Abstract
The mechanism of the thermal cyclization of enyne-carbodiimides 7a–c has been studied computationally by applying the DFT method. The results indicate that enyne-carbodiimides preferentially follow the C2–C6 (Schmittel) cyclization pathway in a concerted fashion although the Myers–Saito diradical formation is kinetically preferred. The experimentally verified preference of the C2–C6 over the Myers–Saito pathway is guided by the inability of the Myers–Saito diradical to kinetically compete in the rate-determining trapping reactions, either inter- or intramolecular, with the concerted C2–C6 cyclization. As demonstrated with enyne-carbodiimide 11, the Myers–Saito channel can be made the preferred pathway if the trapping reaction by hydrogen transfer is no more rate determining.
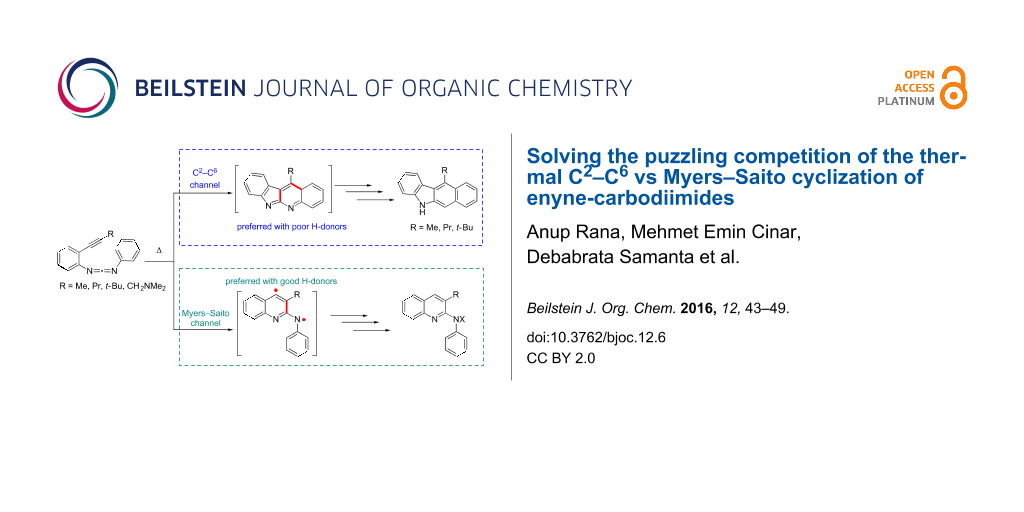
Graphical Abstract
Introduction
The thermal cyclizations of enediynes [1-6], enediallenes [7-10], bisallenes [11], enyne-allenes [12-17], and enyne-carbodiimides [18-25] have received great interest over the last two decades due to their value for organic synthesis and their interesting mechanistic facets [26]. Like for enyne-allenes, the cyclization mode of enyne-carbodiimides 1 (Scheme 1) can be switched between C2–C6 and Myers–Saito reaction channels depending on the substitution at the alkyne terminus, as disclosed by Schmittel [18] and Wang [19]. In 1998, Schmittel et al. [18] reported that the thermolysis of enyne-carbodiimide 1a in toluene/1,4-cyclohexadiene yielded the Myers–Saito product 3a, whereas the thermolysis of enyne-carbodiimide 1b produced the C2–C6 product 6b. In 1999 Wang et al. [19] stated that the thermal cyclization of enyne-carbodiimides 1c furnished C2–C6 (Schmittel) products 6c in p-xylene under reflux conditions. Based on the experimental findings it was postulated that the cyclization mode was shifted to the C2–C6 channel if more sterically demanding substituents than hydrogen were attached to the alkyne terminus, because the resulting strong repulsion of ortho-substituents in diradical 2b,c should disfavor the Myers–Saito pathway. At that time, diradical intermediates were invoked for both pathways based on analogy and computational results [18]. To obtain a deeper understanding of this switch in selectivity, we decided to amalgamate computations and new mechanistic experiments to resolve the puzzle.
Scheme 1: Thermal cyclization modes of enyne-carbodiimides 1a–c.
Scheme 1: Thermal cyclization modes of enyne-carbodiimides 1a–c.
For the computational part of this study, we chose first to interrogate enyne-carbodiimides 7a–c (Scheme 2), the synthesis and thermolysis of which have been reported by Wang and coworkers [19]. The methyl (7a) and tert-butyl groups (7b) at the alkyne terminus are ideal to vary the steric influence and the n-propyl group in 7c offers a realistic option for an intramolecular γ-hydrogen abstraction via a six-membered transition state if the diradical is thermally generated. From the computations (vide infra) we conclude that the thermal cyclization of enyne-carbodiimides 7 follows a concerted mechanism along the C2–C6 (Schmittel) channel and the decision between the two reaction channels for 7 is not necessarily guided by the free activation energy of the two initial steps, but by the inability of the Myers–Saito diradical to compete in follow-up reactions, either inter- or intramolecular, with the C2–C6 (Schmittel) channel.
Scheme 2: Thermolysis of enyne-carbodiimides 7a–c furnishing 8a–c [19].
Scheme 2: Thermolysis of enyne-carbodiimides 7a–c furnishing 8a–c [19].
Results and Discussion
The proper description of organic reactions involving diradical intermediates and transition state structures remains a difficult task in quantum chemistry [27-35]. Singlet diradicals have two electronic configurations that can only be treated accurately by costly multi-reference techniques. An alternative is to apply the unrestricted broken symmetry (U–BS) density functional theory (DFT) approach, which is often in use for larger systems due to its good results [36-49]. Hence, we applied the U–BS DFT approach for the diradical species and the transition states (TSs) connecting them while the restricted method was used for closed shell species. The BLYP functional was chosen for computation because the benchmark studies done by Schreiner showed good applicability of BLYP for the enyne-allene system [3]. Because nitrogen atoms are present in the cumulene core, the enyne-carbodiimide system is expected to be polar and that is why the 6-31+G* basis set was used. All computations were performed in the Gaussian 09 [50] program package. As the reactions take place in a nonpolar solvent (p-xylene), calculations were carried out in vacuum. The nature of all the stationary points was confirmed by frequency analysis. The intrinsic reaction coordinate (IRC) was followed in mass-scaled Cartesian coordinates to validate the TSs. The stability of the wave functions was tested with the stable=opt keyword.
In the computations, we were unable to locate any diradical intermediate along the C2–C6 (Schmittel) channel. Rather the results indicate that all enyne-carbodiimides 7a–c follow a concerted intramolecular Diels–Alder (DA) pathway toward the DA products 9DA (Figure 1), which subsequently rearrange to the isolated final products 8a–c thus avoiding a C2–C6 diradical mechanism. As expected due to the strong steric shielding about the tert-butyl group, enyne-carbodiimide 7b exhibits the highest free energy barrier, i.e., 37.8 kcal mol−1 at 138 °C (= the reaction temperature). The free energies of activation for enyne-carbodiimides 7a and 7c are significantly lower at 32.2 and 32.8 kcal mol−1, respectively. Disturbingly, though, the computations predict in all cases a strong kinetic preference for the Myers–Saito (Figure 1) over the concerted C2–C6 (DA) pathway. The C2–C6 Diels–Alder TS 7a_TSDA is 3.8 kcal mol−1 higher in energy than the corresponding Myers–Saito TS 7a_TSMS, while the difference is 4.8 and 4.3 kcal mol−1 for 7b and 7c, respectively. Thus, at this point, the computational results completely contradict the experimental findings. One plausible explanation for the exclusive formation of 8 is that in absence of any efficient hydrogen donor, the kinetically preferred Myers–Saito diradical intermediate 7_INTMS has to be in a fully reversible equilibrium with the starting material 7, so that eventually product 9 will form. Indeed, at the stage of the Myers–Saito diradical intermediate 7c_INTMS there are two intramolecular radical quenching processes possible and both exhibit a higher lying barrier than that of the C2–C6 cyclization 7c_TSDA (ΔG‡ = 32.8 kcal mol−1 – referenced to 7c). The first one invokes a γ–H abstraction at the propyl chain of 7c_INTMS via the six-membered cyclic TS 7c_TSγHT (ΔG‡ = 36.1 kcal mol−1) and the second possibility an intramolecular β–H abstraction via 7c_TSβHT (ΔG‡ = 39.9 kcal mol−1). As a consequence, enyne-carbodiimide 7c preferentially cyclizes via the C2–C6 pathway to 9cDA, because the Myers–Saito route with its rate-limiting γ- or β-hydrogen abstraction steps is disfavored on the free energy surface (ΔΔG‡ = 3.3 and 7.1 kcal mol−1).
![[1860-5397-12-6-1]](/bjoc/content/figures/1860-5397-12-6-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Reaction profile of 7a–c at BLYP/6-31+G* level. All bold numbers represent the respective free energies in kcal mol–1 at 138 °C. Subscripts “DA”, “MS” and “HT” stand for “Diels–Alder”, “Myers–Saito” and “Hydrogen Transfer”, respectively.
Figure 1: Reaction profile of 7a–c at BLYP/6-31+G* level. All bold numbers represent the respective free ener...
After the insightful computational analysis of the reaction mechanism of 7a–c, we chose to use computations in search for an enyne-carbodiimide with a preferred Myers–Saito pathway that would not carry hydrogen at the alkyne terminus. For any suitable candidate the intermediate Myers–Saito diradical should undergo intramolecular hydrogen abstraction at a lower barrier than that of the concerted C2–C6 cyclization. In order to realize a facile intramolecular H-abstraction in the Myers–Saito diradical via a 6-membered transition state [26,45], we decided to implement the N,N-dimethylaminomethyl group at the alkyne terminus. Two factors should be favorable for effective hydrogen transfer: (i) the nitrogen atom [51] stabilizes the incipient radical center and (ii) six equivalent hydrogens are available for transfer. Radical stabilization by heteroatoms (with lone pairs) has recently gained interest as a reaction design tool [52,53]. Thus, enyne-carbodiimide 11 with its CH2NMe2 group was selected as a target and prepared (vide infra) according to Scheme 3.
Scheme 3: Synthesis of enyne-carbodiimide 11.
Scheme 3: Synthesis of enyne-carbodiimide 11.
Computations at the BLYP/6-31+G* level of theory on the thermal cyclization of 11 (Scheme 4) provided a barrier 11_TSDA of 29.9 kcal mol−1 for the concerted C2-C6 DA cyclization toward 13DA. Notably, both the Myers–Saito diradical cyclization step via 11_INTMS (27.1 kcal mol−1) and the follow-up intramolecular hydrogen abstraction 11_INTMS → 11_INTγHT are favored kinetically over formation of 13DA, suggesting that in the thermolysis the Myers–Saito product 12 should prevail. Due to the low free activation energy for the strongly exergonic intramolecular hydrogen abstraction 11_INTMS → 11_INTγHT of only 1.5 kcal mol−1, the diradical cyclization step is now rate determining in the Myers–Saito pathway.
![[1860-5397-12-6-i4]](/bjoc/content/inline/1860-5397-12-6-i4.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 4: Energetics of the thermal cyclization of enyne-carbodiimide 11 at BLYP/6-31+G* level. Bold numbers in italics are representing respective free energies in kcal mol–1 at 25 °C and other numbers are representing bond distances in angstrom (Å). Subscripts “DA”, “MS”, “HT” and “R” stand for “Diels–Alder”, “Myers–Saito”, “Hydrogen Transfer” and “Rotation”, respectively.
Scheme 4: Energetics of the thermal cyclization of enyne-carbodiimide 11 at BLYP/6-31+G* level. Bold numbers ...
The synthetic strategy (Scheme 3) was to first prepare carbodiimide 10 [24] according to a literature procedure. In the next step, a Sonogashira coupling between 10 and N,N-dimethylprop-2-yn-1-amine afforded the desired enyne-carbodiimide 11 in 24% yield. Thermolysis of 11 (Scheme 5) was carried out in presence of 1,4-cyclohexadiene (1,4-CHD) under toluene reflux condition for 12 h. On heating enyne-carbodiimide 11, the characteristic strong IR absorptions of the carbodiimide unit at 2143 and 2123 cm−1 disappeared, which clearly indicated participation of this moiety during the reaction. Conversion of two methyl groups (NMe2 in 11, 2.23 ppm) to one (NMe in 12, 2.59 ppm) in the 1H NMR spectrum suggested the involvement of one methyl group in the cyclization process. In combination with above two findings, we initially identified the Myers–Saito cyclization product 12, the assignment of which was further confirmed by 1H, 13C, 1H,1H-COSY NMR and also by IR spectroscopy and mass spectrometry. For 14, a broad singlet at 10.02 ppm that is characteristic for its NH proton was observed in the crude NMR mixture. In summary, the Myers–Saito product 12 was produced as major product, 67% yield, whereas only trace amounts of the C2–C6 cyclization product 14 arising from 13DA were obtained. The experimental finding is thus in full agreement with the computational predictions and supports the hypothesis of the Myers–Saito diradical being a kinetically favored species formed in a reversible side equilibrium with the starting enyne-carbodiimide. The diradical can be trapped in good yield by hydrogen transfer and possibly also other reactions (e.g., addition to unsaturated bonds), if this process has a sufficiently low barrier [23].
Scheme 5: Thermolysis of enyne-carbodiimide 11.
Scheme 5: Thermolysis of enyne-carbodiimide 11.
Conclusion
Experimental and computational techniques have been used to shed light on the C2–C6 vs Myers–Saito competition in the thermal cyclization of enyne-carbodiimides. Although in most cases the C2–C6 DA cyclization product is furnished exclusively the results suggest that the Myers–Saito diradical intermediate reversibly forms in a kinetically preferred side equilibrium. This diradical intermediate remains a dead-end route as long as there are no powerful inter- or intramolecular hydrogen transfer pathways available. Considering the computational data, however, also other trapping reactions involving the Myers–Saito diradical should be able to compete with the concerted C2–C6 (DA) pathway, opening new venues for the preparation of heterocycles from carbodiimides [54].
Experimental
General methods. Anhydrous solvents were used to perform reactions under inert atmosphere (argon). Potassium was utilized to dry tetrahydrofuran (THF) and toluene. Triethylamine (Et3N) and dichloromethane (DCM) were dried over calcium hydride. Compounds were purified by flash column chromatography (silica gel, 0.035–0.070 mm). To describe NMR signals, the following abbreviations were utilized: s = singlet, d = doublet, t = triplet, dd = doublet of doublets, tt = triplet of triplets, m = multiplet.
2-(3-(Dimethylamino)prop-1-yn-1-yl)-4-methyl-N-((phenylimino)methylene)aniline (11). To a solution of N-phenyl-N´-(2-iodo-4-methylphenyl)carbodiimide [55] (10, 1.78 g, 5.11 mmol) and N,N-dimethylprop-2-yn-1-amine (637 mg, 820 μL, 7.66 mmol) in 50 mL of dry NEt3 were added PdCl2(PPh3)2 (70.0 mg, 100 μmol) and copper(I) iodide (38.0 mg, 200 μmol) at room temperature under nitrogen atmosphere. The reaction mixture was stirred overnight. Then the solvent was removed under reduced pressure and diethyl ether (40 mL) was added. The suspension was filtered through celite and the filtrate was washed with water and dried over MgSO4. After removal of the solvent, column chromatography over silica gel using (n-hexane/ethyl acetate, 1:1, Rf = 0.28) furnished pure product 11 as a dark-green oil. Yield: 354 mg (1.22 mmol, 24%). Tonset = 119 °C (DSC). 1H NMR (400 MHz, CDCl3) δ 2.25 (s, 6H), 2.30 (s, 3H), 3.14 (s, 2H), 7.02 (d, 3J = 8.2 Hz, 1H), 7.07 (dd, 3J = 8.2 Hz, 4J = 1.6 Hz, 1H), 7.16 (tt, 3J = 7.4 Hz, 4J = 1.2 Hz, 1H), 7.22–7.25 (m, 2H), 7.28 (d, 4J = 1.6 Hz, 1H), 7.30–7.34 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 20.7, 44.1, 48.4, 81.7, 92.2, 119.5, 124.2, 124.3, 125.2, 129.4, 130.0, 133.8, 134.1, 135.1, 136.4, 139.3; IR (KBr) : 2976 (w), 2944 (w), 2864 (w), 2825 (w), 2780 (w), 2143 (s, N=C=N), 2123 (s, N=C=N), 1593 (s), 1519 (m), 1488 (s), 1355 (w), 1324 (w), 1284 (w), 1252 (m), 1213 (s), 1034 (m), 908 (s), 822 (m) cm−1; EIMS (70 eV) m/z (%): 289 (29, M+), 288 (99, M+ − H), 274 (54, M+ − CH3), 246 (47), 245 (100, M+ − N(CH3)2), 244 (20), 243 (26), 231 (14), 218 (15), 203 (18), 183 (62); ESIMS: calcd. for (C19H20N3)+, 290.2; found, 290.2.
3,7-Dimethyl-1-phenyl-1,2,3,4-tetrahydropyrimido[4,5-b]quinoline (12). To 10 mL of dry degassed toluene was added 2-(3-(dimethylamino)prop-1-yn-1-yl)-4-methyl-N-((phenylimino)methylene)aniline (11, 40.0 mg, 138 µmol) and 1,4-CHD (448 mg, 530 μL, 5.60 mmol). The reaction mixture was allowed to reflux for 12 h. After the solvent was removed under reduced pressure, purification was performed by preparative silica gel TLC using n-hexane/ethyl acetate (2:3, Rf = 0.22). Yield 27.0 mg (93.0 µmol, 67%); white solid; mp 124–126 °C; 1H NMR (400 MHz, CDCl3) δ 2.43 (s, 3H), 2.59 (s, 3H), 4.13 (s, 2H), 4.67 (s, 2H), 7.21 (tt, 3J = 6.4 Hz, 4J = 2.1 Hz, 1H), 7.29 (dd, 3J = 8.6 Hz, 4J = 2.0 Hz, 1H), 7.33 (br s, 1H), 7.39–7.45 (m, 4H), 7.52 (d, 3J = 8.6 Hz, 1H), 7.56 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 21.4, 40.6, 55.8, 73.0, 117.2, 124.3, 125.0, 125.8, 125.8, 126.7, 129.0, 131.0, 132.3, 133.6, 144.3, 145.1, 151.7; IR (KBr) : 2945 (m), 2870 (w), 1630 (m), 1594 (m), 1496 (s), 1488 (s), 1455 (m), 1412 (m), 1343 (s, C-N), 1312 (m), 1296 (w), 1225 (w), 1140 (w), 1123 (m), 1068 (m), 1047 (w), 897 (w), 819 (m), 746 (m), 692 (m) cm−1; EIMS (70 eV) m/z (%): 289 (60, M+), 288 (66, M+ − H), 245 (100, M+ − H − CH2NCH3), 244 (11), 91 (17); ESI: calcd. for (C19H20N3)+, 290.2; found, 290.2.
Supporting Information
| Supporting Information File 1: Characterization and computational data. | ||
| Format: PDF | Size: 888.6 KB | Download |
References
-
Jones, R. R.; Bergman, R. G. J. Am. Chem. Soc. 1972, 94, 660–661. doi:10.1021/ja00757a071
Return to citation in text: [1] -
Schmittel, M.; Kiau, S. Chem. Lett. 1995, 24, 953–954. doi:10.1246/cl.1995.953
Return to citation in text: [1] -
Prall, M.; Wittkopp, A.; Schreiner, P. R. J. Phys. Chem. A 2001, 105, 9265–9274. doi:10.1021/jp0028002
Return to citation in text: [1] [2] -
Vavilala, C.; Byrne, N.; Kraml, C. M.; Ho, D. M.; Pascal, R. A., Jr. J. Am. Chem. Soc. 2008, 130, 13549–13551. doi:10.1021/ja803413f
Return to citation in text: [1] -
Kraka, E.; Cremer, D. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2014, 4, 285–324. doi:10.1002/wcms.1174
Return to citation in text: [1] -
Haberhauer, G.; Gleiter, R.; Fabig, S. Org. Lett. 2015, 17, 1425–1428. doi:10.1021/acs.orglett.5b00296
Return to citation in text: [1] -
Braverman, S.; Duar, Y. J. Am. Chem. Soc. 1990, 112, 5830–5837. doi:10.1021/ja00171a024
Return to citation in text: [1] -
Alcaide, B.; Almendros, P.; Aragoncillo, C. Chem. Soc. Rev. 2010, 39, 783–816. doi:10.1039/B913749A
Return to citation in text: [1] -
Samanta, D.; Rana, A.; Schmittel, M. J. Org. Chem. 2014, 79, 8435–8439. doi:10.1021/jo501324w
Return to citation in text: [1] -
Burroughs, L.; Ritchie, J.; Ngwenya, M.; Khan, D.; Lewis, W.; Woodward, S. Beilstein J. Org. Chem. 2015, 11, 273–279. doi:10.3762/bjoc.11.31
Return to citation in text: [1] -
Hopf, H.; Markopoulos, G. Beilstein J. Org. Chem. 2012, 8, 1936–1998. doi:10.3762/bjoc.8.225
Return to citation in text: [1] -
Schmittel, M.; Strittmatter, M.; Kiau, S. Tetrahedron Lett. 1995, 36, 4975–4978. doi:10.1016/00404-0399(50)09378-
Return to citation in text: [1] -
Schmittel, M.; Steffen, J.-P.; Auer, D.; Maywald, M. Tetrahedron Lett. 1997, 38, 6177–6180. doi:10.1016/S0040-4039(97)01393-2
Return to citation in text: [1] -
Schmittel, M.; Vavilala, C. J. Org. Chem. 2005, 70, 4865–4868. doi:10.1021/jo0504971
Return to citation in text: [1] -
Wang, Y.-H.; Bailey, J. F.; Petersen, J. L.; Wang, K. K. Beilstein J. Org. Chem. 2011, 7, 496–502. doi:10.3762/bjoc.7.58
Return to citation in text: [1] -
Cinar, M. E.; Vavilala, C.; Jaquet, R.; Bats, J. W.; Schmittel, M. Eur. J. Org. Chem. 2014, 5166–5177. doi:10.1002/ejoc.201402438
Return to citation in text: [1] -
Samanta, D.; Rana, A.; Schmittel, M. J. Org. Chem. 2014, 79, 2368–2376. doi:10.1021/jo500035b
Return to citation in text: [1] -
Schmittel, M.; Steffen, J.-P.; Engels, B.; Lennartz, C.; Hanrath, M. Angew. Chem., Int. Ed. 1998, 37, 2371–2373. doi:10.1002/(SICI)1521-3773(19980918)37:17<2371::AID-ANIE2371>3.0.CO;2-N
Return to citation in text: [1] [2] [3] [4] -
Shi, C.; Zhang, Q.; Wang, K. K. J. Org. Chem. 1999, 64, 925–932. doi:10.1021/jo981845k
Return to citation in text: [1] [2] [3] [4] [5] -
Zhang, Q.; Shi, C.; Zhang, H.-R.; Wang, K. K. J. Org. Chem. 2000, 65, 7977–7983. doi:10.1021/jo000978e
Return to citation in text: [1] -
Lu, X.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2002, 67, 5412–5415. doi:10.1021/jo0202031
Return to citation in text: [1] -
Lu, X.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2002, 67, 7797–7801. doi:10.1021/jo0204092
Return to citation in text: [1] -
Li, H.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2003, 68, 5512–5518. doi:10.1021/jo020760n
Return to citation in text: [1] [2] -
Schmittel, M.; Steffen, J.-P.; Rodríguez, D.; Engelen, B.; Neumann, E.; Cinar, M. E. J. Org. Chem. 2008, 73, 3005–3016. doi:10.1021/jo701966h
Return to citation in text: [1] [2] -
Rana, A.; Cinar, M. E.; Samanta, D.; Schmittel, M. Org. Lett. 2016, 18, 84–87. doi:10.1021/acs.orglett.5b03310
Return to citation in text: [1] -
Mohamed, R. K.; Peterson, P. W.; Alabugin, I. V. Chem. Rev. 2013, 113, 7089–7129. doi:10.1021/cr4000682
Return to citation in text: [1] [2] -
Allen, W. D.; Horner, D. A.; Dekock, R. L.; Remington, R. B.; Schaefer, H. F., III. Chem. Phys. 1989, 133, 11–45. doi:10.1016/0301-0104(89)80097-7
Return to citation in text: [1] -
Burton, N. A.; Yamaguchi, Y.; Alberts, I. L.; Schaefer, H. F., III. J. Chem. Phys. 1991, 95, 7466–7478. doi:10.1063/1.461372
Return to citation in text: [1] -
Barnes, L. A.; Lindh, R. Chem. Phys. Lett. 1994, 223, 207–214. doi:10.1016/0009-2614(94)00442-0
Return to citation in text: [1] -
Crawford, T. D.; Stanton, J. F.; Allen, W. D.; Schaefer, H. F., III. J. Chem. Phys. 1997, 107, 10626–10632. doi:10.1063/1.474178
Return to citation in text: [1] -
Sherrill, C. D.; Krylov, A. I.; Byrd, E. F. C.; Head-Gordon, M. J. Chem. Phys. 1998, 109, 4171–4181. doi:10.1063/1.477023
Return to citation in text: [1] -
Wenthold, P. G.; Squires, R. R.; Lineberger, W. C. J. Am. Chem. Soc. 1998, 120, 5279–5290. doi:10.1021/ja9803355
Return to citation in text: [1] -
Dehareng, D.; Dive, G. J. Comput. Chem. 2000, 21, 483–504. doi:10.1002/(SICI)1096-987X(20000430)21:6<483::AID-JCC7>3.0.CO;2-O
Return to citation in text: [1] -
Schreiner, P. R.; Navarro-Vázquez, A.; Prall, M. Acc. Chem. Res. 2005, 38, 29–37. doi:10.1021/ar020270h
Return to citation in text: [1] -
Lopez, X.; Ruipérez, F.; Piris, M.; Matxain, J. M.; Ugalde, J. M. ChemPhysChem 2011, 12, 1061–1065. doi:10.1002/cphc.201100136
Return to citation in text: [1] -
Kraka, E.; Cremer, D. J. Am. Chem. Soc. 2000, 122, 8245–8264. doi:10.1021/ja001017k
Return to citation in text: [1] -
Gräfenstein, J.; Hjerpe, A. M.; Kraka, E.; Cremer, D. J. Phys. Chem. A 2000, 104, 1748–1761. doi:10.1021/jp993122q
Return to citation in text: [1] -
Alabugin, I. V.; Manoharan, M.; Kovalenko, S. V. Org. Lett. 2002, 4, 1119–1122. doi:10.1021/ol0255054
Return to citation in text: [1] -
Alabugin, I. V.; Manoharan, M. J. Am. Chem. Soc. 2003, 125, 4495–4509. doi:10.1021/ja029664u
Return to citation in text: [1] -
Chen, W.-C.; Zou, J.-W.; Yu, C.-H. J. Org. Chem. 2003, 68, 3663–3672. doi:10.1021/jo0267246
Return to citation in text: [1] -
Bekele, T.; Christian, C. F.; Lipton, M. A.; Singleton, D. A. J. Am. Chem. Soc. 2005, 127, 9216–9223. doi:10.1021/ja0508673
Return to citation in text: [1] -
Zeidan, T. A.; Manoharan, M.; Alabugin, I. V. J. Org. Chem. 2006, 71, 954–961. doi:10.1021/jo051857n
Return to citation in text: [1] -
Zeidan, T. A.; Kovalenko, S. V.; Manoharan, M.; Alabugin, I. V. J. Org. Chem. 2006, 71, 962–975. doi:10.1021/jo0520801
Return to citation in text: [1] -
Basak, A.; Das, S.; Mallick, D.; Jemmis, E. D. J. Am. Chem. Soc. 2009, 131, 15695–15704. doi:10.1021/ja9023644
Return to citation in text: [1] -
Baroudi, A.; Mauldin, J.; Alabugin, I. V. J. Am. Chem. Soc. 2010, 132, 967–979. doi:10.1021/ja905100u
Return to citation in text: [1] [2] -
Ess, D. H.; Johnson, E. R.; Hu, X.; Yang, W. J. Phys. Chem. A 2011, 115, 76–83. doi:10.1021/jp109280y
Return to citation in text: [1] -
Samanta, D.; Cinar, M. E.; Das, K.; Schmittel, M. J. Org. Chem. 2013, 78, 1451–1462. doi:10.1021/jo302524a
Return to citation in text: [1] -
Samanta, D.; Rana, A.; Schmittel, M. J. Org. Chem. 2015, 80, 2174–2181. doi:10.1021/jo502693b
Return to citation in text: [1] -
Marell, D. J.; Furan, L. R.; Woods, B. P.; Lei, X.; Bendelsmith, A. J.; Cramer, C. J.; Hoye, T. R.; Kuwata, K. T. J. Org. Chem. 2015, 80, 11744–11754. doi:10.1021/acs.joc.5b01356
Return to citation in text: [1] -
Gaussian; Gaussian, Inc.: Wallingford, CT, U.S.A., 2010.
Return to citation in text: [1] -
Brocks, J. J.; Beckhaus, H.-D.; Beckwith, A. L. J.; Rüchardt, C. J. Org. Chem. 1998, 63, 1935–1943. doi:10.1021/jo971940d
Return to citation in text: [1] -
Mohamed, R. K.; Mondal, S.; Gold, B.; Evoniuk, C. J.; Banerjee, K. H.; Alabugin, I. V. J. Am. Chem. Soc. 2015, 137, 6335–6349. doi:10.1021/jacs.5b02373
Return to citation in text: [1] -
Mondal, S.; Gold, B.; Mohamed, R. K.; Alabugin, I. V. Chem. – Eur. J. 2014, 20, 8664–8669. doi:10.1002/chem.201402843
Return to citation in text: [1] -
Samanta, D.; Rana, A.; Bats, J. W.; Schmittel, M. Beilstein J. Org. Chem. 2014, 10, 2989–2996. doi:10.3762/bjoc.10.317
Return to citation in text: [1] -
Lv, X.; Bao, W. J. Org. Chem. 2009, 74, 5618–5621. doi:10.1021/jo900743y
Return to citation in text: [1]
| 51. | Brocks, J. J.; Beckhaus, H.-D.; Beckwith, A. L. J.; Rüchardt, C. J. Org. Chem. 1998, 63, 1935–1943. doi:10.1021/jo971940d |
| 26. | Mohamed, R. K.; Peterson, P. W.; Alabugin, I. V. Chem. Rev. 2013, 113, 7089–7129. doi:10.1021/cr4000682 |
| 45. | Baroudi, A.; Mauldin, J.; Alabugin, I. V. J. Am. Chem. Soc. 2010, 132, 967–979. doi:10.1021/ja905100u |
| 1. | Jones, R. R.; Bergman, R. G. J. Am. Chem. Soc. 1972, 94, 660–661. doi:10.1021/ja00757a071 |
| 2. | Schmittel, M.; Kiau, S. Chem. Lett. 1995, 24, 953–954. doi:10.1246/cl.1995.953 |
| 3. | Prall, M.; Wittkopp, A.; Schreiner, P. R. J. Phys. Chem. A 2001, 105, 9265–9274. doi:10.1021/jp0028002 |
| 4. | Vavilala, C.; Byrne, N.; Kraml, C. M.; Ho, D. M.; Pascal, R. A., Jr. J. Am. Chem. Soc. 2008, 130, 13549–13551. doi:10.1021/ja803413f |
| 5. | Kraka, E.; Cremer, D. Wiley Interdiscip. Rev.: Comput. Mol. Sci. 2014, 4, 285–324. doi:10.1002/wcms.1174 |
| 6. | Haberhauer, G.; Gleiter, R.; Fabig, S. Org. Lett. 2015, 17, 1425–1428. doi:10.1021/acs.orglett.5b00296 |
| 18. | Schmittel, M.; Steffen, J.-P.; Engels, B.; Lennartz, C.; Hanrath, M. Angew. Chem., Int. Ed. 1998, 37, 2371–2373. doi:10.1002/(SICI)1521-3773(19980918)37:17<2371::AID-ANIE2371>3.0.CO;2-N |
| 19. | Shi, C.; Zhang, Q.; Wang, K. K. J. Org. Chem. 1999, 64, 925–932. doi:10.1021/jo981845k |
| 20. | Zhang, Q.; Shi, C.; Zhang, H.-R.; Wang, K. K. J. Org. Chem. 2000, 65, 7977–7983. doi:10.1021/jo000978e |
| 21. | Lu, X.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2002, 67, 5412–5415. doi:10.1021/jo0202031 |
| 22. | Lu, X.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2002, 67, 7797–7801. doi:10.1021/jo0204092 |
| 23. | Li, H.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2003, 68, 5512–5518. doi:10.1021/jo020760n |
| 24. | Schmittel, M.; Steffen, J.-P.; Rodríguez, D.; Engelen, B.; Neumann, E.; Cinar, M. E. J. Org. Chem. 2008, 73, 3005–3016. doi:10.1021/jo701966h |
| 25. | Rana, A.; Cinar, M. E.; Samanta, D.; Schmittel, M. Org. Lett. 2016, 18, 84–87. doi:10.1021/acs.orglett.5b03310 |
| 36. | Kraka, E.; Cremer, D. J. Am. Chem. Soc. 2000, 122, 8245–8264. doi:10.1021/ja001017k |
| 37. | Gräfenstein, J.; Hjerpe, A. M.; Kraka, E.; Cremer, D. J. Phys. Chem. A 2000, 104, 1748–1761. doi:10.1021/jp993122q |
| 38. | Alabugin, I. V.; Manoharan, M.; Kovalenko, S. V. Org. Lett. 2002, 4, 1119–1122. doi:10.1021/ol0255054 |
| 39. | Alabugin, I. V.; Manoharan, M. J. Am. Chem. Soc. 2003, 125, 4495–4509. doi:10.1021/ja029664u |
| 40. | Chen, W.-C.; Zou, J.-W.; Yu, C.-H. J. Org. Chem. 2003, 68, 3663–3672. doi:10.1021/jo0267246 |
| 41. | Bekele, T.; Christian, C. F.; Lipton, M. A.; Singleton, D. A. J. Am. Chem. Soc. 2005, 127, 9216–9223. doi:10.1021/ja0508673 |
| 42. | Zeidan, T. A.; Manoharan, M.; Alabugin, I. V. J. Org. Chem. 2006, 71, 954–961. doi:10.1021/jo051857n |
| 43. | Zeidan, T. A.; Kovalenko, S. V.; Manoharan, M.; Alabugin, I. V. J. Org. Chem. 2006, 71, 962–975. doi:10.1021/jo0520801 |
| 44. | Basak, A.; Das, S.; Mallick, D.; Jemmis, E. D. J. Am. Chem. Soc. 2009, 131, 15695–15704. doi:10.1021/ja9023644 |
| 45. | Baroudi, A.; Mauldin, J.; Alabugin, I. V. J. Am. Chem. Soc. 2010, 132, 967–979. doi:10.1021/ja905100u |
| 46. | Ess, D. H.; Johnson, E. R.; Hu, X.; Yang, W. J. Phys. Chem. A 2011, 115, 76–83. doi:10.1021/jp109280y |
| 47. | Samanta, D.; Cinar, M. E.; Das, K.; Schmittel, M. J. Org. Chem. 2013, 78, 1451–1462. doi:10.1021/jo302524a |
| 48. | Samanta, D.; Rana, A.; Schmittel, M. J. Org. Chem. 2015, 80, 2174–2181. doi:10.1021/jo502693b |
| 49. | Marell, D. J.; Furan, L. R.; Woods, B. P.; Lei, X.; Bendelsmith, A. J.; Cramer, C. J.; Hoye, T. R.; Kuwata, K. T. J. Org. Chem. 2015, 80, 11744–11754. doi:10.1021/acs.joc.5b01356 |
| 12. | Schmittel, M.; Strittmatter, M.; Kiau, S. Tetrahedron Lett. 1995, 36, 4975–4978. doi:10.1016/00404-0399(50)09378- |
| 13. | Schmittel, M.; Steffen, J.-P.; Auer, D.; Maywald, M. Tetrahedron Lett. 1997, 38, 6177–6180. doi:10.1016/S0040-4039(97)01393-2 |
| 14. | Schmittel, M.; Vavilala, C. J. Org. Chem. 2005, 70, 4865–4868. doi:10.1021/jo0504971 |
| 15. | Wang, Y.-H.; Bailey, J. F.; Petersen, J. L.; Wang, K. K. Beilstein J. Org. Chem. 2011, 7, 496–502. doi:10.3762/bjoc.7.58 |
| 16. | Cinar, M. E.; Vavilala, C.; Jaquet, R.; Bats, J. W.; Schmittel, M. Eur. J. Org. Chem. 2014, 5166–5177. doi:10.1002/ejoc.201402438 |
| 17. | Samanta, D.; Rana, A.; Schmittel, M. J. Org. Chem. 2014, 79, 2368–2376. doi:10.1021/jo500035b |
| 3. | Prall, M.; Wittkopp, A.; Schreiner, P. R. J. Phys. Chem. A 2001, 105, 9265–9274. doi:10.1021/jp0028002 |
| 11. | Hopf, H.; Markopoulos, G. Beilstein J. Org. Chem. 2012, 8, 1936–1998. doi:10.3762/bjoc.8.225 |
| 19. | Shi, C.; Zhang, Q.; Wang, K. K. J. Org. Chem. 1999, 64, 925–932. doi:10.1021/jo981845k |
| 7. | Braverman, S.; Duar, Y. J. Am. Chem. Soc. 1990, 112, 5830–5837. doi:10.1021/ja00171a024 |
| 8. | Alcaide, B.; Almendros, P.; Aragoncillo, C. Chem. Soc. Rev. 2010, 39, 783–816. doi:10.1039/B913749A |
| 9. | Samanta, D.; Rana, A.; Schmittel, M. J. Org. Chem. 2014, 79, 8435–8439. doi:10.1021/jo501324w |
| 10. | Burroughs, L.; Ritchie, J.; Ngwenya, M.; Khan, D.; Lewis, W.; Woodward, S. Beilstein J. Org. Chem. 2015, 11, 273–279. doi:10.3762/bjoc.11.31 |
| 27. | Allen, W. D.; Horner, D. A.; Dekock, R. L.; Remington, R. B.; Schaefer, H. F., III. Chem. Phys. 1989, 133, 11–45. doi:10.1016/0301-0104(89)80097-7 |
| 28. | Burton, N. A.; Yamaguchi, Y.; Alberts, I. L.; Schaefer, H. F., III. J. Chem. Phys. 1991, 95, 7466–7478. doi:10.1063/1.461372 |
| 29. | Barnes, L. A.; Lindh, R. Chem. Phys. Lett. 1994, 223, 207–214. doi:10.1016/0009-2614(94)00442-0 |
| 30. | Crawford, T. D.; Stanton, J. F.; Allen, W. D.; Schaefer, H. F., III. J. Chem. Phys. 1997, 107, 10626–10632. doi:10.1063/1.474178 |
| 31. | Sherrill, C. D.; Krylov, A. I.; Byrd, E. F. C.; Head-Gordon, M. J. Chem. Phys. 1998, 109, 4171–4181. doi:10.1063/1.477023 |
| 32. | Wenthold, P. G.; Squires, R. R.; Lineberger, W. C. J. Am. Chem. Soc. 1998, 120, 5279–5290. doi:10.1021/ja9803355 |
| 33. | Dehareng, D.; Dive, G. J. Comput. Chem. 2000, 21, 483–504. doi:10.1002/(SICI)1096-987X(20000430)21:6<483::AID-JCC7>3.0.CO;2-O |
| 34. | Schreiner, P. R.; Navarro-Vázquez, A.; Prall, M. Acc. Chem. Res. 2005, 38, 29–37. doi:10.1021/ar020270h |
| 35. | Lopez, X.; Ruipérez, F.; Piris, M.; Matxain, J. M.; Ugalde, J. M. ChemPhysChem 2011, 12, 1061–1065. doi:10.1002/cphc.201100136 |
| 18. | Schmittel, M.; Steffen, J.-P.; Engels, B.; Lennartz, C.; Hanrath, M. Angew. Chem., Int. Ed. 1998, 37, 2371–2373. doi:10.1002/(SICI)1521-3773(19980918)37:17<2371::AID-ANIE2371>3.0.CO;2-N |
| 18. | Schmittel, M.; Steffen, J.-P.; Engels, B.; Lennartz, C.; Hanrath, M. Angew. Chem., Int. Ed. 1998, 37, 2371–2373. doi:10.1002/(SICI)1521-3773(19980918)37:17<2371::AID-ANIE2371>3.0.CO;2-N |
| 23. | Li, H.; Petersen, J. L.; Wang, K. K. J. Org. Chem. 2003, 68, 5512–5518. doi:10.1021/jo020760n |
| 19. | Shi, C.; Zhang, Q.; Wang, K. K. J. Org. Chem. 1999, 64, 925–932. doi:10.1021/jo981845k |
| 19. | Shi, C.; Zhang, Q.; Wang, K. K. J. Org. Chem. 1999, 64, 925–932. doi:10.1021/jo981845k |
| 54. | Samanta, D.; Rana, A.; Bats, J. W.; Schmittel, M. Beilstein J. Org. Chem. 2014, 10, 2989–2996. doi:10.3762/bjoc.10.317 |
| 18. | Schmittel, M.; Steffen, J.-P.; Engels, B.; Lennartz, C.; Hanrath, M. Angew. Chem., Int. Ed. 1998, 37, 2371–2373. doi:10.1002/(SICI)1521-3773(19980918)37:17<2371::AID-ANIE2371>3.0.CO;2-N |
| 52. | Mohamed, R. K.; Mondal, S.; Gold, B.; Evoniuk, C. J.; Banerjee, K. H.; Alabugin, I. V. J. Am. Chem. Soc. 2015, 137, 6335–6349. doi:10.1021/jacs.5b02373 |
| 53. | Mondal, S.; Gold, B.; Mohamed, R. K.; Alabugin, I. V. Chem. – Eur. J. 2014, 20, 8664–8669. doi:10.1002/chem.201402843 |
| 26. | Mohamed, R. K.; Peterson, P. W.; Alabugin, I. V. Chem. Rev. 2013, 113, 7089–7129. doi:10.1021/cr4000682 |
| 19. | Shi, C.; Zhang, Q.; Wang, K. K. J. Org. Chem. 1999, 64, 925–932. doi:10.1021/jo981845k |
| 24. | Schmittel, M.; Steffen, J.-P.; Rodríguez, D.; Engelen, B.; Neumann, E.; Cinar, M. E. J. Org. Chem. 2008, 73, 3005–3016. doi:10.1021/jo701966h |
© 2016 Rana et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)