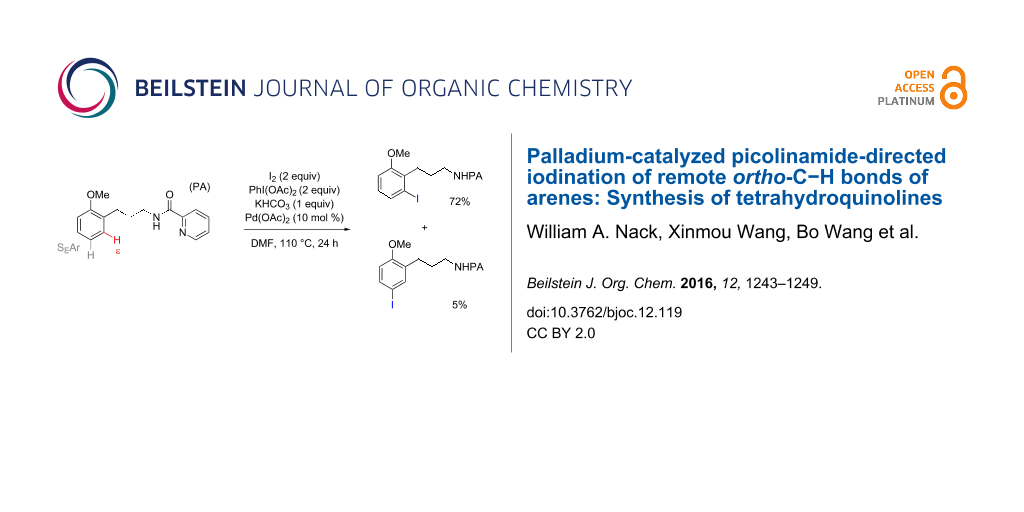
Graphical Abstract
Introduction
Tetrahydroquinoline (THQ) is an important N-heterocyclic scaffold found in many natural products and pharmaceutical agents [1,2]. Efficient and generally applicable methods for the synthesis of THQs with complex substitution patterns are still in great demand [3-7]. Recently, we reported a synthetic strategy for THQs based on picolinamide (PA)-directed sequential C−H functionalization reactions starting from readily accessible aryl iodide and alkylamine precursors (Scheme 1) [8]. Alkylpicolinamides were first subjected to Pd-catalyzed γ-C(sp3)−H arylation with aryl iodides to form γ-arylpropylpicolinamides [9-20]. These γ-arylpropylpicolinamides were then selectively iodinated at the remote ε-C(sp2)−H position via a rarely precedented PA-directed electrophilic aromatic substitution (SEAr) reaction (Scheme 1, reaction 2) [21,22]. Copper-catalyzed intramolecular C−N cyclization of these ortho-iodinated intermediates provided PA-coupled THQ products in good yields.
Scheme 1: New synthetic strategy for THQs via PA-directed C−H functionalization.
Scheme 1: New synthetic strategy for THQs via PA-directed C−H functionalization.
Although ε-C−H iodination via directed SEAr proceeds with excellent yield and mono-selectivity for many γ-arylpropylpicolinamides, the scope of these PA-directed SEAr reactions is limited to arenes bearing moderate electron-donating or withdrawing groups. Arene substrates bearing strongly electron-donating substituents typically gave substantial amounts of undesired iodinated side products via competing innate SEAr processes, and arene substrates bearing strongly electron-withdrawing substituents were often unreactive. Herein, we report our development of a Pd-catalyzed PA-directed iodination reaction of ε-C(sp2)−H bonds of γ-arylpropylpicolinamides. This Pd-catalyzed reaction is complementary in scope to the directed SEAr iodination approach and allows for the efficient synthesis of a broad range of THQs with diverse substitution patterns.
Results and Discussion
Methods for metal-catalyzed halogenation of ortho C−H bonds at the more remote ε position are scarce, in contrast to the large number of ortho C−H halogenation reactions of arenes effected by more proximal directing groups [23-33]. Fundamentally, it is challenging to achieve efficient reactions through kinetically unfavorable seven-membered palladacycle intermediates. Furthermore, the electrophilic reagents used for C–H halogenation can often react with arenes through undirected SEAr pathways, which need to be suppressed for regioselectivity. To address this issue upfront, we commenced our study of Pd-catalyzed ε-C−H halogenation with 3-arylpropylpicolinamide 5 bearing a strongly electron-donating OMe group (Table 1, see Supporting Information File 1 for the preparation of 5). Iodination of 5 under our previous SEAr protocol gave undirected iodination product 7 as the major product; only a trace amount of ortho-iodination product 6 was detected (Table 1, entries 1 and 2). Iodination of 5 under a variety of Pd-catalyzed oxidative conditions gave either low conversion or poor regioselectivity (Table 1, entries 3–5). To our delight, the use of a combination of 2 equiv of I2 and 2 equiv of PhI(OAc)2 in DMF at 110 °C gave the desired product 6 in good yield and moderate selectivity. Similar conditions were reported by Yu to effect the Pd-catalyzed NHTf-directed iodination of δ-C(sp2)−H bonds of β-phenylethyl triflamides [33]. IOAc generated in situ is believed to be the active iodinating species. DMF was found to be the best solvent for this reaction (Table 1, entry 9 vs 11 and 12). Moreover, we found that the choice of alkali carbonate base was important: replacing K2CO3 with KHCO3 or Na2CO3 gave notably improved yields and ortho selectivity (Table 1, entries 9 and 10) [34,35]. By analogy with similar Pd-catalyzed directed C–H halogenation reactions, we speculate that the catalytic cycle follows a sequence of C−H palladation, oxidative addition and reductive elimination [36,37].
Table 1: Optimization of Pd-catalyzed ortho C−H iodination of 5.a
|
|
|||||
| entry | reagents (equiv) | solvent | temperature (°C) | yield (%)b | |
|---|---|---|---|---|---|
| 6 | 7 | ||||
| 1 | NIS (1.5), HBF4·EtO2 (4.0) | T/Dc | 0 | <2 | 68 |
| 2 | NIS (1.5) | T/D | 0 | <2 | 82 |
| 3 | Pd(OAc)2 (10 mol %), NIS (1.5) | chlorobenzene | 110 | <2 | 74 |
| 4 | Pd(OAc)2 (10 mol %), NaI (1.5), NaIO3 (1.5), K2S2O8 (2.0) | n-BuOH | 110 | <2 | <2 |
| 5 | Pd(OAc)2 (10 mol %), I2 (2.0), K2S2O8 (2) | DMF | 110 | <2 | 60 |
| 6 | Pd(OAc)2 (10 mol %), I2 (2.0), PhI(OAc)2 (2.0) | DMF | 110 | 43 | 25 |
| 7 | Pd(OAc)2 (10 mol %), I2 (2.0), PhI(OAc)2 (2.0), K2CO3 (1.0) | DMF | 110 | 14 | 11 |
| 8 | Pd(OAc)2 (10 mol %), I2 (2.0), PhI(OAc)2 (2.0), KHCO3 (2.0) | DMF | 110 | 45 | 12 |
| 9 | Pd(OAc)2 (10 mol %), I2 (2.0), PhI(OAc)2 (2.0), KHCO3 (1.0) | DMF | 110 |
75
(72)d |
9
(5)d |
| 10 | Pd(OAc)2 (10 mol %), I2 (2.0), PhI(OAc)2 (2.0), Na2CO3 (1.0) | DMF | 110 | 80 | 8 |
| 11 | Pd(OAc)2 (10 mol %), I2 (2.0), PhI(OAc)2 (2.0), KHCO3 (1.0) | dichloroethane | 110 | 16 | 58 |
| 12 | Pd(OAc)2 (10 mol %), I2 (2.0), PhI(OAc)2 (2.0), KHCO3 (1.0) | dioxane | 110 | 13 | 65 |
| 13 | I2 (2.0), PhI(OAc)2 (2.0), KHCO3 (1.0) | DMF | 110 | <2 | 64 |
aAll screening reactions were carried out in a 10 mL glass vial on a 0.2 mmol scale: bYields are based on 1H NMR analysis of the reaction mixture using CH2Br2 as internal standard; cT/D: TFA (T)/CH2Cl2 (D); disolated yield.
With the best conditions in hand (Table 1, entries 9 and 10), we then examined the substrate scope of this Pd-catalyzed iodination of γ-arylpropylpicolinamides (Table 2). The γ-arylpropylpicolinamides were prepared from the corresponding N-alkylpicolinamides and aryl iodides under our (BnO)2PO2H-promoted Pd-catalyzed γ-C(sp3)−H arylation conditions (see Supporting Information File 1 for details). The substrate scope was chosen to complement the SEAr method, which is notably incompatible with NO2, F and OMe substituents. In contrast to the mono-selectivity of the directed SEAr approach (reaction 2, Scheme 1), iodination of γ-phenylpropylpicolinamide 2 bearing two equivalent ortho C−H bonds under Pd-catalyzed conditions A gave a mixture of mono-iodinated 3 and ortho diiodinated product 4. However, no para-iodinated side product was formed. With 4 equiv of PhI(OAc)2/I2 and 1 equiv of KHCO3, 4 can be formed as the major product in 69% yield.
Table 2: Substrate scope of Pd-catalyzed ε-C−H iodination and Cu-catalyzed C−N cyclization to form THQsa.
|
|
|||
| C–H arylationb | iodination | C–N cyclization | |
|---|---|---|---|
| Pd catalyzed | directed SEAr | ||
|
2 (67%) |
3 (mono-I, 47%) + 4 (di-I, 25%)c |
3 (76%)
+ 4 (6%) |
8 (93%) |
|
9 (81%) |
10 (75%) |
10 (60%)
(o/x = 5:3)c |
11 (96%) |
|
12 (81%) |
13 (68%) |
13 (50%)
(o/x = 5:4)c |
14 (94%) |
|
15 (28%) |
16 (68%) |
NR |
17 (47%) |
|
18 (60%) |
19 (56%) |
19 (20%)
(o/x = 1:4)c |
20 (85%) |
|
21 (95%) |
22 (53% or 85%d) |
23 (90%) X-ray |
24 (78%) |
aYields are based on isolated product on a 0.2 mmol scale; bsee reaction 1 in Scheme 1B for conditions for Pd-catalyzed C−H arylation; cdi: ortho-diiodinated isomer, x: mixture of other iodinated isomers; dconditions B: I2 (2 equiv), PhI(OAc)2 (2 equiv), Pd(OAc)2 (10 mol %), Na2CO3 (1 equiv), DMF, 110 °C, 24 h.
Arenes bearing meta-substituents (e.g., 12) were selectively iodinated at the less hindered ortho position. Pd-catalyzed iodination of substrate 15 bearing a strongly electron-withdrawing NO2 group also proceeded smoothly to give 16; this substrate is unreactive to directed SEAr. The rigid arylnorbornane scaffold 18 is incompatible with directed SEAr, but was iodinated selectively at the ortho position under Pd-catalyzed conditions without the formation of regioisomeric side products. The strong para-directing effect exerted by aryl fluoride substituents overrides directed SEAr selectivity [38,39]. Thus, we observed only para-iodinated compound 23 when 21 was subjected to the directed SEAr protocol. In contrast, using our Pd-catalyzed iodination (conditions B), ortho-iodinated product 22 was obtained via Pd-catalyzed iodination as the only product in excellent yield. The iodinated intermediates could be readily cyclized under our previously reported Cu-catalyzed conditions to give PA-coupled THQ products with various substitution patterns in good yields (Scheme 2) [8].
Scheme 2: Preparation of iodo-substituted THQs via PA-directed C−H functionalization strategy. a) ArI (2 equiv), Pd(OAc)2 (10 mol %), (BnO)2PO2H (20 mol %), Ag2CO3 (1.5 equiv), t-AmylOH, 110 °C, 24h; b) Pd(OAc)2 (10 mol %), I2 (4 equiv), PhI(OAc)2 (4 equiv), KHCO3 (1 equiv), 130 °C, DMF, 24 h; c) NIS (1.1 equiv), HBF4.OEt2 (4), TFA/DCM (1:9), 2.5 mM, 0 °C, 4 h; d) CuI (10 mol %), CsOAc (2.5 equiv), DMSO, Ar, 90 °C, 20 h; e) NIS (1.1 equiv), TFA/DCM (1:9), 2.5 mM, rt, 16 h; f) Pd(OAc)2 (15 mol %), NIS (2.5 equiv), α,α,α-trifluorotoluene, Ar, 100 °C, 24 h.
Scheme 2: Preparation of iodo-substituted THQs via PA-directed C−H functionalization strategy. a) ArI (2 equi...
As shown in Scheme 2, Pd-catalyzed PA-directed ε-C−H iodination can be used in concert with PA-directed γ-C−H arylation, PA-directed SEAr iodination, and undirected SEAr iodination to quickly access THQs 27–30 bearing iodo groups at different positions on the arene ring [40-42]. Ortho-diiodinated product 4 was obtained from 2 in 69% yield using optimized Pd-catalyzed ε-C–H iodination conditions, and Cu-catalyzed C–N cyclization of 4 gave 5-iodo-THQ 27. PA-THQ 8 was susceptible to iodination at two positions. Under undirected SEAr conditions, 6-iodo-THQ 28 was produced in excellent yield and regioselectivity. Alternatively, a Pd-catalyzed C–H iodination reaction of 8 was developed which provides 8-iodo-THQ 29. Pd-catalyzed C−H arylation of 1 with para-diiodobenzene under the standard arylation conditions gave 25 in moderate yield. Iodination of 25 via PA-directed SEAr gave diiodinated compound 26, which was cyclized under Cu catalysis to give 7-iodo-THQ 30 in good yield. The PA group of 8-iodo-THQ 29 was readily removed with LiBHEt3 to give 31 (Scheme 3) [10].
Scheme 3: Removal of PA auxiliary from THQ product.
Scheme 3: Removal of PA auxiliary from THQ product.
Conclusion
In summary, we have developed a new palladium-catalyzed picolinamide (PA)-directed iodination reaction of ε-C(sp2)−H bonds of γ-arylpropylamine substrates. This method works well for arenes with a broad range of substituents and offers a complementary scope to our previously reported PA-directed SEAr approach. This Pd-catalyzed PA-directed ε-C−H iodination can be used in concert with the PA-directed γ-C−H arylation, PA-directed SEAr iodination, undirected SEAr iodination, and Cu-catalyzed C−N cyclization to quickly access tetrahydroquinolines bearing diverse substitution patterns from readily accessible starting materials.
Supporting Information
| Supporting Information File 1: Detailed synthetic procedures and characterizations of all new compounds. | ||
| Format: PDF | Size: 5.6 MB | Download |
References
-
Katritzky, A. R.; Rachwal, S.; Rachwal, B. Tetrahedron 1996, 51, 15031. doi:10.1016/S0040-4020(96)00911-8
Return to citation in text: [1] -
Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157. doi:10.1021/cr100307m
Return to citation in text: [1] -
Akiyama, T.; Morita, H.; Fuchibe, K. J. Am. Chem. Soc. 2006, 128, 13070. doi:10.1021/ja064676r
Return to citation in text: [1] -
Liu, H.; Dagousset, G.; Masson, G.; Retailleau, P.; Zhu, J. J. Am. Chem. Soc. 2009, 131, 4598. doi:10.1021/ja900806q
Return to citation in text: [1] -
Han, Z.-Y.; Xiao, H.; Chen, X.-H.; Gong, L.-Z. J. Am. Chem. Soc. 2009, 131, 9182. doi:10.1021/ja903547q
Return to citation in text: [1] -
Rousseaux, S.; Liégault, B.; Fagnou, K. Chem. Sci. 2012, 3, 244. doi:10.1039/C1SC00458A
Return to citation in text: [1] -
Saget, T.; Cramer, N. Angew. Chem., Int. Ed. 2012, 51, 12842. doi:10.1002/anie.201207959
Return to citation in text: [1] -
Nack, W. A.; He, G.; Zhang, S.-Y.; Chen, G. Org. Lett. 2013, 15, 3440. doi:10.1021/ol4015078
Return to citation in text: [1] [2] -
Zaitsev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127, 13154. doi:10.1021/ja054549f
Return to citation in text: [1] -
Nadres, E. T.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 7. doi:10.1021/ja210959p
Return to citation in text: [1] [2] -
Nadres, E. T.; Santos, G. I. F.; Shabashov, D.; Daugulis, O. J. Org. Chem. 2013, 78, 9689. doi:10.1021/jo4013628
Return to citation in text: [1] -
He, G.; Chen, G. Angew. Chem., Int. Ed. 2011, 50, 5192. doi:10.1002/anie.201100984
Return to citation in text: [1] -
Zhao, Y.; Chen, G. Org. Lett. 2011, 13, 4850. doi:10.1021/ol201930e
Return to citation in text: [1] -
He, G.; Zhao, Y.; Zhang, S.; Lu, C.; Chen, G. J. Am. Chem. Soc. 2012, 134, 3. doi:10.1021/ja210660g
Return to citation in text: [1] -
Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313. doi:10.1021/ja3023972
Return to citation in text: [1] -
He, G.; Lu, C.; Zhao, Y.; Nack, W. A.; Chen, G. Org. Lett. 2012, 14, 2944. doi:10.1021/ol301352v
Return to citation in text: [1] -
Zhao, Y.; He, G.; Nack, W. A.; Chen, G. Org. Lett. 2012, 14, 2948. doi:10.1021/ol301214u
Return to citation in text: [1] -
Zhang, S.-Y.; He, G.; Nack, W. A.; Zhao, Y.; Li, Q.; Chen, G. J. Am. Chem. Soc. 2013, 135, 2124. doi:10.1021/ja312277g
Return to citation in text: [1] -
Roman, D. S.; Charette, A. B. Org. Lett. 2013, 15, 4394. doi:10.1021/ol401931s
Return to citation in text: [1] -
Huang, L.; Li, Q.; Wang, C.; Qi, C. J. Org. Chem. 2013, 78, 3030. doi:10.1021/jo400017v
Return to citation in text: [1] -
Barluenga, J.; Álvarez-Gutiérrez, J. M.; Ballesteros, A.; González, J. M. Angew. Chem., Int. Ed. 2007, 46, 1281. doi:10.1002/anie.200603631
Return to citation in text: [1] -
Espuña, G.; Arsequell, G.; Valencia, G.; Barluenga, J.; Álvarez-Gutiérrez, J. M.; Ballesteros, A.; González, J. M. Angew. Chem., Int. Ed. 2004, 43, 325. doi:10.1002/anie.200352464
Return to citation in text: [1] -
Lu, C.; Zhang, S.-Y.; He, G.; Nack, W. A.; Chen, G. Tetrahedron 2014, 70, 4197. doi:10.1016/j.tet.2014.02.070
See for our recent report on Pd-catalyzed PA-directed ortho C−H halogenation of benzylamine substrates.
Return to citation in text: [1] -
Kalyani, D.; Dick, A. R.; Anani, W. Q.; Sanford, M. S. Tetrahedron 2006, 62, 11483. doi:10.1016/j.tet.2006.06.075
Return to citation in text: [1] -
Wan, X.; Ma, Z.; Li, B.; Zhang, K.; Cao, S.; Zhang, S.; Shi, Z. J. Am. Chem. Soc. 2006, 128, 7416. doi:10.1021/ja060232j
Return to citation in text: [1] -
Mei, T.-S.; Giri, R.; Maugel, N.; Yu, J.-Q. Angew. Chem., Int. Ed. 2008, 47, 5215. doi:10.1002/anie.200705613
Return to citation in text: [1] -
Mo, F.; Yan, J. M.; Qiu, D.; Li, F.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2010, 49, 2028. doi:10.1002/anie.200906699
Return to citation in text: [1] -
Schröder, N.; Wencel-Delord, J.; Glorius, F. J. Am. Chem. Soc. 2012, 134, 8298. doi:10.1021/ja302631j
Return to citation in text: [1] -
Hennings, D. D.; Iwasa, S.; Rawal, V. H. J. Org. Chem. 1997, 62, 2. doi:10.1021/jo961876k
Return to citation in text: [1] -
Leblanc, M.; Fagnou, K. Org. Lett. 2005, 7, 2849. doi:10.1021/ol0505959
Return to citation in text: [1] -
Li, J.-J.; Giri, R.; Yu, J.-Q. Tetrahedron 2008, 64, 6979. doi:10.1016/j.tet.2008.03.026
Return to citation in text: [1] -
Leow, D.; Li, G.; Mei, T.-S.; Yu, J.-Q. Nature 2012, 486, 518. doi:10.1038/nature11158
Return to citation in text: [1] -
Li, J.-J.; Mei, T.-S.; Yu, J.-Q. Angew. Chem., Int. Ed. 2008, 47, 6452. doi:10.1002/anie.200802187
Return to citation in text: [1] [2] -
Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094. doi:10.1002/anie.200806273
Return to citation in text: [1] -
Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147. doi:10.1021/cr900184e
Return to citation in text: [1] -
Zhang, X.-G.; Dai, H.-X.; Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2012, 134, 11948. doi:10.1021/ja305259n
Alkali cations (e.g., Na+, K+) might play a useful role in this PA-directed C–H functionalization reaction. See for mechanistic investigations on related reaction systems.
Return to citation in text: [1] -
Ano, Y.; Tobisu, M.; Chatani, N. J. Am. Chem. Soc. 2011, 133, 12984. doi:10.1021/ja206002m
Return to citation in text: [1] -
Ault, A. J. Chem. Educ. 1966, 43, 329. doi:10.1021/ed043p329
Return to citation in text: [1] -
Rosenthal, J.; Schuster, D. I. J. Chem. Educ. 2003, 80, 679. doi:10.1021/ed080p679
Return to citation in text: [1] -
Thansandote, P.; Lautens, M. Chem. – Eur. J. 2009, 15, 5874. doi:10.1002/chem.200900281
Return to citation in text: [1] -
Mei, T.-S.; Kou, L.; Ma, S.; Engle, K. M.; Yu, J.-Q. Synthesis 2012, 44, 1778. doi:10.1055/s-0031-1289766
Return to citation in text: [1] -
Nack, W. A.; Chen, G. Synlett 2015, 26, 2505. doi:10.1055/s-0034-1381051
Return to citation in text: [1]
| 1. | Katritzky, A. R.; Rachwal, S.; Rachwal, B. Tetrahedron 1996, 51, 15031. doi:10.1016/S0040-4020(96)00911-8 |
| 2. | Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157. doi:10.1021/cr100307m |
| 21. | Barluenga, J.; Álvarez-Gutiérrez, J. M.; Ballesteros, A.; González, J. M. Angew. Chem., Int. Ed. 2007, 46, 1281. doi:10.1002/anie.200603631 |
| 22. | Espuña, G.; Arsequell, G.; Valencia, G.; Barluenga, J.; Álvarez-Gutiérrez, J. M.; Ballesteros, A.; González, J. M. Angew. Chem., Int. Ed. 2004, 43, 325. doi:10.1002/anie.200352464 |
| 9. | Zaitsev, V. G.; Shabashov, D.; Daugulis, O. J. Am. Chem. Soc. 2005, 127, 13154. doi:10.1021/ja054549f |
| 10. | Nadres, E. T.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 7. doi:10.1021/ja210959p |
| 11. | Nadres, E. T.; Santos, G. I. F.; Shabashov, D.; Daugulis, O. J. Org. Chem. 2013, 78, 9689. doi:10.1021/jo4013628 |
| 12. | He, G.; Chen, G. Angew. Chem., Int. Ed. 2011, 50, 5192. doi:10.1002/anie.201100984 |
| 13. | Zhao, Y.; Chen, G. Org. Lett. 2011, 13, 4850. doi:10.1021/ol201930e |
| 14. | He, G.; Zhao, Y.; Zhang, S.; Lu, C.; Chen, G. J. Am. Chem. Soc. 2012, 134, 3. doi:10.1021/ja210660g |
| 15. | Zhang, S.-Y.; He, G.; Zhao, Y.; Wright, K.; Nack, W. A.; Chen, G. J. Am. Chem. Soc. 2012, 134, 7313. doi:10.1021/ja3023972 |
| 16. | He, G.; Lu, C.; Zhao, Y.; Nack, W. A.; Chen, G. Org. Lett. 2012, 14, 2944. doi:10.1021/ol301352v |
| 17. | Zhao, Y.; He, G.; Nack, W. A.; Chen, G. Org. Lett. 2012, 14, 2948. doi:10.1021/ol301214u |
| 18. | Zhang, S.-Y.; He, G.; Nack, W. A.; Zhao, Y.; Li, Q.; Chen, G. J. Am. Chem. Soc. 2013, 135, 2124. doi:10.1021/ja312277g |
| 19. | Roman, D. S.; Charette, A. B. Org. Lett. 2013, 15, 4394. doi:10.1021/ol401931s |
| 20. | Huang, L.; Li, Q.; Wang, C.; Qi, C. J. Org. Chem. 2013, 78, 3030. doi:10.1021/jo400017v |
| 8. | Nack, W. A.; He, G.; Zhang, S.-Y.; Chen, G. Org. Lett. 2013, 15, 3440. doi:10.1021/ol4015078 |
| 10. | Nadres, E. T.; Daugulis, O. J. Am. Chem. Soc. 2012, 134, 7. doi:10.1021/ja210959p |
| 3. | Akiyama, T.; Morita, H.; Fuchibe, K. J. Am. Chem. Soc. 2006, 128, 13070. doi:10.1021/ja064676r |
| 4. | Liu, H.; Dagousset, G.; Masson, G.; Retailleau, P.; Zhu, J. J. Am. Chem. Soc. 2009, 131, 4598. doi:10.1021/ja900806q |
| 5. | Han, Z.-Y.; Xiao, H.; Chen, X.-H.; Gong, L.-Z. J. Am. Chem. Soc. 2009, 131, 9182. doi:10.1021/ja903547q |
| 6. | Rousseaux, S.; Liégault, B.; Fagnou, K. Chem. Sci. 2012, 3, 244. doi:10.1039/C1SC00458A |
| 7. | Saget, T.; Cramer, N. Angew. Chem., Int. Ed. 2012, 51, 12842. doi:10.1002/anie.201207959 |
| 36. |
Zhang, X.-G.; Dai, H.-X.; Wasa, M.; Yu, J.-Q. J. Am. Chem. Soc. 2012, 134, 11948. doi:10.1021/ja305259n
Alkali cations (e.g., Na+, K+) might play a useful role in this PA-directed C–H functionalization reaction. See for mechanistic investigations on related reaction systems. |
| 37. | Ano, Y.; Tobisu, M.; Chatani, N. J. Am. Chem. Soc. 2011, 133, 12984. doi:10.1021/ja206002m |
| 8. | Nack, W. A.; He, G.; Zhang, S.-Y.; Chen, G. Org. Lett. 2013, 15, 3440. doi:10.1021/ol4015078 |
| 34. | Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094. doi:10.1002/anie.200806273 |
| 35. | Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147. doi:10.1021/cr900184e |
| 40. | Thansandote, P.; Lautens, M. Chem. – Eur. J. 2009, 15, 5874. doi:10.1002/chem.200900281 |
| 41. | Mei, T.-S.; Kou, L.; Ma, S.; Engle, K. M.; Yu, J.-Q. Synthesis 2012, 44, 1778. doi:10.1055/s-0031-1289766 |
| 42. | Nack, W. A.; Chen, G. Synlett 2015, 26, 2505. doi:10.1055/s-0034-1381051 |
| 33. | Li, J.-J.; Mei, T.-S.; Yu, J.-Q. Angew. Chem., Int. Ed. 2008, 47, 6452. doi:10.1002/anie.200802187 |
| 23. |
Lu, C.; Zhang, S.-Y.; He, G.; Nack, W. A.; Chen, G. Tetrahedron 2014, 70, 4197. doi:10.1016/j.tet.2014.02.070
See for our recent report on Pd-catalyzed PA-directed ortho C−H halogenation of benzylamine substrates. |
| 24. | Kalyani, D.; Dick, A. R.; Anani, W. Q.; Sanford, M. S. Tetrahedron 2006, 62, 11483. doi:10.1016/j.tet.2006.06.075 |
| 25. | Wan, X.; Ma, Z.; Li, B.; Zhang, K.; Cao, S.; Zhang, S.; Shi, Z. J. Am. Chem. Soc. 2006, 128, 7416. doi:10.1021/ja060232j |
| 26. | Mei, T.-S.; Giri, R.; Maugel, N.; Yu, J.-Q. Angew. Chem., Int. Ed. 2008, 47, 5215. doi:10.1002/anie.200705613 |
| 27. | Mo, F.; Yan, J. M.; Qiu, D.; Li, F.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2010, 49, 2028. doi:10.1002/anie.200906699 |
| 28. | Schröder, N.; Wencel-Delord, J.; Glorius, F. J. Am. Chem. Soc. 2012, 134, 8298. doi:10.1021/ja302631j |
| 29. | Hennings, D. D.; Iwasa, S.; Rawal, V. H. J. Org. Chem. 1997, 62, 2. doi:10.1021/jo961876k |
| 30. | Leblanc, M.; Fagnou, K. Org. Lett. 2005, 7, 2849. doi:10.1021/ol0505959 |
| 31. | Li, J.-J.; Giri, R.; Yu, J.-Q. Tetrahedron 2008, 64, 6979. doi:10.1016/j.tet.2008.03.026 |
| 32. | Leow, D.; Li, G.; Mei, T.-S.; Yu, J.-Q. Nature 2012, 486, 518. doi:10.1038/nature11158 |
| 33. | Li, J.-J.; Mei, T.-S.; Yu, J.-Q. Angew. Chem., Int. Ed. 2008, 47, 6452. doi:10.1002/anie.200802187 |
| 38. | Ault, A. J. Chem. Educ. 1966, 43, 329. doi:10.1021/ed043p329 |
| 39. | Rosenthal, J.; Schuster, D. I. J. Chem. Educ. 2003, 80, 679. doi:10.1021/ed080p679 |
© 2016 Nack et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)