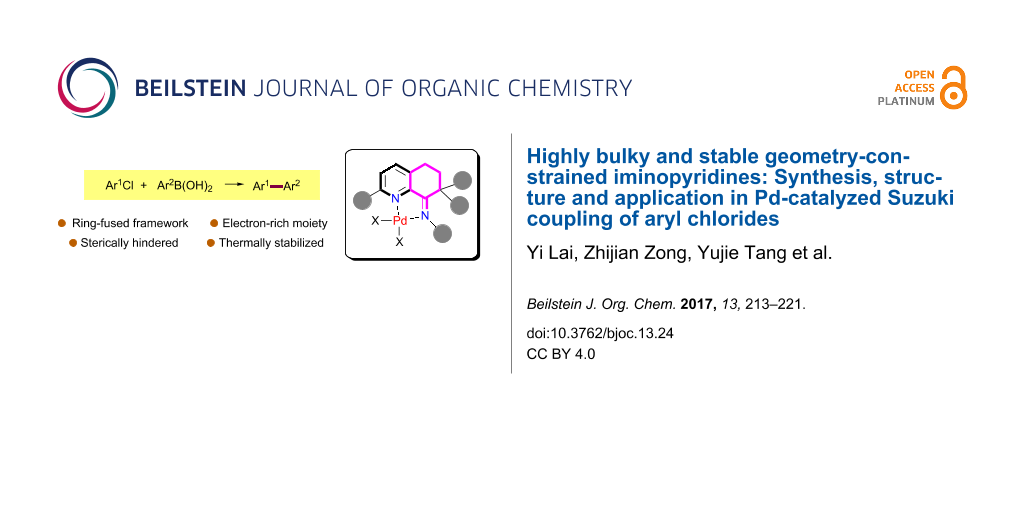
Abstract
A series of bulky geometry-constrained iminopyridylpalladium chlorides were developed. The steric environment adjacent to the nitrogen atom in the pyridine rings and diimine parts enhanced the thermal stability of the palladium species. Bulkier groups at the imino group stabilized the palladium species and the corresponding palladium chlorides showed high activities in the coupling reaction of aryl chlorides.
Graphical Abstract
Introduction
Palladium-promoted C–C coupling reactions are recognized as one of the most powerful tools in organic syntheses, pharmaceutical processes and biological modifications [1-4]. The achievement is largely beneficial to organic chemists for their parallel contributions in Pd-catalyzed transformations as well as for discovering new reactions. The improvement of the efficiency of noble metal catalysts is still highly attractive from both the academic and the industrial point of view [5]. Targeting the handling and applicability of palladium catalysts, various species have been developed through the use of diversified ligands [6-12].
Typical ligands that successfully enhanced the reactivity of palladium species towards the oxidative addition of aryl or alkyl halides include phosphine ligands independently developed by, for example, Buchwald [13-15], Hartwig [16,17], Fu [18-20], Kwong [21-23], Tang [24-27] and Lundgren [28], and N-heterocyclic carbene ligands due to the inherent strong sigma-donating property [29]. Alternatively, the palladacycles reported by Herrmann and Beller in the 1990s [30] effectively elongated the lifetime of the active Pd species through employing bidentate or multidentate ligands [31-36]. Meanwhile nitrogen ligands are also widely used for palladium catalysts in coupling reactions with the advantage of convenient synthetic methods, easy functional group modifications and better stability [37-43]. Pyridine, azole and imine-based N(sp2) ligands received considerable attention, especially bidentate N,N-ligands. In general, most bidentate N,N(sp2) ligands comprise symmetrical frameworks, such as bipyridine [44-46], biimidazole [47,48], and diimine [49-53]. Moreover iminopyridines were attractive for a more straightforward preparation, the condensation of pyridine-2-carboxaldehyde or ketone and the relevant primary amines. By this work a wide variety of different substituents were introduced leading to a diversity with regard to steric and electronic aspects [54-59].
Recently, we have developed a series of geometry-constrained iminopyridyl compounds [60-64] and their corresponding palladium complexes showed a good catalytic efficiency for both Suzuki and Heck cross-coupling reactions. The favorable effect originated from the ring-fused framework being able to establish a strained environment for better stability. Efficient couplings were achieved with aryl bromides or iodides as substrates, however, it is still not favored for the cheaper and more widely available aryl chloride substrates. For C–Cl bond activation, the major efforts have focused on using extraordinarily electron-rich ligands to promote the oxidative addition. We assumed that the coupling of aryl chlorides could also be furnished under high temperature, if the palladium catalyst is stable enough. The formation of palladium black was observed in reactions of aryl chlorides using the reported palladium catalyst system, which inspired us to improve the thermal stability of the palladium complex. The steric environment adjacent to the nitrogen in pyridine rings and diimine parts enhanced the thermal stability of metal species, albeit under harsh conditions [65-68]. Therefore, Figure 1 schematically illustrates various substituents of iminopyridines constraining the geometrical influence around the palladium center. In this report, the modifications using various substituents with different electronic and steric factors have been explored, and fortunately thermally stable palladium chlorides are obtained demonstrating high activities toward the coupling reaction of aryl chlorides.
Figure 1: The steric geometry-constrained iminopyridyl–palladium complexes.
Figure 1: The steric geometry-constrained iminopyridyl–palladium complexes.
Results and Discussion
According to Figure 1, the geometry-constrained iminopyridyl skeleton could be modified at three sites: Ca, Cb and Nc. Both the derivation of the Ca position and adjusting the imino part are expected to protect the active palladium center from decomposition during the catalytic cycle. Nevertheless, multiple substitutions at Cb probably prohibit any tautomerization and result to increase the stability of iminopyridines and their palladium catalysts.
6,7-Dihydroquinolin-8(5H)-one (1) and 2-chloro-6,7-dihydroquinolin-8(5H)-one (2) could be prepared in large amounts following the previously reported synthetic procedures [60,62,69-71]. Geminal dimethylation of 1 with NaH and MeI in THF afforded 7,7-dimethyl-6,7-dihydroquinolin-8(5H)-one (3) easily in good yield. Meanwhile, the Ni-catalyzed Kumada coupling of 2 with methylmagnesium chloride produced 2-methyl-6,7-dihydroquinolin-8(5H)-one (4) efficiently with quantitative yield. With compounds 1, 2, 3 and 4 in hand, further condensations with different arylamines and direct coordination to PdCl2 in one pot smoothly in the presence of TsOH. For this transformation, we found that vigorous stirring during the reaction was very important, which increased the yields from medium to quantitative results [72]. Following a similar procedure as described in the Experimental section, complexes Pd1 to Pd5 were synthesized and could be isolated by simple work-up (Scheme 1). All of these complexes have been fully characterized and confirmed by NMR and HRMS, and the analyses are in good agreement with that of the previously reported palladium complexes [72]. All of these complexes could be stored open to air without any decomposition for several months except Pd4. Their melting points [>240 °C] indicated their high thermostability, especially Pd2 with a melting point up to 325 °C.
Scheme 1: Preparation of the bulky iminopyridyl–palladium complexes.
Scheme 1: Preparation of the bulky iminopyridyl–palladium complexes.
With these sterically hindered iminopyridine–palladium complexes Pd1 to Pd5 in hand, we firstly investigated their catalytic activity directly in Suzuki cross-coupling reactions with chlorobenzene as the electrophile. The reactions were performed under the previously reported conditions without any change except at the higher reaction temperature. As shown in Table 1, using complex Pd1, which has exhibited an impressive efficiency in Pd-catalyzed Suzuki coupling of aryl bromides, only delivered 18% conversion of chlorobenzene. The modification of the Cb position by importing two methyl groups of Pd1 to Pd3 did not give any improvement while with much lower conversion. The efforts changing the steric and electronic effect from the ortho-position of pyridyl (Pd4 and Pd5) also failed to promote C–Cl bond activation. To our delight, complex Pd2, with a bulkier version of dipp (2,6-diisopropylphenyl) in the imino motif, displayed significant enhance with the conversion up to 44%. The results in Table 1 and Figure 1 matched well with our proposal that improving the stability of palladium species favored C–Cl bond activation under higher temperature.
Table 1: Pd-catalyzed coupling of chlorobenzene and 4-tolylboronic acid using Pd1–Pd5 as the catalyst.a
|
|
|||||
| Cat. | Pd1 | Pd2 | Pd3 | Pd4 | Pd5 |
|---|---|---|---|---|---|
| Conv.(%)b | 18 | 44 | 5 | 4 | 18 |
aReaction conditions: chlorobenzene (1.0 mmol), 4-methylphenylboronic acid (1.2 mmol), toluene (3.0 mL), Pd catalyst (1 mol %), 18 h, purged with N2 for 2 min. bConversions were determined by GC.
Single crystals of complex Pd2 were obtained by slow diffusion of diethyl ether to a saturated dichloromethane solution and the molecular structure in solid state was determined by X-ray diffraction [73]. Presented in Figure 2, the molecular structure and the predicted conformation was unambiguously confirmed. The same as before, the complexes also exhibit square-planar coordination geometry around the palladium centre with a slight distortion. Selected bond lengths and angles listed in Table 2 suggest that both the imino double bond N3–C3 (1.304 Å) and Pd–N3 (2.037 Å) were slightly longer than that of our previous reported iminopyridyl–palladium complexes (1.27–1.30 Å, 2.02–2.03 Å, respectively) [57]. The structure clearly showed a flexible steric bulky effect from the replacement of two methyl groups of iPr by phenyl rings and the palladium center could be protected very well.
![[1860-5397-13-24-2]](/bjoc/content/figures/1860-5397-13-24-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: ORTEP drawing of Pd2 with thermal ellipsoids at 30% probability level. Hydrogen atoms and the solvent CH2Cl2 have been omitted for clarity.
Figure 2: ORTEP drawing of Pd2 with thermal ellipsoids at 30% probability level. Hydrogen atoms and the solve...
Table 2: Selected bond lengths [Å] and angles [°] for complexes Pd2.
| Bond lengths (Å) | Bond angles (°) | ||
|---|---|---|---|
| Pd(1)–N(2) | 2.036 (3) | N(3)–Pd(1)–N(2) | 80.45(9) |
| Pd(1)–N(3) | 2.038(2) | N(3)–Pd(1)–Cl(1) | 174.33(7) |
| Pd(1)–Cl(2) | 2.2772(10) | N(3)–Pd(1)–Cl(2) | 94.80(6) |
| Pd(1)–Cl(1) | 2.2938(9) | N(2)–Pd(1)–Cl(2) | 175.22(7) |
| N(2)–C(2) | 1.373(4) | N(2)–Pd(1)–Cl(2) | 93.99(7) |
| N(2)–C(41) | 1.327(4) | Cl(2)–Pd(1)–Cl(1) | 90.75(3) |
| N(3)–C(3) | 1.304(3) | ||
| N(3)–C(5) | 1.437(3) | ||
With Pd2 as the catalyst, we then put our efforts on simply optimizing the reaction conditions for the coupling between chlorobenzene and 4-methylphenylboronic acid. We noticed that water could promote the conversion of chlorobenzene to 62% which might be due to the better solubility of the base in toluene/H2O (2.5:1) mixed solvents (Table 3, entry 1). Bases have been proven to play an important role in Suzuki coupling reactions [74]. Then, in mixed solvents, various bases were examined (Table 3, entries 1–10). Compared with K2CO3, the other tested bases did not show any advantage. Increasing the amount of base to 2.5 equivalents or elevating the temperature to 140 °C could be an advantage for the reaction. Without any other screening, we finally determined the reaction conditions as following for the next substrates scope test: Pd2 (1 mol %), toluene/H2O (5:1), 24 h, 140 °C.
Table 3: The optimization of conditions for Pd-catalyzed coupling reactions between chlorobenzene and phenylboronic acid a.
|
|
|||
| Entry | Base (equiv) | Temperature (°C) | Conversion.(%)b |
|---|---|---|---|
| 1 | K2CO3 (2.0) | 130 | 62 |
| 2 | Cs2CO3 (2.0) | 130 | 31 |
| 3 | t-BuOK (2.0) | 130 | trace |
| 4 | NaOH (2.0) | 130 | 16 |
| 5 | DIPEA (2.0) | 130 | NR |
| 6 | KOAc (2.0) | 130 | 8 |
| 7 | Na2CO3 (2.0) | 130 | 22 |
| 8 | NaF (2.0) | 130 | NR |
| 9 | NaOAc (2.0) | 130 | NR |
| 10 | EtONa (2.0) | 130 | 30 |
| 11 | K2CO3 (2.2) | 130 | 69 |
| 12 | K2CO3 (2.5) | 130 | 72 |
| 13 | K2CO3 (2.2) | 100 | 52 |
| 14 | K2CO3 (2.2) | 120 | 54 |
| 15 | K2CO3 (2.2) | 140 | 77 |
| 16 | K2CO3 (2.2) | 150 | 74 |
aReaction conditions: chlorobenzene (1.0 mmol), 4-methylphenylboronic acid (1.2 mmol), toluene/H2O (2.5/0.5, v/v), Pd2 (1 mol %), 18 h, purged with N2 for 2 min. bConversions were determined by GC. DIPEA = diisopropylethylamine.
With the optimized reaction conditions, a standard reaction in a 5 mmol scale between chlorobenzene and phenylboronic acid was carried out and 64% isolated yield was obtained (Table 4, entry 1). Subsequently, different aryl chlorides and arylboronic acids were tested. The results were listed in Table 4. Aryl chlorides with an electron-withdrawing functional group, such as nitro (Table 4, entry 2), carbonyl (Table 4, entries 4–6), nitrile and CF3 (Table 4, entries 6 and 7) could be coupled with 4-methylphenylboric acid in good to excellent yields. Aryl chlorides with an electron-donating functional group (Table 4, entries 9 and 10) and steric hindered 2-chlorotoluene (Table 4, entry 11), which are usually regarded as reluctant substrates due to the sluggish oxidative addition of Pd(0) to aryl chlorides, proceeded successfully with moderate yields. Heterocyclic aryl chloride, for example, 2-chloropyridine, was also well tolerated and 84% isolated yields was obtained (Table 4, entries 13). Different arylboronic acids were also examined to react with 4-chloroacetophenone in this system. No matter what kind of boronic acids were used: electron-deficient (Table 4, entry 14), electron-rich (Table 4, entries 15 and 16) or sterically hindered (Table 4, entry 17) arylboronic acids, high conversions and excellent isolated yields were achieved.
Table 4: Pd-catalyzed coupling between various aryl chlorides and arylboronic acids.a
|
|
|||
| Entry | ArCl | ArB(OH)2 | Conv/Yield (%)b |
|---|---|---|---|
| 1 |
1-1 |
2-1 |
3-1 71/64 |
| 2 |
1-2 |
2-1 | 3-2 64/58 |
| 3 |
1-3 |
2-1 | 3-3 99/99 |
| 4 |
1-4 |
2-1 | 3-4 86/85 |
| 5 |
1-5 |
2-1 | 3-5 82/69 |
| 6 |
1-6 |
2-1 | 3-6 94/90 |
| 7 |
1-7 |
2-1 | 3-7 93/86 |
| 8 |
1-8 |
2-1 | 3-8 80/75 |
| 9 |
1-9 |
2-1 | 3-9 60/49 |
| 10 |
1-10 |
2-1 | 3-10 59/51 |
| 11 |
1-11 |
2-1 | 3-11 64/57 |
| 12 |
1-12 |
2-1 | 3-12 91/91 |
| 13 |
1-13 |
2-1 | 3-13 92/84 |
| 14 | 1-3 |
2-2 |
3-14 99/95 |
| 15 | 1-3 |
2-3 |
3-15 99/99 |
| 16 | 1-3 |
2-4 |
3-16 96/89 |
| 17 | 1-3 |
2-5 |
3-17 95/93 |
aReaction conditions: aryl chlorides (5.0 mmol), arylboronic acid (6.0 mmol), K2CO3 (11.0 mmol), toluene/H2O (10 mL/2 mL), Pd2 (1 mol %), 140 °C, 24 h, purged with N2 for 2 min. bConversions were determined by GC and yields were isolated results.
Conclusion
In summary, a series of geometrically-constrained iminopyridine–palladium complexes were prepared with various substituents. They promoted efficiently the Suzuki cross coupling of aryl chlorides. To result good catalytic activities the importance of bulkier groups at the imino moiety for the stabilization of the palladium species was sown. A variety of aryl chlorides and arylboronic acids were successfully coupled in high yields and selectivity.
Experimental
General procedures
All reactions were carried out under air atmosphere unless otherwise noted. All 1H and 13C NMR spectra were recorded on a Bruker AVANCE III 500 MHz spectrometer in deuterated solvents with tetramethylsilane (TMS) as internal standard. NMR multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, q = quartet, sept = septet, m = multiplet, br = broad signal. Chemical shifts are given in ppm and are referenced to SiMe4 (1H, 13C) and all spectra were obtained at 25 °C in the solvent indicated. Coupling constants J are given in Hz. GC analyses were performed on Agilent 6890 instrument with FID detector using an HP-5 capillary column (30 m × 0.32 mm (i.d.), 0.25 μ). High-resolution mass spectra were recorded in the EI mode on Agilent 6210 TOF mass spectrometry. Flash column chromatography was performed on neutral SiO2 (200–300 mesh) with ethyl acetate/petroleum as eluent. Melting points were determined on BÜCHI M-565 apparatus. X-ray crystallography were performed on a Bruker Smart Apex CCD area detector diffractometer using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). Details of the X-ray structure determinations and refinements are provided in Table 5. According to the synthetic procedures in the literature, the organic compounds 1, 2, 3, and 4 were prepared in good yield [69,71,75].
Table 5: Crystal data and structure refinement for Pd2.
| Identification code | Pd2 | Identification code | Pd2 |
|---|---|---|---|
| empirical formula | C42H34N2Cl2Pd·CH2Cl2 | ρcalcg/cm3 | 1.4237 |
| formula weight | 829.00 | μ/mm−1 | 0.789 |
| crystal system | orthorhombic | F(000) | 3373.8 |
| temperature/K | 173 | radiation | Mo Kα (λ = 0.71073) |
| space group | Pbca | 2θ range for data collection/o | 3.58 to 56.6 |
| a/Å | 18.996(3) | index ranges | −24 ≤ h ≤25, −26 ≤ k ≤ 26, −27 ≤ l ≤ 23 |
| b/Å | 19.832(4) | reflections collected | 66946 |
| c/Å | 20.531(4) | independent reflections | 9585 [Rint = 0.0438, Rsigma = 0.0275] |
| α/o | 90 | data/restraints/parameters | 9585/0/470 |
| β/o | 90 | goodness-of-fit on F2 | 1.123 |
| γ/o | 90 | final R indexes [I>=2σ (I)] | R1 = 0.0412, wR2 = 0.1026 |
| volume/Å3 | 7735(2) | final R indexes [all data] | R1 = 0.0714, wR2 = 0.1295 |
| Z | 8 | largest diff. peak/hole/e Å−3 | 1.09/−0.68 |
Synthesis of Pd2
Toluene (100 mL) was added to a mixture of 6,7-dihydroquinolin-8(5H)-one (1.57 g, 11 mmol), 2,6-dibenzhydryl-4-methylaniline (5.27 g, 12 mmol), PdCl2 (1.77 g, 10 mmol) and TsOH·H2O (0.95 g, 5 mmol). After being degassed by N2 for 2 min, the reaction mixture was sealed and stirred at 130 °C for 36 hours. Then the tube was allowed to cool to room temperature. After filtration and washing with toluene, the resulted residue was purified by column chromatography with dichloromethane as the eluent. The complex Pd2 was obtained as yellow solid (7.1 g, yield 95%). Mp (dec.) 325 °C. 1H NMR (CDCl3, 500 MHz) δ 9.42 (t, J = 3.4 Hz, 1H), 7.70 (d, J = 3.4 Hz, 2H), 7.63 (m, 16H), 7.30 (d, J = 7.4Hz, 4H), 6.72 (s, 2H), 6.22 (s, 2H), 2.14 (s, 5H), 0.48 (t, J = 6.5 Hz, 2H), 0.17 (m, 2H); 13C NMR (CDCl3, 125 MHz) δ 182.1, 151.9, 149.9, 143.2, 142.4, 141.4, 140.3, 137.3, 137.0, 130.1, 129.9, 129.3, 129.0, 128.2, 127.0, 126.5, 53.6, 52.3, 31.6, 31.0, 27.6, 21.7, 19.8; HRMS (ESI+) m/z: [M – Cl + CH3CN]+ calcd for C42H36ClN2Pd·CH3CN, 750.1874; found, 750.1897.
Synthesis of Pd3
Using xylene as the solvent, and following a similar procedure to that described for Pd2 at 160 °C, resulted Pd3 with 29% yield as yellow powder. Mp 246 °C; 1H NMR (DMSO-d6, 500 MHz) δ 9.10 (d, J = 5.2 Hz, 1H), 8.22 (d, J = 7.8 Hz, 1H), 7.87 (m, 1H), 7.26 (m, 1H), 7.14 (d, J = 7.7 Hz, 2H), 3.06 (m, 4H), 1.86 (t, J = 6.1 Hz, 2H), 1.45 (d, J = 6.7 Hz, 6H), 1.26 (d, J = 6.7 Hz, 6H), 0.89 (s, 6H); 13C NMR (DMSO-d6, 125 MHz) δ 181.7, 153.4, 150.2, 142.5, 140.7, 140.5, 139.6, 128.4, 128.3, 123.4, 42.5, 37.7, 29.3, 29.0, 27.4, 25.7, 24.8, 24.5, 23.7; HRMS (ESI+) m/z: [M – Cl + CH3CN]+ calcd for C23H30ClN2Pd·CH3CN, 518.1392; found, 518.1378.
Synthesis of Pd4:
Toluene (4.0 mL) was added to a mixture of 2-chloro-6,7-dihydroquinolin-8(5H)-one (181 mg, 1 mmol), 2,6-dibenzhydryl-4-methylaniline (468 mg, 1.1 mmol), and TsOH·H2O (57 mg, 0.3 mmol). After degassed by N2 for 2 min, the reaction mixture was sealed and stirred at 130 °C for 24 hours. Then the tube was allowed to cool to room temperature and PdCl2 (160 mg, 0.9 mmol) was added. After stirring at 50 °C for additional 24 hours, filtration and washing with toluene, the resulted residue was purified by column chromatography with dichloromethane as the eluent. The complex Pd4 was obtained as yellow solid (380 mg, yield 82%). Mp (dec.) 306 °C. Pd4 decomposes slowly when exposed open to air. 1H NMR (CDCl3, 500 MHz) δ 7.90 (d, J = 8.1 Hz, 1H), 7.69 (d, J = 8.1 Hz, 1H), 7.34 (d, J = 7.6 Hz, 1H), 7.20 (d, J = 7.6 Hz, 2H), 3.13 (m, 2H), 3.03 (m, 2H), 2.44 (m, 2H), 1.93 (m, 2H), 1.44 (d, J = 6.7 Hz, 6H), 1.15 (d, J = 6.7 Hz, 6H); HRMS (ESI+) m/z: [M − Cl + CH3CN]+ calcd for C21H25Cl2N2Pd·CH3CN, 524.0684; found, 524.0679.
Synthesis of Pd5:
In a similar procedure to that described for Pd2 with 81% yield as yellow powder. Mp (dec.) 282 °C; 1H NMR (DMSO-d6, 500 MHz) δ 8.05 (d, J = 7.8 Hz, 1H), 7.70 (d, J = 7.8 Hz, 1H), 7.30 (t, J = 8.1 Hz, 1H), 7.19 (t, J = 8.1 Hz, 2H), 3.11 (m, 2H), 2.97 (d, J = 8.3 Hz, 5H), 2.40 (t, J = 6.0 Hz, 2H), 1.82 (t, J = 6.2 Hz, 2H), 1.33 (d, J = 6.9 Hz, 5H), 1.10 (d, J = 6.8 Hz, 6H); 13C NMR (DMSO-d6, 125 MHz) δ 163.8, 151.6, 141.2, 140.9, 140.2, 139.8, 132.1, 127.9, 123.4, 31.9, 27.9, 27.7, 27.2, 23.6, 23.5, 21.0; HRMS (ESI+) m/z: [M – Cl + CH3CN]+ calcd for C22H28ClN2Pd·CH3CN, 504.1235; found, 504.1231.
General procedure for Pd-catalyzed Suzuki cross-coupling reactions
To a Young tube, aryl chlorides (5.0 mmol), K2CO3 (1.5 g, 11 mmol), arylboric acid (6 mmol), complex Pd2 (37.5 mg, 1 mol %), toluene (10 mL) and H2O (2 mL) were added. The mixture was degassed for 2 min. Then, the sealed Young tube was set into the pre-heated 140 °C oil bath. After stirring for 24 hours, the Young tube was allowed to cool to room temperature. After filtration and extraction with toluene (50 mL), the resulted solution was concentrated under vacuum and the desired biaryl was isolated by column chromatography.
Supporting Information
| Supporting Information File 1: NMR spectra of palladium complexes and products. | ||
| Format: PDF | Size: 1.3 MB | Download |
References
-
Negishi, E.-i. Handbook of organopalladium chemistry for organic synthesis; Wiley: New York Chichester, 2002.
Return to citation in text: [1] -
Wu, X.-F.; Anbarasan, P.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2010, 49, 9047–9050. doi:10.1002/anie.201006374
Return to citation in text: [1] -
Magano, J.; Dunetz, J. R. Chem. Rev. 2011, 111, 2177–2250. doi:10.1021/cr100346g
Return to citation in text: [1] -
Budarin, V. L.; Shuttleworth, P. S.; Clark, J. H.; Luque, R. Curr. Org. Synth. 2010, 7, 614–627. doi:10.2174/157017910794328529
Return to citation in text: [1] -
Farina, V. Adv. Synth. Catal. 2004, 346, 1553–1582. doi:10.1002/adsc.200404178
Return to citation in text: [1] -
Engle, K. M.; Yu, J.-Q. J. Org. Chem. 2013, 78, 8927–8955. doi:10.1021/jo400159y
Return to citation in text: [1] -
Li, H.; Johansson Seechurn, C. C. C.; Colacot, T. J. ACS Catal. 2012, 2, 1147–1164. doi:10.1021/cs300082f
Return to citation in text: [1] -
Fleckenstein, C. A.; Plenio, H. Chem. Soc. Rev. 2010, 39, 694–711. doi:10.1039/B903646F
Return to citation in text: [1] -
Elsevier, C. J.; Reedijk, J.; Walton, P. H.; Ward, M. D. Dalton Trans. 2003, 1869–1880. doi:10.1039/b303975g
Return to citation in text: [1] -
Hanhan, M. E. Appl. Organomet. Chem. 2008, 22, 270–275. doi:10.1002/aoc.1389
Return to citation in text: [1] -
Gildner, P. G.; Colacot, T. J. Organometallics 2015, 34, 5497–5508. doi:10.1021/acs.organomet.5b00567
Return to citation in text: [1] -
Miura, M. Angew. Chem., Int. Ed. 2004, 43, 2201–2203. doi:10.1002/anie.200301753
Return to citation in text: [1] -
Walker, S. D.; Barder, T. E.; Martinelli, J. R.; Buchwald, S. L. Angew. Chem., Int. Ed. 2004, 43, 1871–1876. doi:10.1002/anie.200353615
Return to citation in text: [1] -
Wolfe, J. P.; Singer, R. A.; Yang, B. H.; Buchwald, S. L. J. Am. Chem. Soc. 1999, 121, 9550–9561. doi:10.1021/ja992130h
Return to citation in text: [1] -
Martin, R.; Buchwald, S. L. Acc. Chem. Res. 2008, 41, 1461–1473. doi:10.1021/ar800036s
Return to citation in text: [1] -
Kataoka, N.; Shelby, Q.; Stambuli, J. P.; Hartwig, J. F. J. Org. Chem. 2002, 67, 5553–5566. doi:10.1021/jo025732j
Return to citation in text: [1] -
Hartwig, J. F. Synlett 2006, 1283–1294. doi:10.1055/s-2006-939728
Return to citation in text: [1] -
Tang, H.; Menzel, K.; Fu, G. C. Angew. Chem., Int. Ed. 2003, 42, 5079–5082. doi:10.1002/anie.200352668
Return to citation in text: [1] -
Hills, I. D.; Netherton, M. R.; Fu, G. C. Angew. Chem., Int. Ed. 2003, 42, 5749–5752. doi:10.1002/anie.200352858
Return to citation in text: [1] -
Fu, G. C. Acc. Chem. Res. 2008, 41, 1555–1564. doi:10.1021/ar800148f
Return to citation in text: [1] -
So, C. M.; Lau, C. P.; Kwong, F. Y. Org. Lett. 2007, 9, 2795–2798. doi:10.1021/ol070898y
Return to citation in text: [1] -
Chung, K. H.; So, C. M.; Wong, S. M.; Luk, C. H.; Zhou, Z.; Lau, C. P.; Kwong, F. Y. Chem. Commun. 2012, 48, 1967–1969. doi:10.1039/c2cc15972d
Return to citation in text: [1] -
Wong, S. M.; Yuen, O. Y.; Choy, P. Y.; Kwong, F. Y. Coord. Chem. Rev. 2015, 293–294, 158–186. doi:10.1016/j.ccr.2015.01.017
Return to citation in text: [1] -
Tang, W.; Capacci, A. G.; Wei, X.; Li, W.; White, A.; Patel, N. D.; Savoie, J.; Gao, J. J.; Rodriguez, S.; Qu, B.; Haddad, N.; Lu, B. Z.; Krishnamurthy, D.; Yee, N. K.; Senanayake, C. H. Angew. Chem., Int. Ed. 2010, 49, 5879–5883. doi:10.1002/anie.201002404
Return to citation in text: [1] -
Tang, W.; Keshipeddy, S.; Zhang, Y.; Wei, X.; Savoie, J.; Patel, N. D.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2011, 13, 1366–1369. doi:10.1021/ol2000556
Return to citation in text: [1] -
Li, C.; Xiao, G.; Zhao, Q.; Liu, H.; Wang, T.; Tang, W. Org. Chem. Front. 2014, 1, 225–229. doi:10.1039/c4qo00024b
Return to citation in text: [1] -
Li, C.; Chen, T.; Li, B.; Xiao, G.; Tang, W. Angew. Chem., Int. Ed. 2015, 54, 3792–3796. doi:10.1002/anie.201411518
Return to citation in text: [1] -
Lundgren, R. J.; Stradiotto, M. Chem. – Eur. J. 2012, 18, 9758–9769. doi:10.1002/chem.201201195
Return to citation in text: [1] -
Herrmann, W. A.; Elison, M.; Fischer, J.; Köcher, C.; Artus, G. R. J. Angew. Chem., Int. Ed. Engl. 1995, 34, 2371–2374. doi:10.1002/anie.199523711
Return to citation in text: [1] -
Herrmann, W. A.; Brossmer, C.; Öfele, K.; Reisinger, C.-P.; Priermeier, T.; Beller, M.; Fischer, H. Angew. Chem., Int. Ed. Engl. 1995, 34, 1844–1848. doi:10.1002/anie.199518441
Return to citation in text: [1] -
Yan, X.; Liu, Y.; Xi, C. Appl. Organomet. Chem. 2008, 22, 341–345. doi:10.1002/aoc.1396
Return to citation in text: [1] -
Alonso, D. A.; Nájera, C. Chem. Soc. Rev. 2010, 39, 2891–2902. doi:10.1039/b821314n
Return to citation in text: [1] -
Hamasaka, G.; Sakurai, F.; Uozumi, Y. Chem. Commun. 2015, 51, 3886–3888. doi:10.1039/C4CC09726B
Return to citation in text: [1] -
Moreno, I.; SanMartin, R.; Herrero, M. T.; Dominguez, E. Curr. Top. Catal. 2009, 8, 91–102.
Return to citation in text: [1] -
Beletskaya, I. P.; Cheprakov, A. V. J. Organomet. Chem. 2004, 689, 4055–4082. doi:10.1016/j.jorganchem.2004.07.054
Return to citation in text: [1] -
Bruneau, A.; Roche, M.; Alami, M.; Messaoudi, S. ACS Catal. 2015, 5, 1386–1396. doi:10.1021/cs502011x
Return to citation in text: [1] -
Chen, W.; Xi, C.; Wu, Y. J. Organomet. Chem. 2007, 692, 4381–4388. doi:10.1016/j.jorganchem.2007.07.006
Return to citation in text: [1] -
Terrasson, V.; Prim, D.; Marrot, J. Eur. J. Inorg. Chem. 2008, 2008, 2739–2745. doi:10.1002/ejic.200800154
Return to citation in text: [1] -
Nájera, C.; Gil-Moltó, J.; Karlström, S. Adv. Synth. Catal. 2004, 346, 1798–1811. doi:10.1002/adsc.200404195
Return to citation in text: [1] -
Li, F.; Hor, T. S. A. Adv. Synth. Catal. 2008, 350, 2391–2400. doi:10.1002/adsc.200800356
Return to citation in text: [1] -
Yu, J.; Wang, L.; Liu, M.; Qiu, J.; Shen, Q.; Fang, L.; Tang, J. Chin. J. Chem. 2012, 30, 1114–1118. doi:10.1002/cjoc.201100544
Return to citation in text: [1] -
Saiyed, A. S.; Joshi, R. S.; Bedekar, A. V. J. Chem. Res. 2011, 35, 408–411. doi:10.3184/174751911X13100589976564
Return to citation in text: [1] -
Mukherjee, A.; Sarkar, A. Tetrahedron Lett. 2005, 46, 15–18. doi:10.1016/j.tetlet.2004.11.051
Return to citation in text: [1] -
Silberg, J.; Schareina, T.; Kempe, R.; Wurst, K.; Buchmeiser, M. R. J. Organomet. Chem. 2001, 622, 6–18. doi:10.1016/S0022-328X(00)00783-X
Return to citation in text: [1] -
Zhong, H.; Wang, J.; Li, L.; Wang, R. Dalton Trans. 2014, 43, 2098–2103. doi:10.1039/C3DT52970C
Return to citation in text: [1] -
Ishii, H.; Goyal, M.; Ueda, M.; Takeuchi, K.; Asai, M. Appl. Catal., A 2000, 201, 101–105. doi:10.1016/S0926-860X(00)00421-X
Return to citation in text: [1] -
Park, S. B.; Alper, H. Org. Lett. 2003, 5, 3209–3212. doi:10.1021/ol030071d
Return to citation in text: [1] -
Xiao, J.-C.; Twamley, B.; Shreeve, J. M. Org. Lett. 2004, 6, 3845–3847. doi:10.1021/ol048327i
Return to citation in text: [1] -
Weng, C.-M.; Hong, F.-E. Dalton Trans. 2011, 40, 6458–6468. doi:10.1039/c1dt10233h
Return to citation in text: [1] -
Chitanda, J. M.; Prokopchuk, D. E.; Quail, J. W.; Foley, S. R. Organometallics 2008, 27, 2337–2345. doi:10.1021/om800080e
Return to citation in text: [1] -
Ceder, R. M.; Muller, G.; Ordinas, M.; Font-Bardia, M.; Solans, X. Dalton Trans. 2003, 3052–3059. doi:10.1039/B302375C
Return to citation in text: [1] -
Paul, F.; Moulin, S.; Piechaczyk, O.; Le Floch, P.; Osborn, J. A. J. Am. Chem. Soc. 2007, 129, 7294–7304. doi:10.1021/ja068291k
Return to citation in text: [1] -
Grasa, G. A.; Hillier, A. C.; Nolan, S. P. Org. Lett. 2001, 3, 1077–1080. doi:10.1021/ol015676t
Return to citation in text: [1] -
Chen, W.; Xi, C.; Yang, K. Appl. Organomet. Chem. 2007, 21, 641–644. doi:10.1002/aoc.1224
Return to citation in text: [1] -
Bozic-Weber, B.; Constable, E. C.; Housecroft, C. E.; Neuburger, M.; Price, J. R. Dalton Trans. 2010, 39, 3585–3594. doi:10.1039/b925623g
Return to citation in text: [1] -
Hotze, A. C. G.; Faiz, J. A.; Mourtzis, N.; Pascu, G. I.; Webber, P. R. A.; Clarkson, G. J.; Yannakopoulou, K.; Pikramenou, Z.; Hannon, M. J. Dalton Trans. 2006, 3025–3034. doi:10.1039/b518027a
Return to citation in text: [1] -
Davies, D. L.; Lelj, F.; Lowe, M. P.; Ryder, K. S.; Singh, K.; Singh, S. Dalton Trans. 2014, 43, 4026–4039. doi:10.1039/c3dt52975d
Return to citation in text: [1] [2] -
Ouali, A.; Spindler, J.-F.; Jutand, A.; Taillefer, M. Adv. Synth. Catal. 2007, 349, 1906–1916. doi:10.1002/adsc.200600628
Return to citation in text: [1] -
Huang, Y.-T.; Tang, X.; Yang, Y.; Shen, D.-S.; Tan, C.; Liu, F.-S. Appl. Organomet. Chem. 2012, 26, 701–706. doi:10.1002/aoc.2913
Return to citation in text: [1] -
Huang, F.; Sun, Z.; Du, S.; Yue, E.; Ba, J.; Hu, X.; Liang, T.; Galland, G. B.; Sun, W.-H. Dalton Trans. 2015, 44, 14281–14292. doi:10.1039/C5DT01831E
Return to citation in text: [1] [2] -
Sun, W.-H.; Kong, S.; Chai, W.; Shiono, T.; Redshaw, C.; Hu, X.; Guo, C.; Hao, X. Appl. Catal., A: Gen. 2012, 447–448, 67–73. doi:10.1016/j.apcata.2012.09.011
Return to citation in text: [1] -
Huang, F.; Xing, Q.; Liang, T.; Flisak, Z.; Ye, B.; Hu, X.; Yang, W.; Sun, W.-H. Dalton Trans. 2014, 43, 16818–16829. doi:10.1039/C4DT02102A
Return to citation in text: [1] [2] -
Huang, F.; Zhang, W.; Yue, E.; Liang, T.; Hu, X.; Sun, W.-H. Dalton Trans. 2016, 45, 657–666. doi:10.1039/C5DT03779D
Return to citation in text: [1] -
Ye, B.; Wang, L.; Hu, X.; Redshaw, C.; Sun, W.-H. Inorg. Chim. Acta 2013, 407, 281–288. doi:10.1016/j.ica.2013.08.008
Return to citation in text: [1] -
Grasa, G. A.; Singh, R.; Stevens, E. D.; Nolan, S. P. J. Organomet. Chem. 2003, 687, 269–279. doi:10.1016/S0022-328X(03)00375-9
Return to citation in text: [1] -
John, L. C.; Gunay, A.; Wood, A. J.; Emmert, M. H. Tetrahedron 2013, 69, 5758–5764. doi:10.1016/j.tet.2013.03.011
Return to citation in text: [1] -
Dai, S.; Sui, X.; Chen, C. Angew. Chem., Int. Ed. 2015, 54, 9948–9953. doi:10.1002/anie.201503708
Return to citation in text: [1] -
Gholinejad, M.; Karimkhani, V.; Kim, I. Appl. Organomet. Chem. 2014, 28, 221–224. doi:10.1002/aoc.3110
Return to citation in text: [1] -
Xie, Y.; Huang, H.; Mo, W.; Fan, X.; Shen, Z.; Shen, Z.; Sun, N.; Hu, B.; Hu, X. Tetrahedron: Asymmetry 2009, 20, 1425–1432. doi:10.1016/j.tetasy.2009.05.014
Return to citation in text: [1] [2] -
Chai, W.; Yu, J.; Wang, L.; Hu, X.; Redshaw, C.; Sun, W.-H. Inorg. Chim. Acta 2012, 385, 21–26. doi:10.1016/j.ica.2011.12.008
Return to citation in text: [1] -
Ba, J.; Du, S.; Yue, E.; Hu, X.; Flisak, Z.; Sun, W.-H. RSC Adv. 2015, 5, 32720–32729. doi:10.1039/C5RA04722F
Return to citation in text: [1] [2] -
Tang, Y.; Zeng, Y.; Hu, Q.; Huang, F.; Jin, L.; Mo, W.; Sun, N.; Hu, B.; Shen, Z.; Hu, X.; Sun, W.-H. Adv. Synth. Catal. 2016, 358, 2642–2651. doi:10.1002/adsc.201600294
Return to citation in text: [1] [2] -
CCDC 1422920 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Return to citation in text: [1] -
Liu, L.; Zhang, S.; Chen, H.; Lv, Y.; Zhu, J.; Zhao, Y. Chem. – Asian J. 2013, 8, 2592–2595. doi:10.1002/asia.201300688
Return to citation in text: [1] -
Yu, J.; Hu, X.; Zeng, Y.; Zhang, L.; Ni, C.; Hao, X.; Sun, W.-H. New J. Chem. 2011, 35, 178–183. doi:10.1039/C0NJ00516A
Return to citation in text: [1]
| 1. | Negishi, E.-i. Handbook of organopalladium chemistry for organic synthesis; Wiley: New York Chichester, 2002. |
| 2. | Wu, X.-F.; Anbarasan, P.; Neumann, H.; Beller, M. Angew. Chem., Int. Ed. 2010, 49, 9047–9050. doi:10.1002/anie.201006374 |
| 3. | Magano, J.; Dunetz, J. R. Chem. Rev. 2011, 111, 2177–2250. doi:10.1021/cr100346g |
| 4. | Budarin, V. L.; Shuttleworth, P. S.; Clark, J. H.; Luque, R. Curr. Org. Synth. 2010, 7, 614–627. doi:10.2174/157017910794328529 |
| 16. | Kataoka, N.; Shelby, Q.; Stambuli, J. P.; Hartwig, J. F. J. Org. Chem. 2002, 67, 5553–5566. doi:10.1021/jo025732j |
| 17. | Hartwig, J. F. Synlett 2006, 1283–1294. doi:10.1055/s-2006-939728 |
| 47. | Park, S. B.; Alper, H. Org. Lett. 2003, 5, 3209–3212. doi:10.1021/ol030071d |
| 48. | Xiao, J.-C.; Twamley, B.; Shreeve, J. M. Org. Lett. 2004, 6, 3845–3847. doi:10.1021/ol048327i |
| 13. | Walker, S. D.; Barder, T. E.; Martinelli, J. R.; Buchwald, S. L. Angew. Chem., Int. Ed. 2004, 43, 1871–1876. doi:10.1002/anie.200353615 |
| 14. | Wolfe, J. P.; Singer, R. A.; Yang, B. H.; Buchwald, S. L. J. Am. Chem. Soc. 1999, 121, 9550–9561. doi:10.1021/ja992130h |
| 15. | Martin, R.; Buchwald, S. L. Acc. Chem. Res. 2008, 41, 1461–1473. doi:10.1021/ar800036s |
| 49. | Weng, C.-M.; Hong, F.-E. Dalton Trans. 2011, 40, 6458–6468. doi:10.1039/c1dt10233h |
| 50. | Chitanda, J. M.; Prokopchuk, D. E.; Quail, J. W.; Foley, S. R. Organometallics 2008, 27, 2337–2345. doi:10.1021/om800080e |
| 51. | Ceder, R. M.; Muller, G.; Ordinas, M.; Font-Bardia, M.; Solans, X. Dalton Trans. 2003, 3052–3059. doi:10.1039/B302375C |
| 52. | Paul, F.; Moulin, S.; Piechaczyk, O.; Le Floch, P.; Osborn, J. A. J. Am. Chem. Soc. 2007, 129, 7294–7304. doi:10.1021/ja068291k |
| 53. | Grasa, G. A.; Hillier, A. C.; Nolan, S. P. Org. Lett. 2001, 3, 1077–1080. doi:10.1021/ol015676t |
| 6. | Engle, K. M.; Yu, J.-Q. J. Org. Chem. 2013, 78, 8927–8955. doi:10.1021/jo400159y |
| 7. | Li, H.; Johansson Seechurn, C. C. C.; Colacot, T. J. ACS Catal. 2012, 2, 1147–1164. doi:10.1021/cs300082f |
| 8. | Fleckenstein, C. A.; Plenio, H. Chem. Soc. Rev. 2010, 39, 694–711. doi:10.1039/B903646F |
| 9. | Elsevier, C. J.; Reedijk, J.; Walton, P. H.; Ward, M. D. Dalton Trans. 2003, 1869–1880. doi:10.1039/b303975g |
| 10. | Hanhan, M. E. Appl. Organomet. Chem. 2008, 22, 270–275. doi:10.1002/aoc.1389 |
| 11. | Gildner, P. G.; Colacot, T. J. Organometallics 2015, 34, 5497–5508. doi:10.1021/acs.organomet.5b00567 |
| 12. | Miura, M. Angew. Chem., Int. Ed. 2004, 43, 2201–2203. doi:10.1002/anie.200301753 |
| 37. | Chen, W.; Xi, C.; Wu, Y. J. Organomet. Chem. 2007, 692, 4381–4388. doi:10.1016/j.jorganchem.2007.07.006 |
| 38. | Terrasson, V.; Prim, D.; Marrot, J. Eur. J. Inorg. Chem. 2008, 2008, 2739–2745. doi:10.1002/ejic.200800154 |
| 39. | Nájera, C.; Gil-Moltó, J.; Karlström, S. Adv. Synth. Catal. 2004, 346, 1798–1811. doi:10.1002/adsc.200404195 |
| 40. | Li, F.; Hor, T. S. A. Adv. Synth. Catal. 2008, 350, 2391–2400. doi:10.1002/adsc.200800356 |
| 41. | Yu, J.; Wang, L.; Liu, M.; Qiu, J.; Shen, Q.; Fang, L.; Tang, J. Chin. J. Chem. 2012, 30, 1114–1118. doi:10.1002/cjoc.201100544 |
| 42. | Saiyed, A. S.; Joshi, R. S.; Bedekar, A. V. J. Chem. Res. 2011, 35, 408–411. doi:10.3184/174751911X13100589976564 |
| 43. | Mukherjee, A.; Sarkar, A. Tetrahedron Lett. 2005, 46, 15–18. doi:10.1016/j.tetlet.2004.11.051 |
| 44. | Silberg, J.; Schareina, T.; Kempe, R.; Wurst, K.; Buchmeiser, M. R. J. Organomet. Chem. 2001, 622, 6–18. doi:10.1016/S0022-328X(00)00783-X |
| 45. | Zhong, H.; Wang, J.; Li, L.; Wang, R. Dalton Trans. 2014, 43, 2098–2103. doi:10.1039/C3DT52970C |
| 46. | Ishii, H.; Goyal, M.; Ueda, M.; Takeuchi, K.; Asai, M. Appl. Catal., A 2000, 201, 101–105. doi:10.1016/S0926-860X(00)00421-X |
| 28. | Lundgren, R. J.; Stradiotto, M. Chem. – Eur. J. 2012, 18, 9758–9769. doi:10.1002/chem.201201195 |
| 30. | Herrmann, W. A.; Brossmer, C.; Öfele, K.; Reisinger, C.-P.; Priermeier, T.; Beller, M.; Fischer, H. Angew. Chem., Int. Ed. Engl. 1995, 34, 1844–1848. doi:10.1002/anie.199518441 |
| 24. | Tang, W.; Capacci, A. G.; Wei, X.; Li, W.; White, A.; Patel, N. D.; Savoie, J.; Gao, J. J.; Rodriguez, S.; Qu, B.; Haddad, N.; Lu, B. Z.; Krishnamurthy, D.; Yee, N. K.; Senanayake, C. H. Angew. Chem., Int. Ed. 2010, 49, 5879–5883. doi:10.1002/anie.201002404 |
| 25. | Tang, W.; Keshipeddy, S.; Zhang, Y.; Wei, X.; Savoie, J.; Patel, N. D.; Yee, N. K.; Senanayake, C. H. Org. Lett. 2011, 13, 1366–1369. doi:10.1021/ol2000556 |
| 26. | Li, C.; Xiao, G.; Zhao, Q.; Liu, H.; Wang, T.; Tang, W. Org. Chem. Front. 2014, 1, 225–229. doi:10.1039/c4qo00024b |
| 27. | Li, C.; Chen, T.; Li, B.; Xiao, G.; Tang, W. Angew. Chem., Int. Ed. 2015, 54, 3792–3796. doi:10.1002/anie.201411518 |
| 31. | Yan, X.; Liu, Y.; Xi, C. Appl. Organomet. Chem. 2008, 22, 341–345. doi:10.1002/aoc.1396 |
| 32. | Alonso, D. A.; Nájera, C. Chem. Soc. Rev. 2010, 39, 2891–2902. doi:10.1039/b821314n |
| 33. | Hamasaka, G.; Sakurai, F.; Uozumi, Y. Chem. Commun. 2015, 51, 3886–3888. doi:10.1039/C4CC09726B |
| 34. | Moreno, I.; SanMartin, R.; Herrero, M. T.; Dominguez, E. Curr. Top. Catal. 2009, 8, 91–102. |
| 35. | Beletskaya, I. P.; Cheprakov, A. V. J. Organomet. Chem. 2004, 689, 4055–4082. doi:10.1016/j.jorganchem.2004.07.054 |
| 36. | Bruneau, A.; Roche, M.; Alami, M.; Messaoudi, S. ACS Catal. 2015, 5, 1386–1396. doi:10.1021/cs502011x |
| 21. | So, C. M.; Lau, C. P.; Kwong, F. Y. Org. Lett. 2007, 9, 2795–2798. doi:10.1021/ol070898y |
| 22. | Chung, K. H.; So, C. M.; Wong, S. M.; Luk, C. H.; Zhou, Z.; Lau, C. P.; Kwong, F. Y. Chem. Commun. 2012, 48, 1967–1969. doi:10.1039/c2cc15972d |
| 23. | Wong, S. M.; Yuen, O. Y.; Choy, P. Y.; Kwong, F. Y. Coord. Chem. Rev. 2015, 293–294, 158–186. doi:10.1016/j.ccr.2015.01.017 |
| 18. | Tang, H.; Menzel, K.; Fu, G. C. Angew. Chem., Int. Ed. 2003, 42, 5079–5082. doi:10.1002/anie.200352668 |
| 19. | Hills, I. D.; Netherton, M. R.; Fu, G. C. Angew. Chem., Int. Ed. 2003, 42, 5749–5752. doi:10.1002/anie.200352858 |
| 20. | Fu, G. C. Acc. Chem. Res. 2008, 41, 1555–1564. doi:10.1021/ar800148f |
| 29. | Herrmann, W. A.; Elison, M.; Fischer, J.; Köcher, C.; Artus, G. R. J. Angew. Chem., Int. Ed. Engl. 1995, 34, 2371–2374. doi:10.1002/anie.199523711 |
| 65. | Grasa, G. A.; Singh, R.; Stevens, E. D.; Nolan, S. P. J. Organomet. Chem. 2003, 687, 269–279. doi:10.1016/S0022-328X(03)00375-9 |
| 66. | John, L. C.; Gunay, A.; Wood, A. J.; Emmert, M. H. Tetrahedron 2013, 69, 5758–5764. doi:10.1016/j.tet.2013.03.011 |
| 67. | Dai, S.; Sui, X.; Chen, C. Angew. Chem., Int. Ed. 2015, 54, 9948–9953. doi:10.1002/anie.201503708 |
| 68. | Gholinejad, M.; Karimkhani, V.; Kim, I. Appl. Organomet. Chem. 2014, 28, 221–224. doi:10.1002/aoc.3110 |
| 54. | Chen, W.; Xi, C.; Yang, K. Appl. Organomet. Chem. 2007, 21, 641–644. doi:10.1002/aoc.1224 |
| 55. | Bozic-Weber, B.; Constable, E. C.; Housecroft, C. E.; Neuburger, M.; Price, J. R. Dalton Trans. 2010, 39, 3585–3594. doi:10.1039/b925623g |
| 56. | Hotze, A. C. G.; Faiz, J. A.; Mourtzis, N.; Pascu, G. I.; Webber, P. R. A.; Clarkson, G. J.; Yannakopoulou, K.; Pikramenou, Z.; Hannon, M. J. Dalton Trans. 2006, 3025–3034. doi:10.1039/b518027a |
| 57. | Davies, D. L.; Lelj, F.; Lowe, M. P.; Ryder, K. S.; Singh, K.; Singh, S. Dalton Trans. 2014, 43, 4026–4039. doi:10.1039/c3dt52975d |
| 58. | Ouali, A.; Spindler, J.-F.; Jutand, A.; Taillefer, M. Adv. Synth. Catal. 2007, 349, 1906–1916. doi:10.1002/adsc.200600628 |
| 59. | Huang, Y.-T.; Tang, X.; Yang, Y.; Shen, D.-S.; Tan, C.; Liu, F.-S. Appl. Organomet. Chem. 2012, 26, 701–706. doi:10.1002/aoc.2913 |
| 60. | Huang, F.; Sun, Z.; Du, S.; Yue, E.; Ba, J.; Hu, X.; Liang, T.; Galland, G. B.; Sun, W.-H. Dalton Trans. 2015, 44, 14281–14292. doi:10.1039/C5DT01831E |
| 61. | Sun, W.-H.; Kong, S.; Chai, W.; Shiono, T.; Redshaw, C.; Hu, X.; Guo, C.; Hao, X. Appl. Catal., A: Gen. 2012, 447–448, 67–73. doi:10.1016/j.apcata.2012.09.011 |
| 62. | Huang, F.; Xing, Q.; Liang, T.; Flisak, Z.; Ye, B.; Hu, X.; Yang, W.; Sun, W.-H. Dalton Trans. 2014, 43, 16818–16829. doi:10.1039/C4DT02102A |
| 63. | Huang, F.; Zhang, W.; Yue, E.; Liang, T.; Hu, X.; Sun, W.-H. Dalton Trans. 2016, 45, 657–666. doi:10.1039/C5DT03779D |
| 64. | Ye, B.; Wang, L.; Hu, X.; Redshaw, C.; Sun, W.-H. Inorg. Chim. Acta 2013, 407, 281–288. doi:10.1016/j.ica.2013.08.008 |
| 69. | Xie, Y.; Huang, H.; Mo, W.; Fan, X.; Shen, Z.; Shen, Z.; Sun, N.; Hu, B.; Hu, X. Tetrahedron: Asymmetry 2009, 20, 1425–1432. doi:10.1016/j.tetasy.2009.05.014 |
| 71. | Ba, J.; Du, S.; Yue, E.; Hu, X.; Flisak, Z.; Sun, W.-H. RSC Adv. 2015, 5, 32720–32729. doi:10.1039/C5RA04722F |
| 75. | Yu, J.; Hu, X.; Zeng, Y.; Zhang, L.; Ni, C.; Hao, X.; Sun, W.-H. New J. Chem. 2011, 35, 178–183. doi:10.1039/C0NJ00516A |
| 57. | Davies, D. L.; Lelj, F.; Lowe, M. P.; Ryder, K. S.; Singh, K.; Singh, S. Dalton Trans. 2014, 43, 4026–4039. doi:10.1039/c3dt52975d |
| 74. | Liu, L.; Zhang, S.; Chen, H.; Lv, Y.; Zhu, J.; Zhao, Y. Chem. – Asian J. 2013, 8, 2592–2595. doi:10.1002/asia.201300688 |
| 72. | Tang, Y.; Zeng, Y.; Hu, Q.; Huang, F.; Jin, L.; Mo, W.; Sun, N.; Hu, B.; Shen, Z.; Hu, X.; Sun, W.-H. Adv. Synth. Catal. 2016, 358, 2642–2651. doi:10.1002/adsc.201600294 |
| 73. | CCDC 1422920 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. |
| 60. | Huang, F.; Sun, Z.; Du, S.; Yue, E.; Ba, J.; Hu, X.; Liang, T.; Galland, G. B.; Sun, W.-H. Dalton Trans. 2015, 44, 14281–14292. doi:10.1039/C5DT01831E |
| 62. | Huang, F.; Xing, Q.; Liang, T.; Flisak, Z.; Ye, B.; Hu, X.; Yang, W.; Sun, W.-H. Dalton Trans. 2014, 43, 16818–16829. doi:10.1039/C4DT02102A |
| 69. | Xie, Y.; Huang, H.; Mo, W.; Fan, X.; Shen, Z.; Shen, Z.; Sun, N.; Hu, B.; Hu, X. Tetrahedron: Asymmetry 2009, 20, 1425–1432. doi:10.1016/j.tetasy.2009.05.014 |
| 70. | Chai, W.; Yu, J.; Wang, L.; Hu, X.; Redshaw, C.; Sun, W.-H. Inorg. Chim. Acta 2012, 385, 21–26. doi:10.1016/j.ica.2011.12.008 |
| 71. | Ba, J.; Du, S.; Yue, E.; Hu, X.; Flisak, Z.; Sun, W.-H. RSC Adv. 2015, 5, 32720–32729. doi:10.1039/C5RA04722F |
| 72. | Tang, Y.; Zeng, Y.; Hu, Q.; Huang, F.; Jin, L.; Mo, W.; Sun, N.; Hu, B.; Shen, Z.; Hu, X.; Sun, W.-H. Adv. Synth. Catal. 2016, 358, 2642–2651. doi:10.1002/adsc.201600294 |
© 2017 Lai et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)