Abstract
Cationic biaryl derivatives were synthesized by Suzuki–Miyaura coupling of 3-bromonaphtho[1,2-b]quinolizinium bromide with arylboronic acids. The resulting cationic biaryl derivatives exhibit pronounced fluorosolvatochromic properties. First photophysical studies in different solvents showed that the emission energy of the biaryl derivatives decreases with increasing solvent polarity. This red-shifted emission in polar solvents is explained by a charge shift (CS) in the excited state and subsequent solvent relaxation. Furthermore, the polarity of protic polar and aprotic polar solvents affects the emission energy to different extent, which indicates a major influence of hydrogen bonding on the stabilization of the ground and excited states.
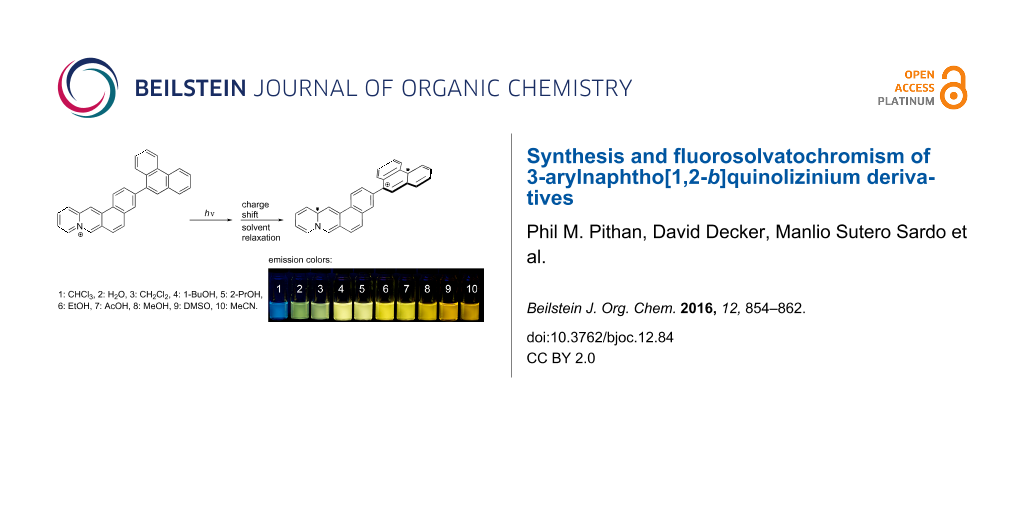
Graphical Abstract
Introduction
Dyes that change their absorption and emission properties, especially their color, in different media are considered as helpful optical probes, because they may be applied for the characterization and identification of either bulk media or microscopic environments with relatively simple spectrometric analyses [1-3]. For example, distinct solvent properties, such as hydrogen-bonding ability, polarity or polarizability can be determined even quantitatively by means of solvatochromic optical probes [4-7]. In this context, fluorosolvatochromic probes appear to be especially attractive as indicators for the surrounding medium, because emission spectroscopy is a highly sensitive method that allows to determine three different physical quantities, namely emission quantum yield, emission energy and emission lifetime. Notably, a considerable number of solvatochromic molecules is based on charge-transfer (CT) processes in the excited state resulting from a pronounced donor–acceptor interplay within the fluorophore [8]. Thus, upon excitation of such compounds, a CT – or in charged species a charge shift (CS) – takes place that results in a significantly different electron distribution of the molecule in the excited state as compared to the ground state. As a consequence, the solvent molecules reorganize to re-establish an optimal stabilization of the molecules in the excited state, which is usually referred to as solvent relaxation. Thus, the different absorption and emission energies of solvatochromic compounds may result from different energies of the ground and excited state that are caused by the different stabilizing (or destabilizing) interactions between the solvent and the solutes [9-12].
Along these lines, we established the annelated quinolizinium ion as a versatile platform for the investigation of cationic chemosensors, especially when the chromophore is incorporated within a donor–acceptor system [13]. In particular, we observed that biaryl-type quinolizinium derivatives such as 1a–f (Figure 1) exhibit fluorosolvatochromic properties that are especially pronounced with donor-substituted aryl substituents [14,15].
Figure 1: Structures of biaryl-type benzo[b]quinolizinium derivatives 1a–f.
Figure 1: Structures of biaryl-type benzo[b]quinolizinium derivatives 1a–f.
In contrast, simple donor-substituted benzo[b]quinolizinium derivatives only show a relatively moderate solvatochromic behavior [16,17], which implies that the biaryl structure is an important feature that supports the solvatochromic behavior, most likely as it facilitates the CS in a twisted biaryl conformation [14,15]. To explore this property of annelated aryl-substituted quinolizinium derivatives further with a main focus on the fluorosolvatochromic probes, we developed novel 3-arylnaphtho[1,2-b]quinolizinium derivatives. Firstly, we chose the naphthoquinolizinium fluorophore because it should have essentially the same ability to act as an acceptor in the photoinduced CS process as the benzo[b]quinolizinium [18]. But due to its more extended π-system it may be better suited to delocalize the radical that is formed after the CS. The quinolizinium is further attached with unsubstituted aryl substituents, because it was shown that the latter may act as electron donating units in aryl-substituted acridinium and quinolinium derivatives [19-25]. We refrained from using additional donor functionalities such as the amino group, because we observed in a previous work that these substituents cause significant fluorescence quenching and lead to only weakly fluorescent derivatives [14]. Herein, we report the synthesis of novel 3-arylnaphtho[1,2-b]quinolizinium derivatives and demonstrate that some of these compounds have fluorosolvatochromic properties.
Results
Synthesis
The 3-arylnaphtho[1,2-b]quinolizinium derivatives 6a–e were prepared by Suzuki–Miyaura coupling reactions with the 3-bromonaphtho[1,2-b]quinolizinium bromide (4). The latter was synthesized from the known 2-bromo-6-(bromomethyl)naphthalene (2) which was prepared in two steps according to published procedures [26] from commercially available methyl 6-bromo-2-naphthoate. The reaction of the (bromomethyl)naphthalene 2 with (1,3-dioxolan-2-yl)pyridine yielded the N-benzylpyridinium derivative 3 and the subsequent cyclodehydration [27] in refluxing HBr (w = 48%) gave the bromonaphtho[1,2-b]quinolizinium 4 in 57% overall yield (Scheme 1).
Scheme 1: Synthesis of 3-bromonaphtho[1,2-b]quinolizinium bromide (4).
Scheme 1: Synthesis of 3-bromonaphtho[1,2-b]quinolizinium bromide (4).
The Suzuki–Miyaura coupling reactions of 3-bromonaphthoquinolizinium derivative 4 with the arylboronic acids 5a–e were performed under reaction conditions optimized for quinolizinium derivatives [28] with Pd(PPh3)2Cl2 or Pd(dppf)2Cl2·CH2Cl2 as catalyst and KF as mild base to give the respective aryl-substituted naphthoquinolizinium derivatives 6a–e in 17–49% yield (Scheme 2). The structures of the new compounds 3, 4 and 6a–e were confirmed by NMR spectroscopic analysis (1H, 13C, COSY, HSQC, HMBC), mass-spectrometric data and elemental analysis.
Scheme 2: Synthesis of the 3-aryl-substituted naphtho[1,2-b]quinolizinium derivatives 6a–e.
Scheme 2: Synthesis of the 3-aryl-substituted naphtho[1,2-b]quinolizinium derivatives 6a–e.
Absorption and emission properties
The 3-arylnaphthoquinolizinium derivatives 6a–e are moderately soluble in protic polar and aprotic polar solvents and show the characteristic long-wavelength absorption band of the parent naphtho[1,2-b]quinolizinium [29] with two local maxima between 380 and 420 nm. The shifts of the absorption maxima of these compounds are almost independent from the solvent. For example, the long-wavelength absorption maximum of the phenyl-substituted derivative 6a ranges from 407 nm in MeOH to 414 nm in CHCl3 (Table 1, Figure 2). As an exception, the absorption band of derivative 6e in dimethoxyethane (DME) is significantly red-shifted (λabs = 438 nm). Notably, all compounds have particularly low extinction coefficients in H2O.
Table 1: Absorption and emission properties of quinolizinium derivatives 6a–e.
| 6a | 6b | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Solventa | λabsb | lg εc | λFd | ΦFe / 10−2 | λabsb | lg εc | λFd | ΦFe / 10−2 | |
| H2O | 408 | 3.67 | 421 | 34 | 407 | 3.91 | 471 | 53 | |
| MeOH | 407 | 4.09 | 423 | 45 | 408 | 4.05 | 465 | 66 | |
| DMSO | 410 | 4.13 | 425 | 3.4 | 411 | 4.16 | 456 | 6.0 | |
| CHCl3 | 414 | 4.02 | 428 | 1.4 | 415 | 4.09 | 455 | 3.1 | |
| 6c | 6d | ||||||||
| Solventa | λabsb | lg εc | λFd | ΦFf / 10−2 | λabsb | lg εc | λFd | ΦFf / 10−2 | |
| H2O | 405 | 3.92 | 546 | 1.9 | 416 | 3.84 | 524 | 19 | |
| MeOH | 406 | 4.04 | 545 | 25 | 416 | 4.34 | 526 | 43 | |
| DMSO | 408 | 4.09 | 559 | 17 | 412 | 4.24 | 538 | 18 | |
| CHCl3 | 413 | 4.07 | 473 | 4.2 | 416 | 4.23 | 470 | 3.4 | |
| 6e | |||||||||
| Solventa | λabsb | lg εc | λFd | ΦFf / 10−2 | |||||
| H2O | 404 | 3.72 | 531 | 9.2 | |||||
| MeOH | 406 | 4.11 | 553 | 21 | |||||
| EtOH | 407 | 4.09 | 547 | 29 | |||||
| AcOH | 407 | 4.11 | 548 | 28 | |||||
| BuOH | 407 | 4.11 | 546 | 34 | |||||
| 2-PrOH | 407 | 4.06 | 544 | 34 | |||||
| MeCN | 405 | 4.08 | 563 | 24 | |||||
| DMSO | 407 | 4.04 | 562 | 23 | |||||
| Aceton | 406 | 3.92 | 556 | 22 | |||||
| CH2Cl2 | 411 | 4.04 | 538 | 17 | |||||
| CHCl3 | 413 | 4.10 | 485 | 7.1 | |||||
| DME | 438 | 3.64 | 492 | 14 | |||||
aSolvents arranged in order of decreasing ET30 values. bLong-wavelength absorption maximum in nm; c = 20 µM. cMolar extinction coefficient in cm–1 M–1. dFluorescence emission maximum (Abs. = 0.10 at excitation wavelength); 6a–d: λex = 365 nm; 6e: λex = 385 nm. eFluorescence quantum yield relative to coumarin 1 [30,31]. fFluorescence quantum yield relative to coumarin 153 [30,31]; estimated error for fluorescence quantum yields: ±10%.
![[1860-5397-12-84-2]](/bjoc/content/figures/1860-5397-12-84-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Absorption spectra of derivatives 6a (A), 6c (B), 6d (C), and 6e (D); c = 20 µM; solvents: H2O (red), MeOH (black), DMSO (blue), CHCl3 (orange), DME (green).
Figure 2: Absorption spectra of derivatives 6a (A), 6c (B), 6d (C), and 6e (D); c = 20 µM; solvents: H2O (red...
All derivatives 6a–e are fluorescent. The emission maxima of the phenyl-substituted derivatives 6a and 6b deviate just slightly in different solvents (6a: 421–428 nm; 6b: 455–471 nm). In contrast, the long-wavelength emission maxima of the naphthyl-substituted derivatives 6c and 6d vary, for instance, from 473 nm or 470 nm in CHCl3 to 559 nm or 538 in DMSO, respectively (Table 1, Figure 3A–D). Derivative 6e is also fluorosolvatochromic, whereas the emission band is red-shifted by 78 nm from CHCl3 (λfl = 485 nm) to MeCN (λfl = 563 nm) (Figure 3D, Figure 4). Moreover, 6e exhibits the highest Stokes shift in MeCN (Δλ = 63 000 cm−1). Unfortunately, the emission properties of 6a–d in THF could not be determined due the very low solubility in this solvent. Derivate 6e was moderately soluble in THF; however, it was not possible to record reproducible absorption and emission spectra due to slow partial decomposition of the compound in this solvent. For the derivatives 6a–d the quantum yields were the highest in MeOH and the lowest in CHCl3. The phenanthrenyl-substituted derivative 6e exhibits the highest fluorescence quantum yields in 2-PrOH and 1-BuOH (ΦF = 0.34) and the lowest in CHCl3 (ΦF = 0.071).
![[1860-5397-12-84-3]](/bjoc/content/figures/1860-5397-12-84-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Normalized emission spectra of derivatives 6a (A), 6c (B), 6d (C) and 6e (D) (Abs. = 0.10 at excitation wavelength); 6a, 6c, 6d: λex = 365 nm; 6e: λex = 385 nm; solvents: H2O (red), MeOH (black), DMSO (blue), CHCl3 (orange), DME (green).
Figure 3: Normalized emission spectra of derivatives 6a (A), 6c (B), 6d (C) and 6e (D) (Abs. = 0.10 at excita...
![[1860-5397-12-84-4]](/bjoc/content/figures/1860-5397-12-84-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Fluorescence colors of derivative 6e in various solvents; λex = 366 nm. 1: CHCl3, 2: H2O, 3: CH2Cl2, 4: 1-BuOH, 5: 2-PrOH, 6: EtOH, 7: AcOH, 8: MeOH, 9: DMSO, 10: MeCN.
Figure 4: Fluorescence colors of derivative 6e in various solvents; λex = 366 nm. 1: CHCl3, 2: H2O, 3: CH2Cl2...
Discussion
In most of the tested solvents the absorption maxima of the biaryl derivatives are only slightly red-shifted relative to the ones of the parent naphthoquinolizinium ion (e.g., λabs = 403 nm in MeOH) [32]. In the chloroalkane solvents CH2Cl2 and CHCl3 the red shift is, however, slightly more pronounced, which is commonly observed for cationic dyes and explained with the high polarizability of these solvents [33,34]. Furthermore, the derivative 6e has a significantly red-shifted absorption in dimethoxyethane. Apart from these exceptional cases, however, the absorption properties depend only marginally on the solvent properties, such as the parent compound. This behavior indicates that the initial absorption does not contain a significant contribution of a charge-shift component and almost exclusively leads to the first local excited state (LE). The low extinction coefficients of all derivatives in water suggested aggregation in this solvent, as for example reported for 9-(4-dimethylaminophenyl)benzo[b]quinolizinium in aqueous solution [14]. Though, exemplary control experiments with derivative 6c showed that the absorption of this compound depends linearly on the concentration (cf. Supporting Information File 1), which contradicts aggregation. Furthermore, the absorption band of 6c is essentially maintained with increasing concentration. This behavior also indicates the absence of aggregates as the latter are characterized by the formation of red- or blue-shifted absorption bands or shoulders [35,36].
The emission properties of the phenyl-substituted compound 6a are also essentially independent of the solvent and resemble the fluorescence spectra of the parent naphthoquinolizinium (e.g., λF = 420 nm in MeOH) [34], which indicates emission from the LE state without specific stabilization or destabilization of the excited molecule after solvent relaxation. In contrast, the biaryl derivatives 6b–e have significantly red-shifted emission bands, especially in more polar solvents. Notably, this effect is more pronounced with increasing ring size and for that matter with the electron-donating ability of the substituent (tolyl < naphthyl ≈ phenanthryl). Such a red shift of the emission bands was already observed for acridinium- and benzo[b]quinolizinium-containing biaryl derivatives and shown to result from a photoinduced electron transfer from the electron-donating aryl unit to the excited cationic hetarene, thus leading to a charge shift (CS) in the excited state [19-25]. Thus, the results of the absorption and steady-state emission experiments of compounds 6b–e, along with literature precedence, point to an initial excitation of the ground state molecule to the LE state, followed by a charge shift (CS) to generate an intermediate excited species, that is a combination of charge neutral radical, i.e., a quinolizinyl radical, and the radical cation of the aromatic substituent (Scheme 3). The proposed photoinduced charge shift in derivatives 6b–e is supported by observations that even the intermolecular electron transfer reactions between electron rich aromatic compounds, such as naphthalene and phenanthrene, and the excited benzo[b]quinolizinium fluorophore were shown to be efficient processes [37].
Scheme 3: Intramolecular charge shift upon excitation in derivatives 6b–e (see Scheme 2 for assignment of substituents).
Scheme 3: Intramolecular charge shift upon excitation in derivatives 6b–e (see Scheme 2 for assignment of substituent...
The biaryl derivatives 6b–e exhibit fluorosolvatochromism that is characteristic of donor–acceptor dyes, namely a cumulative red shift of the emission maximum with increasing solvent polarity, along with a broad, unstructured band structure [1,2]. To assess the effect of the solvent on the emission of compound 6e several different solvents were employed. Unfortunately, many solvents – mainly less polar or non-polar – could not be used because of the limited solubility of the analyte. Remarkably, neither the emission maxima, nor the Stokes shifts correlate well with common solvent parameters, such as the hydrogen bond (HB) donating properties, α, HB accepting properties, β, the Taft parameter π*, the dipole moment, μ, the acidity, SA, the basicity, SB, the dipolarity and polarizability SPP, the polarizability, SP, or the dipolarity SDP (cf. Supporting Information File 1). In all cases, the plots of the solvent parameters versus the emission energy do not disclose an obvious relationship. This behavior is in agreement with that of other donor–acceptor biaryl-type dyes, such as for example para-hetaryl-substituted benzophenone derivatives, whose emission properties were demonstrated to depend on a complex interplay of different solvent parameters [38]. Unfortunately, the limited solubility of the derivatives 6b–e did not permit to employ a large series of solvents that is required for such a multiparameter analysis of these compounds. Nevertheless, the separation of the solvents into protic and aprotic solvents reveals at least a general trend of the solvent effect on the emission properties of 6e. Namely, the emission maxima shift to lower energy with increasing polarity of the aprotic solvents (Figure 5). This qualitative relationship denotes a larger dipole moment of the molecule in the excited state than in the ground state, as usually observed in donor–acceptor dyes [3]. However, the linear regression analysis of a plot of the emission energy versus solvent polarity, as quantified by the ET(30) value, did not produce a good correlation, which indicates that solvent properties other than the polarity also contribute to some extent to the stabilization or destabilization of the ground and/or excited state of 6e.
![[1860-5397-12-84-5]](/bjoc/content/figures/1860-5397-12-84-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Plot of the emission energy of 6e versus the solvent polarity parameter ET(30).
Figure 5: Plot of the emission energy of 6e versus the solvent polarity parameter ET(30).
Of special note are the different emission properties of 6e in protic and aprotic polar solvents (Figure 5). In particular, these two classes of solvents differ in their hydrogen bond (H-bond) donating properties. The latter usually stabilize negative polarized or charged species, which is most likely the bromide counter ion in the case of the salt 6e. In fact, the fate of the counter ion during the photoinduced CS in cationic biaryl derivatives was already discussed [24,39]. Specifically, it was proposed that after the CS, and likely during solvent relaxation, the counter anion migrates to the radical cation unit at the aryl substituent. At the same time, this mechanism implies that directly after the "back CS" the bromide anion is still located in the vicinity of the aryl substituent and therefore no longer compensated by a nearby cationic charge before it moves back to the cationic hetarene in a subsequent relaxation process (Scheme 4). In protic solvents, the bromide ion is still stabilized by H-bonding. In contrast, in aprotic polar solvents the anion cannot be stabilized sufficiently which leads to an increased energy of this state. As a result the emission from the CS state in aprotic solvents has a lower energy and exhibits an emission maximum that is red-shifted relative to the emission in protic solvents.
Scheme 4: State diagram of the photoexcitation and deactivation pathways of 3-aryl-naphthoquinolizinium derivatives.
Scheme 4: State diagram of the photoexcitation and deactivation pathways of 3-aryl-naphthoquinolizinium deriv...
Unfortunately, these results did not enable a quantitative assessment of the solvent parameters that govern the steady-state emission energy of biaryl-type quinolizinium derivatives 6a–e; but the most relevant solvent properties were identified that cause the fluorosolvatochromism of these derivatives. Interestingly, attempts to compare these results with the ones of related aryl-substituted cationic hetarenes and to draw a consistent picture of the solvatochromism of this class of compounds turned out to be rather difficult. Specifically, different cationic biaryl derivatives (Figure 6) show significantly varying trends regarding the influence of the solvent on the steady-state emission. For example, it was reported that the emission maximum of the CS emission of (benzoylamino)phenyl-10-methylacridinium (7a) is shifted to lower energy in solvents with decreasing polarity of the solvent, presumably as in this case the ground state dipole is larger than the one in the excited state [40]. In contrast, however, it was stated that there is no significant difference between the ground and excited-state dipole of the 9-mesityl-10-methylacridinium (7b), because the emission energies do not correlate with the Lippert–Mataga solvent parameter [41]. Furthermore, the 9-thienyl-10-methylacridinium (7c) is not solvatochromic at all, which was explained by a similar delocalization of the charge in the ground and excited state [21]. Overall, these results, along with ones observed in this work, clearly show that the steady-state fluorosolvatochromism of biaryl-type cationic hetarenes depends on a very fine balance of the donor–acceptor interplay in the ground and excited state, specifically the resulting delocalization of the charge. Unfortunately, detailed investigations of the steady-state solvatochromism of this class of compounds are rather scarce, so far, as most photophysical studies of 9-aryl-9-methylacridinium or 4-aryl-N-methylquinolinium have a strong emphasis on the dynamics and the charge separation in the excited state. Therefore, the work presented here may initiate investigations along these lines to further understand and fully explore the useful solvatochromic properties of this class of compounds.
Figure 6: Structures of biaryl derivatives 7a–c.
Figure 6: Structures of biaryl derivatives 7a–c.
Conclusion
In summary, we present a new class of solvatochromic cationic biaryl derivatives whose emission properties depend strongly on the solvent properties. The synthesis of these compounds is straightforward, and variations of substrate structures should allow the synthesis of further derivatives. First photophysical studies showed that the emission energy of the biaryl derivatives 6b–e is progressively red shifted with increasing polarity of the solvent, which is explained by a charge shift in the excited state and subsequent solvent relaxation. Notably, the polarity of protic polar and aprotic polar solvents affects the emission energy to a different degree, thus denoting a major influence of hydrogen bonding on the stabilization of the ground and excited states. Based on these results it is concluded that the solvent-sensitive emission properties of biaryl-type quinolizinium fluorophores are a promising structural motif for the development of novel solvatochromic probes.
Supporting Information
The Supporting Information contains the experimental section (synthesis, determination of fluorescence quantum yields); absorption and emission spectra of 6b; 1H NMR spectra of compounds 6a–e; plots of emission energies of 6e versus selected solvent parameters; plot of the absorbance of 6c in H2O at λabs = 405 nm versus concentration.
| Supporting Information File 1: Experimental and analytical data. | ||
| Format: PDF | Size: 1004.0 KB | Download |
References
-
Lakowicz, J. R. Principles of Fluorescence Spectroscopy; Springer, 2006. doi:10.1007/978-0-387-46312-4
Return to citation in text: [1] [2] -
Valeur, B.; Berberan-Santos, M. N. Molecular fluorescence. Principles and applications, 2nd ed.; Wiley-VCH: Weinheim, 2012. doi:10.1002/9783527650002
Return to citation in text: [1] [2] -
Suppan, P.; Ghoneim, N. Solvatochromism; RSC: Cambridge, 1997.
Return to citation in text: [1] [2] -
Orozco, M.; Luque, F. J. Chem. Rev. 2000, 100, 4187–4226. doi:10.1021/cr990052a
Return to citation in text: [1] -
Cramer, C. J.; Truhlar, D. G. Chem. Rev. 1999, 99, 2161–2200. doi:10.1021/cr960149m
Return to citation in text: [1] -
Katritzky, A. R.; Fara, D. C.; Yang, H.; Tämm, K. Chem. Rev. 2004, 104, 175–198. doi:10.1021/cr020750m
Return to citation in text: [1] -
Reichardt, C. Chem. Rev. 1994, 94, 2319–2358. doi:10.1021/cr00032a005
Return to citation in text: [1] -
Grabowski, Z. R.; Rotkiewicz, K. Chem. Rev. 2003, 103, 3899–4032. doi:10.1021/cr940745l
Return to citation in text: [1] -
Kudo, K.; Momotake, A.; Kanna, Y.; Nishimura, Y.; Arai, T. Chem. Commun. 2011, 47, 3867–3869. doi:10.1039/c1cc10183h
Return to citation in text: [1] -
Marini, A.; Muñoz-Losa, A.; Biancardi, A.; Mennucci, B. J. Phys. Chem. B 2010, 114, 17128–17135. doi:10.1021/jp1097487
Return to citation in text: [1] -
Mennucci, B.; Caricato, M.; Ingrosso, F.; Cappelli, C.; Cammi, R.; Tomasi, J.; Scalmani, G.; Frisch, J. M. J. Phys. Chem. B 2008, 112, 414–423. doi:10.1021/jp076138m
Return to citation in text: [1] -
Moyon, N. S.; Mitra, S. J. Phys. Chem. A 2011, 115, 2456–2464. doi:10.1021/jp1102687
Return to citation in text: [1] -
Granzhan, A.; Ihmels, H.; Tian, M. ARKIVOC 2015, No. (vi), 494–523. doi:10.3998/ark.5550190.p009.339
Return to citation in text: [1] -
Bortolozzi, R.; Ihmels, H.; Thomas, L.; Tian, M.; Viola, G. Chem. – Eur. J. 2013, 19, 8736–8741. doi:10.1002/chem.201301164
Return to citation in text: [1] [2] [3] [4] -
Tian, M.; Ihmels, H.; Ye, S. Org. Biomol. Chem. 2012, 10, 3010–3018. doi:10.1039/c2ob06948b
Return to citation in text: [1] [2] -
Faulhaber, K.; Granzhan, A.; Ihmels, H.; Otto, D.; Thomas, L.; Wells, S. Photochem. Photobiol. Sci. 2011, 10, 1535–1545. doi:10.1039/c1pp05106g
Return to citation in text: [1] -
Ihmels, H.; Engels, B.; Faulhaber, K.; Lennartz, C. Chem. – Eur. J. 2000, 6, 2854–2864. doi:10.1002/1521-3765(20000804)6:15<2854::AID-CHEM2854>3.0.CO;2-5
Return to citation in text: [1] -
Mitzner, R.; Bendig, J.; Ziebig, R.; Graichen, F.; Kreysig, D.; Pragst, F. J. Prakt. Chem. 1985, 327, 241–250. doi:10.1002/prac.19853270209
Return to citation in text: [1] -
Kotani, H.; Ohkubo, K.; Fukuzumi, S. Faraday Discuss. 2012, 155, 89–102. doi:10.1039/C1FD00084E
Return to citation in text: [1] [2] -
Li, X.; Liang, M.; Chakraborty, A.; Kondo, M.; Maroncelli, M. J. Phys. Chem. B 2011, 115, 6592–6607. doi:10.1021/jp200339e
Return to citation in text: [1] [2] -
Hu, J.; Xia, B.; Bao, D.; Ferreira, A.; Wan, J.; Jones, G., II; Vullev, V. I. J. Phys. Chem. A 2009, 113, 3096–3107. doi:10.1021/jp810909v
Return to citation in text: [1] [2] [3] -
Verhoeven, J. W.; van Ramesdonk, H. J.; Groeneveld, M. M.; Benniston, A. C.; Harriman, A. ChemPhysChem 2005, 6, 2251–2260. doi:10.1002/cphc.200500029
Return to citation in text: [1] [2] -
Jones, G., II; Yan, D.-X.; Gosztola, D. J.; Greenfield, S. R.; Wasielewski, M. R. J. Am. Chem. Soc. 1999, 121, 11016–11017. doi:10.1021/ja9927319
Return to citation in text: [1] [2] -
Horng, M. L.; Dahl, K.; Jones, G., II; Maroncelli, M. Chem. Phys. Lett. 1999, 315, 363–370. doi:10.1016/S0009-2614(99)01258-0
Return to citation in text: [1] [2] [3] -
Jones, G., II; Farahat, M. S.; Greenfield, S. R.; Gosztola, D. J.; Wasielewski, M. R. Chem. Phys. Lett. 1994, 229, 40–46. doi:10.1016/0009-2614(94)00996-1
Return to citation in text: [1] [2] -
Pu, Y.-M.; Ku, Y.-Y.; Grieme, T.; Black, L. A.; Bhatia, A. V.; Cowart, M. Org. Process Res. Dev. 2007, 11, 1004–1009. doi:10.1021/op700102k
Return to citation in text: [1] -
Bradsher, C. K.; Parham, J. C. J. Org. Chem. 1963, 28, 83–85. doi:10.1021/jo01036a018
Return to citation in text: [1] -
Tian, M.; Ihmels, H. Synthesis 2009, 24, 4226–4234. doi:10.1055/s-0029-1217060
Return to citation in text: [1] -
Viola, G.; Bressanini, M.; Gabellini, N.; Vedaldi, D.; Dall'Acqua, F.; Ihmels, H. Photochem. Photobiol. Sci. 2002, 1, 882–889. doi:10.1039/B204275D
Return to citation in text: [1] -
Jones, G., II; Jackson, W. R.; Choi, C. Y.; Bergmark, W. R. J. Phys. Chem. 1985, 89, 294–300. doi:10.1021/j100248a024
Return to citation in text: [1] [2] -
Crosby, G. A.; Demas, J. N. J. Phys. Chem. 1971, 75, 991–1024. doi:10.1021/j100678a001
Return to citation in text: [1] [2] -
Ihmels, H.; Mohrschladt, C. J.; Schmitt, A.; Bressanini, M.; Leusser, D.; Stalke, D. Eur. J. Org. Chem. 2002, 2624–2632. doi:10.1002/1099-0690(200208)2002:15<2624::AID-EJOC2624>3.0.CO;2-Q
Return to citation in text: [1] -
van den Berg, O.; Jager, W. F.; Picken, S. J. J. Org. Chem. 2006, 71, 2666–2676. doi:10.1021/jo052441c
Return to citation in text: [1] -
Granzhan, A.; Ihmels, H.; Viola, G. J. Am. Chem. Soc. 2007, 129, 1254–1267. doi:10.1021/ja0668872
Return to citation in text: [1] [2] -
Würthner, F.; Kaiser, T. E.; Saha-Möller, C. R. Angew. Chem. 2011, 123, 3436–3473. doi:10.1002/ange.201002307
Return to citation in text: [1] -
Knapp, E. W. Chem. Phys. 1984, 85, 73–82.
Return to citation in text: [1] -
Bendig, J.; Geppert, B.; Helm, S.; Kreysig, D. Theor. Exp. Chem. 1978, 14, 488–495. doi:10.1007/BF01004352
Teor. Eksp. Khim. 1978, 14, 629–637.
Return to citation in text: [1] -
Friebe, N.; Schreiter, K.; Kübel, J.; Dietzek, B.; Moszner, N.; Burtscher, P.; Oehlke, A.; Spange, S. New J. Chem. 2015, 39, 5171–5179. doi:10.1039/C5NJ00256G
Return to citation in text: [1] -
Zilberg, S. Phys. Chem. Chem. Phys. 2010, 12, 10292–10294. doi:10.1039/C0CP00491J
Return to citation in text: [1] -
Jones, G., II; Yan, D.-X.; Greenfield, S. R.; Gosztola, D. J.; Wasielewski, M. R. J. Phys. Chem. A 1997, 101, 4939–4942. doi:10.1021/jp970518y
Return to citation in text: [1] -
Benniston, A. C.; Harriman, A.; Li, P.; Rostron, J. P.; van Ramesdonk, H. J.; Groeneveld, M. M.; Zhang, H.; Verhoeven, J. W. J. Am. Chem. Soc. 2005, 127, 16054–16064. doi:10.1021/ja052967e
Return to citation in text: [1]
| 24. | Horng, M. L.; Dahl, K.; Jones, G., II; Maroncelli, M. Chem. Phys. Lett. 1999, 315, 363–370. doi:10.1016/S0009-2614(99)01258-0 |
| 39. | Zilberg, S. Phys. Chem. Chem. Phys. 2010, 12, 10292–10294. doi:10.1039/C0CP00491J |
| 40. | Jones, G., II; Yan, D.-X.; Greenfield, S. R.; Gosztola, D. J.; Wasielewski, M. R. J. Phys. Chem. A 1997, 101, 4939–4942. doi:10.1021/jp970518y |
| 41. | Benniston, A. C.; Harriman, A.; Li, P.; Rostron, J. P.; van Ramesdonk, H. J.; Groeneveld, M. M.; Zhang, H.; Verhoeven, J. W. J. Am. Chem. Soc. 2005, 127, 16054–16064. doi:10.1021/ja052967e |
| 1. | Lakowicz, J. R. Principles of Fluorescence Spectroscopy; Springer, 2006. doi:10.1007/978-0-387-46312-4 |
| 2. | Valeur, B.; Berberan-Santos, M. N. Molecular fluorescence. Principles and applications, 2nd ed.; Wiley-VCH: Weinheim, 2012. doi:10.1002/9783527650002 |
| 3. | Suppan, P.; Ghoneim, N. Solvatochromism; RSC: Cambridge, 1997. |
| 13. | Granzhan, A.; Ihmels, H.; Tian, M. ARKIVOC 2015, No. (vi), 494–523. doi:10.3998/ark.5550190.p009.339 |
| 29. | Viola, G.; Bressanini, M.; Gabellini, N.; Vedaldi, D.; Dall'Acqua, F.; Ihmels, H. Photochem. Photobiol. Sci. 2002, 1, 882–889. doi:10.1039/B204275D |
| 9. | Kudo, K.; Momotake, A.; Kanna, Y.; Nishimura, Y.; Arai, T. Chem. Commun. 2011, 47, 3867–3869. doi:10.1039/c1cc10183h |
| 10. | Marini, A.; Muñoz-Losa, A.; Biancardi, A.; Mennucci, B. J. Phys. Chem. B 2010, 114, 17128–17135. doi:10.1021/jp1097487 |
| 11. | Mennucci, B.; Caricato, M.; Ingrosso, F.; Cappelli, C.; Cammi, R.; Tomasi, J.; Scalmani, G.; Frisch, J. M. J. Phys. Chem. B 2008, 112, 414–423. doi:10.1021/jp076138m |
| 12. | Moyon, N. S.; Mitra, S. J. Phys. Chem. A 2011, 115, 2456–2464. doi:10.1021/jp1102687 |
| 30. | Jones, G., II; Jackson, W. R.; Choi, C. Y.; Bergmark, W. R. J. Phys. Chem. 1985, 89, 294–300. doi:10.1021/j100248a024 |
| 31. | Crosby, G. A.; Demas, J. N. J. Phys. Chem. 1971, 75, 991–1024. doi:10.1021/j100678a001 |
| 8. | Grabowski, Z. R.; Rotkiewicz, K. Chem. Rev. 2003, 103, 3899–4032. doi:10.1021/cr940745l |
| 27. | Bradsher, C. K.; Parham, J. C. J. Org. Chem. 1963, 28, 83–85. doi:10.1021/jo01036a018 |
| 4. | Orozco, M.; Luque, F. J. Chem. Rev. 2000, 100, 4187–4226. doi:10.1021/cr990052a |
| 5. | Cramer, C. J.; Truhlar, D. G. Chem. Rev. 1999, 99, 2161–2200. doi:10.1021/cr960149m |
| 6. | Katritzky, A. R.; Fara, D. C.; Yang, H.; Tämm, K. Chem. Rev. 2004, 104, 175–198. doi:10.1021/cr020750m |
| 7. | Reichardt, C. Chem. Rev. 1994, 94, 2319–2358. doi:10.1021/cr00032a005 |
| 28. | Tian, M.; Ihmels, H. Synthesis 2009, 24, 4226–4234. doi:10.1055/s-0029-1217060 |
| 18. | Mitzner, R.; Bendig, J.; Ziebig, R.; Graichen, F.; Kreysig, D.; Pragst, F. J. Prakt. Chem. 1985, 327, 241–250. doi:10.1002/prac.19853270209 |
| 14. | Bortolozzi, R.; Ihmels, H.; Thomas, L.; Tian, M.; Viola, G. Chem. – Eur. J. 2013, 19, 8736–8741. doi:10.1002/chem.201301164 |
| 14. | Bortolozzi, R.; Ihmels, H.; Thomas, L.; Tian, M.; Viola, G. Chem. – Eur. J. 2013, 19, 8736–8741. doi:10.1002/chem.201301164 |
| 15. | Tian, M.; Ihmels, H.; Ye, S. Org. Biomol. Chem. 2012, 10, 3010–3018. doi:10.1039/c2ob06948b |
| 26. | Pu, Y.-M.; Ku, Y.-Y.; Grieme, T.; Black, L. A.; Bhatia, A. V.; Cowart, M. Org. Process Res. Dev. 2007, 11, 1004–1009. doi:10.1021/op700102k |
| 16. | Faulhaber, K.; Granzhan, A.; Ihmels, H.; Otto, D.; Thomas, L.; Wells, S. Photochem. Photobiol. Sci. 2011, 10, 1535–1545. doi:10.1039/c1pp05106g |
| 17. | Ihmels, H.; Engels, B.; Faulhaber, K.; Lennartz, C. Chem. – Eur. J. 2000, 6, 2854–2864. doi:10.1002/1521-3765(20000804)6:15<2854::AID-CHEM2854>3.0.CO;2-5 |
| 21. | Hu, J.; Xia, B.; Bao, D.; Ferreira, A.; Wan, J.; Jones, G., II; Vullev, V. I. J. Phys. Chem. A 2009, 113, 3096–3107. doi:10.1021/jp810909v |
| 14. | Bortolozzi, R.; Ihmels, H.; Thomas, L.; Tian, M.; Viola, G. Chem. – Eur. J. 2013, 19, 8736–8741. doi:10.1002/chem.201301164 |
| 15. | Tian, M.; Ihmels, H.; Ye, S. Org. Biomol. Chem. 2012, 10, 3010–3018. doi:10.1039/c2ob06948b |
| 19. | Kotani, H.; Ohkubo, K.; Fukuzumi, S. Faraday Discuss. 2012, 155, 89–102. doi:10.1039/C1FD00084E |
| 20. | Li, X.; Liang, M.; Chakraborty, A.; Kondo, M.; Maroncelli, M. J. Phys. Chem. B 2011, 115, 6592–6607. doi:10.1021/jp200339e |
| 21. | Hu, J.; Xia, B.; Bao, D.; Ferreira, A.; Wan, J.; Jones, G., II; Vullev, V. I. J. Phys. Chem. A 2009, 113, 3096–3107. doi:10.1021/jp810909v |
| 22. | Verhoeven, J. W.; van Ramesdonk, H. J.; Groeneveld, M. M.; Benniston, A. C.; Harriman, A. ChemPhysChem 2005, 6, 2251–2260. doi:10.1002/cphc.200500029 |
| 23. | Jones, G., II; Yan, D.-X.; Gosztola, D. J.; Greenfield, S. R.; Wasielewski, M. R. J. Am. Chem. Soc. 1999, 121, 11016–11017. doi:10.1021/ja9927319 |
| 24. | Horng, M. L.; Dahl, K.; Jones, G., II; Maroncelli, M. Chem. Phys. Lett. 1999, 315, 363–370. doi:10.1016/S0009-2614(99)01258-0 |
| 25. | Jones, G., II; Farahat, M. S.; Greenfield, S. R.; Gosztola, D. J.; Wasielewski, M. R. Chem. Phys. Lett. 1994, 229, 40–46. doi:10.1016/0009-2614(94)00996-1 |
| 33. | van den Berg, O.; Jager, W. F.; Picken, S. J. J. Org. Chem. 2006, 71, 2666–2676. doi:10.1021/jo052441c |
| 34. | Granzhan, A.; Ihmels, H.; Viola, G. J. Am. Chem. Soc. 2007, 129, 1254–1267. doi:10.1021/ja0668872 |
| 30. | Jones, G., II; Jackson, W. R.; Choi, C. Y.; Bergmark, W. R. J. Phys. Chem. 1985, 89, 294–300. doi:10.1021/j100248a024 |
| 31. | Crosby, G. A.; Demas, J. N. J. Phys. Chem. 1971, 75, 991–1024. doi:10.1021/j100678a001 |
| 32. | Ihmels, H.; Mohrschladt, C. J.; Schmitt, A.; Bressanini, M.; Leusser, D.; Stalke, D. Eur. J. Org. Chem. 2002, 2624–2632. doi:10.1002/1099-0690(200208)2002:15<2624::AID-EJOC2624>3.0.CO;2-Q |
| 38. | Friebe, N.; Schreiter, K.; Kübel, J.; Dietzek, B.; Moszner, N.; Burtscher, P.; Oehlke, A.; Spange, S. New J. Chem. 2015, 39, 5171–5179. doi:10.1039/C5NJ00256G |
| 37. |
Bendig, J.; Geppert, B.; Helm, S.; Kreysig, D. Theor. Exp. Chem. 1978, 14, 488–495. doi:10.1007/BF01004352
Teor. Eksp. Khim. 1978, 14, 629–637. |
| 1. | Lakowicz, J. R. Principles of Fluorescence Spectroscopy; Springer, 2006. doi:10.1007/978-0-387-46312-4 |
| 2. | Valeur, B.; Berberan-Santos, M. N. Molecular fluorescence. Principles and applications, 2nd ed.; Wiley-VCH: Weinheim, 2012. doi:10.1002/9783527650002 |
| 34. | Granzhan, A.; Ihmels, H.; Viola, G. J. Am. Chem. Soc. 2007, 129, 1254–1267. doi:10.1021/ja0668872 |
| 19. | Kotani, H.; Ohkubo, K.; Fukuzumi, S. Faraday Discuss. 2012, 155, 89–102. doi:10.1039/C1FD00084E |
| 20. | Li, X.; Liang, M.; Chakraborty, A.; Kondo, M.; Maroncelli, M. J. Phys. Chem. B 2011, 115, 6592–6607. doi:10.1021/jp200339e |
| 21. | Hu, J.; Xia, B.; Bao, D.; Ferreira, A.; Wan, J.; Jones, G., II; Vullev, V. I. J. Phys. Chem. A 2009, 113, 3096–3107. doi:10.1021/jp810909v |
| 22. | Verhoeven, J. W.; van Ramesdonk, H. J.; Groeneveld, M. M.; Benniston, A. C.; Harriman, A. ChemPhysChem 2005, 6, 2251–2260. doi:10.1002/cphc.200500029 |
| 23. | Jones, G., II; Yan, D.-X.; Gosztola, D. J.; Greenfield, S. R.; Wasielewski, M. R. J. Am. Chem. Soc. 1999, 121, 11016–11017. doi:10.1021/ja9927319 |
| 24. | Horng, M. L.; Dahl, K.; Jones, G., II; Maroncelli, M. Chem. Phys. Lett. 1999, 315, 363–370. doi:10.1016/S0009-2614(99)01258-0 |
| 25. | Jones, G., II; Farahat, M. S.; Greenfield, S. R.; Gosztola, D. J.; Wasielewski, M. R. Chem. Phys. Lett. 1994, 229, 40–46. doi:10.1016/0009-2614(94)00996-1 |
| 14. | Bortolozzi, R.; Ihmels, H.; Thomas, L.; Tian, M.; Viola, G. Chem. – Eur. J. 2013, 19, 8736–8741. doi:10.1002/chem.201301164 |
| 35. | Würthner, F.; Kaiser, T. E.; Saha-Möller, C. R. Angew. Chem. 2011, 123, 3436–3473. doi:10.1002/ange.201002307 |
| 36. | Knapp, E. W. Chem. Phys. 1984, 85, 73–82. |
© 2016 Pithan et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)