Abstract
Electronic differentiations in Pd-catalyzed allylic substitutions are assessed computationally from transition structure models with electronically modified phospha-benzene-pyridine ligands. Although donor/acceptor substitutions at P and N ligand sites were expected to increase the site selectivity, i.e. the preference for "trans to P" attack at the allylic intermediate, acceptor/acceptor substitution yields the highest selectivity. Energetic and geometrical analyses of transition structures show that the sensitivity for electronic differentiation is crucial for this site selectivity. Early transition structures with acceptor substituted ligands give rise to more intensive Pd-allyl interactions, which transfer electronic P,N differentiation of the ligand more efficiently to the allyl termini and hence yield higher site selectivities.
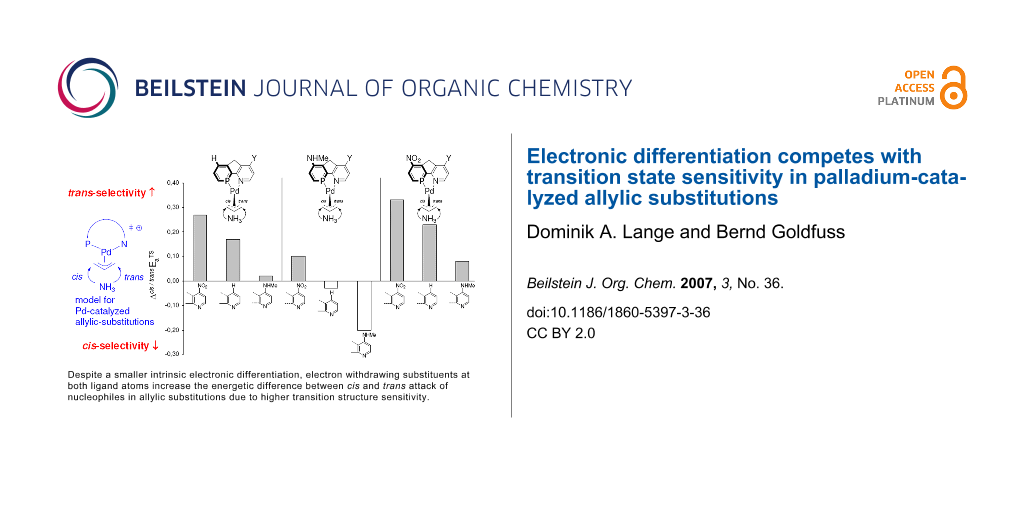
Graphical Abstract
Introduction
Palladium-catalyzed allylic substitutions allow very selective and mild allylations of C-,N- and O-nucleophiles. [1-13] The selectivity derives from steric and electronic properties of substrate and catalyst structures. "Side arm guidance" of nucleophiles with multifunctional phosphinoferrocenes [14-18] or "chiral pockets" in C2-symmetric diphosphanes based on 2-(diphenyl-phosphino)benzoic acid amides [19-22] were applied especially successfully. Chiral P,N-ligands (e.g. phosphinooxazolines, phox) [23-27] provide in addition to steric control the possibility for "electronic differentiation", originating from the trans-influence [28] of different donor atoms. Nucleophiles (e.g. dimethylmalonate) normally favour addition to the "trans to phosphorus" position at the Pd-η3-allylic intermediate (Scheme 1). [29-42] This "trans to P" rule is supported by X-ray and computational analyses of Pd-η3-allylic intermediates, which exhibit longer and hence weaker Pd-Callyl bonds trans to P (i.e. the stronger π-acceptor vs. N) and hence are more susceptible to nucleophilic attack (Scheme 1). [29-41] This electronic differentiation contributes to the high selectivity in Pd-catalyzed asymmetric allylic substitutions[19] and provides also an explanation for α-memory effects. [42,43] Computational model systems for P,N-ligands, i.e. PH3 and para-substituted pyridines, have shown that cis-trans differentiations, i.e. the electronic site selectivity, of nucleophilic additions to Pd-η3-allylic intermediates is highest for electron poor pyridine ligands.[45]
Scheme 1: Electronic and steric differentiations provide the basis for the high selectivity of P,N-ligands in Pd-catalyzed allylic substitutions. Effects are studied with P-N-model ligands with para-substituted, coplanar phosphabenzene and pyridine moieties.
Scheme 1: Electronic and steric differentiations provide the basis for the high selectivity of P,N-ligands in...
To further explore origins of site selectivities based on electronic differentiations in Pd-catalyzed allylic substitutions, we here employ a more advanced model system with phosphabenzene, [45-48] and pyridine moieties for the crucial step of Pd-catalyzed allylic substitutions. Both P- and N-coordination sites are tuned electronically with para-substituents to reveal energetic and geometrical effects on cis- vs. trans- additions of nucleophiles to the Pd-η3-allylic intermediates (Scheme 1).
Results and Discussion
Electron donating or withdrawing groups (i. e. X, Y = HNMe, H, NO2) in para-positions of phosphabenzene (X) and pyridine (Y) units tune electronic characteristics of P,N-ligand models in Pd-catalyzed allylic substitutions (Scheme 1). The phosphabenzene and pyridine moieties are linked via Car-Car bonds and a methylene bridge retains planarity and limits conformational flexibility. NHMe rather than higher substituted NMe2 was employed as donor group, to retain lp-aryl conjugation. Ammonia serves as model nucleophile and attacks the Pd-η3-allylic intermediate cis or trans to phosphorus. This cis vs. trans site selectivity is employed as measure for electronic differentiation induced by the ligand system (Scheme 2).
Scheme 2: Activation (ΔEa) and reaction (ΔEr) energies (kcal mol-1), computed for the P,N-ligand model with tuneable electronic differentiation.
Scheme 2: Activation (ΔEa) and reaction (ΔEr) energies (kcal mol-1), computed for the P,N-ligand model with t...
The lowest activation energies (Ea, Table 1) for ammonia addition to the Pd-η3-allylic intermediate are apparent for strong electron withdrawing para-substituted phosphabenzene and pyridine units, i.e. X, Y = NO2 (Figure 1 and Figure 2, Eatrans = 2.19, Eacis = 2.52 kcal mol-1, Table 1). The highest activation energies result from electron donating amino groups X, Y = NHMe (Figure 3 and Figure 4, Eatrans = 10.67, Eacis = 10.47 kcal mol-1, Table 1, Scheme 2). Such electronic tunings of the ligands strongly affect the reactivity and give rise to increased or decreased electrophilicity of Pd-allyl intermediates.
Table 1: Activation (Ea) and reaction energies (Er) reflecting electronic differentiations in transition structures (ΔEacis-trans) and Pd-ene products relative to Pd-allyl and NH3 reactands (pb = phosphabenzene; py = pyridine moieties)[a]
| pb-X | py-Y | Ea | TS | ΔEaTS | ErProd | ΔErProd |
|---|---|---|---|---|---|---|
| H | HNMe | cis | 8.55 | 0.03 | 7.81 | 0.55 |
| trans | 8.52 | 8.36 | ||||
| H | H | cis | 6.38 | 0.17 | 5.14 | 0.52 |
| trans | 6.21 | 5.67 | ||||
| H | NO2 | cis | 4.47 | 0.27 | 2.48 | 0.54 |
| trans | 4.20 | 3.02 | ||||
| HNMe | HNMe | cis | 10.47 | -0.20 [b] | 10.33 | 0.65 |
| trans | 10.67 | 10.98 | ||||
| HNMe | H | cis | 8.43 | -0.03[b] | 7.80 | 0.60 |
| trans | 8.46 | 8.40 | ||||
| HNMe | NO2 | cis | 6.61 | 0.10 | 5.34 | 0.65 |
| trans | 6.51 | 5.99 | ||||
| NO2 | HNMe | cis | 6.34 | 0.08 | 5.05 | 0.53 |
| trans | 6.26 | 5.58 | ||||
| NO2 | H | cis | 4.24 | 0.23 | 2.26 | 0.43 |
| trans | 4.01 | 2.70 | ||||
| NO2 | NO2 | cis | 2.52 | 0.33 | -0.25[c] | 0.54 |
| trans | 2.19 | 0.29 | ||||
[a] B3LYP/6-31G* (C, H, N, P, O), /SDD (Pd) optimized structures. Energies include ZPE corrections scaled by 0.9806; [b] Negative ΔEaTS with Eacis < Eatrans; [c] exothermic reaction energy.
Figure 1: Transition structure for the energetically favored trans to phosphorus addition of ammonia at the Pd-η3-allylic intermediate (B3LYP/6-31G* (C, H, N, P, O), /SDD (Pd)). Bond distances are given in Å.
Figure 1: Transition structure for the energetically favored trans to phosphorus addition of ammonia at the P...
Figure 2: Transition structure for the energetically disfavored cis to phosphorus addition of ammonia at the Pd-η3-allylic intermediate (B3LYP/6-31G* (C, H, N, P, O), /SDD (Pd)). Bond distances are given in Å.
Figure 2: Transition structure for the energetically disfavored cis to phosphorus addition of ammonia at the ...
Figure 3: Transition structure for the energetically disfavored trans to phosphorus addition of ammonia at the Pd-η3-allylic intermediate (B3LYP/6-31G* (C, H, N, P, O), /SDD (Pd)). Bond distances are given in Å.
Figure 3: Transition structure for the energetically disfavored trans to phosphorus addition of ammonia at th...
Figure 4: Transition structure for the energetically favored cis to phosphorus addition of ammonia at the Pd-η3-allylic intermediate (B3LYP/6-31G* (C, H, N, P, O), /SDD (Pd)). Bond distances are given in Å.
Figure 4: Transition structure for the energetically favored cis to phosphorus addition of ammonia at the Pd-η...
The reaction energies (Er) for ammonia addition to the Pd-η3-allylic intermediate show a similar preference: Pd-ene-adduct formation is favoured most for X, Y = NO2 (Ertrans = 0.29, Ercis = -0.25 kcal mol-1) and becomes most unfavourable (i.e. endothermic) for X, Y = NHMe (Ertrans = 10.98, Ercis = 10.33 kcal mol-1, Table 1, Scheme 2). This points to a more π-donating character of the ene product relative to the allyl-cation reactant.
In agreement with the "trans to phosphorus" rule, [23-28] attack of ammonia is preferred for most X, Y combinations trans to P, due to the stronger π*/σ* acidity at P in phosphabenzene relative to N in pyridine (Table 1).[44] Surprisingly however, this electronic site selectivity, as it is measured from relative energies of the transition structures (ΔEaTS), is not largest for different X, Y donor-acceptor combinations (Figure 5, Figure 6, Figure 7 and Figure 8), but is highest for X and Y = NO2 (ΔEaTS = 0.33 kcal mol-1, Table 1). Likewise, the smallest electronic site "trans to P" selectivity is not found for X, Y donor-acceptor combinations, but for strong donating X and Y = NHMe. Here, the selectivity is so low, that it even inverts to "cis to P" (ΔEaTS = -0.20 kcal mol-1, Table 1).
Figure 5: Transition structure for the energetically favored trans to phosphorus addition of ammonia at the Pd-η3-allylic intermediate (B3LYP/6-31G* (C, H, N, P, O), /SDD (Pd)). Bond distances are given in Å.
Figure 5: Transition structure for the energetically favored trans to phosphorus addition of ammonia at the P...
Figure 6: Transition structure for the energetically disfavored cis to phosphorus addition of ammonia at the Pd-η3-allylic intermediate (B3LYP/6-31G* (C, H, N, P, O), /SDD (Pd)). Bond distances are given in Å.
Figure 6: Transition structure for the energetically disfavored cis to phosphorus addition of ammonia at the ...
Figure 7: Transition structure for the energetically disfavored cis to phosphorus addition of ammonia at the Pd-η3-allylic intermediate (B3LYP/6-31G* (C, H, N, P, O), /SDD (Pd)). Bond distances are given in Å.
Figure 7: Transition structure for the energetically disfavored cis to phosphorus addition of ammonia at the ...
Figure 8: Transition structure for the energetically favored trans to phosphorus addition of ammonia at the Pd-η3-allylic intermediate (B3LYP/6-31G* (C, H, N, P, O), /SDD (Pd)). Bond distances are given in Å.
Figure 8: Transition structure for the energetically favored trans to phosphorus addition of ammonia at the P...
For each phosphabenzene moiety with X = H or NHMe or NO2, the "trans to P" site selectivity ΔEaTS increases for pyridine substituents Y in the order NHMe < H < NO2 (Figure 9, Table 1). Hence, there is apparently an additional effect, which controls the site selectivity ΔEaTS besides the electronic donor vs. acceptor properties of different ligand atoms, i.e. P vs. N. Via this effect; electron withdrawing groups (e.g. NO2) give rise to the highest site-selectivities.
Figure 9: For each phosphabenzene moiety, the site selectivities ΔEaTS increase with more electron withdrawing pyridine substituents (Y) in the order HNMe < H < NO2 (cf. Table 1).
Figure 9: For each phosphabenzene moiety, the site selectivities ΔEaTS increase with more electron withdrawin...
NO2-substituted ligands give rise to earlier transition structures with longer (forming) H3N-Cα bonds (Table 2, Figure 1 to Figure 8), e.g. trans-TS with X = Y = NO2: H3N-Cα = 2.04 Å (Figure 1). In contrast, amino-donor substitution leads to later transition structures with shorter H3N-Cα distances, e.g. trans-TS with X = Y = NHMe: H3N-Cα = 1.866 Å (Figure 3). This agrees with the more electrophilic properties of cationic Pd-allyl intermediates induced by electron withdrawing ligands.
Table 2: H3N-Cα, H3N+-Cα and Pd-Cα distances (Å) of transition states and Pd-ene product complexes (pb = phosphabenzene; py = pyridine)[a]
| Transition structures | Pd-ene product complexes | ||||
|---|---|---|---|---|---|
| Pb-X | py-Y | Pd-Cα | H3N-Cα | H3N+-Cα | |
| H | HNMe | cis | 2.754 | 1.930 | 1.594 |
| trans | 2.834 | 1.906 | 1.604 | ||
| H | H | cis | 2.728 | 1.968 | 1.588 |
| trans | 2.815 | 1.947 | 1.598 | ||
| H | NO2 | cis | 2.696 | 2.010 | 1.583 |
| trans | 2.797 | 1.989 | 1.592 | ||
| HNMe | HNMe | cis | 2.767 | 1.898 | 1.598 |
| trans | 2.850 | 1.866 | 1.611 | ||
| HNMe | H | cis | 2.745 | 1.932 | 1.593 |
| trans | 2.840 | 1.902 | 1.603 | ||
| HNMe | NO2 | cis | 2.718 | 1.969 | 1.588 |
| trans | 2.824 | 1.940 | 1.598 | ||
| NO2 | HNMe | cis | 2.733 | 1.970 | 1.587 |
| trans | 2.805 | 1.957 | 1.596 | ||
| NO2 | H | cis | 2.703 | 2.012 | 1.582 |
| trans | 2.787 | 1.997 | 1.590 | ||
| NO2 | NO2 | cis | 2.674 | 2.051 | 1.578 |
| trans | 2.765 | 2.040 | 1.586 | ||
[a] B3LYP/6-31G* (C, H, N, P, O), /SDD (Pd) optimized structures. Energies include ZPE corrections scaled by 0.9806.
These positions on the reaction coordinate indeed correspond to the site selectivity of the transition structures, i.e. ΔEaTS: earlier transition structures have higher, later transition structures exhibit lower "trans to P" selectivities (Figure 10).
Figure 10: Higher site selectivities, i.e. larger ΔEaTS values, are found for earlier transition structures with larger H3N-Cα distances.
Figure 10: Higher site selectivities, i.e. larger ΔEaTS values, are found for earlier transition structures wi...
The distance between Pd and the allylic systems decreases from early (allyl cation like) to late (ene like) positions on the reaction coordinate. A closer, more intense Pd-Cα contact (e.g. 2.674 Å, Figure 2, Table 2) stronger delivers electronic differentiation of the ligand, and hence "trans to P" selectivity. Hence, higher electronic site selectivity closely corresponds to intense Pd-allyl interactions with short Pd-Cα distances (Figure 11).
Figure 11: Higher site selectivities, i.e. larger ΔEaTS values, are found for transition structures with closer, more intense Pd-Cα contacts.
Figure 11: Higher site selectivities, i.e. larger ΔEaTS values, are found for transition structures with close...
Apparently, the positions on the reaction coordinate influence the site selectivity even stronger than the electronic differentiation between P and N ligand atoms: No substitution (X = Y = H) gives rise to even higher ΔEaTS than more pronounced electronic differentiations with X, Y = NO2 or NHMe (Figure 11), due to higher TS-sensitivity originating from closer Pd-allyl contact.
Conclusion
In Pd-catalyzed allylic substitutions, the electronic site selectivity, i.e. the preference for "trans to P" addition, is affected by the intrinsic electronic differentiation of the ligand atoms, e.g. P vs. N. However, the sensitivity for this electronic differentiation depends on the intensity of the Pd-allyl interaction. A close Pd-allyl distance in an early, allyl cation like transition structure delivers the electronic differentiation of the ligand system more efficiently to the allylic termini (Cα) than a more distant Pd-allyl (more ene like) unit of a late transition structure. Electron withdrawing (e.g. NO2) substituents in the ligand system generate earlier transition structures with more intense Pd-allyl interactions and higher sensitivity for electronic differentiations. Hence, both intrinsic electronic differentiation in the ligand and high TS-sensitivity appear to be crucial for high site-selectivity in Pd-catalyzed allylic substitutions.
Computational details
All structures were fully optimized and characterized by frequency computations as minima or transition structures using Gaussian 03[49] with standard basis sets [50,51] and the B3LYP [52-55] hybrid-DFT method. Zero point energies and thermochemical analysis were scaled by 0.9806.[56]
Acknowledgements
We are grateful to the Fonds der Chemischen Industrie for financial support as well as for a Dozenten-Stipendium to B.G. We especially thank the Deutsche Forschungsgemeinschaft (DFG) for funding (GO-930/9, GO-930/7 and GO-930/5) as well as the Bayer AG, the BASF AG, the Wacker AG, the Degussa AG, the Raschig GmbH, the Symrise GmbH, the Solvay GmbH and the OMG AG for generous support.
References
-
Tsuji, J. Acc. Chem. Res. 1969, 2, 144–152. doi:10.1021/ar50017a003
Return to citation in text: [1] -
Trost, B. M.; Fullerton, T. J. J. Am. Chem. Soc. 1973, 95, 292–294. doi:10.1021/ja00782a080
Return to citation in text: [1] -
Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395–422. doi:10.1021/cr9409804
Return to citation in text: [1] -
Pfaltz, A.; Lautens, M. In Comprehensive Asymmetric Catalysis; Jacobsen, E. N.; Pfaltz, A.; Yamamoto, H., Eds.; Springer: Heidelberg, 1999; Vol. Chapter 24, pp 2–49.
Return to citation in text: [1] -
Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921–2944. doi:10.1021/cr020027w
Return to citation in text: [1] -
Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2004, 126, 15044–15045. doi:10.1021/ja044812x
Return to citation in text: [1] -
Trost, B. M.; Xu, J. J. Am. Chem. Soc. 2005, 127, 2846–2847. doi:10.1021/ja043472c
Return to citation in text: [1] -
Goldfuss, B.; Löschmann, T.; Kop-Weiershausen, T.; Neudörfl, J.; Rominger, F. Beilstein J. Org. Chem. 2006, 2, No. 7. doi:10.1186/1860-5397-2-7
Return to citation in text: [1] -
Savoia, D.; Alvaro, G.; Di Fabio, R.; Fiorelli, C.; Gualandi, A.; Monari, M.; Piccinelli, F. Adv. Synth. Catal. 2006, 348, 1883–1893. doi:10.1002/adsc.200606109
Return to citation in text: [1] -
Braun, M.; Meier, T. Angew. Chem. 2006, 118, 7106–7109. doi:10.1002/ange.200602169
Angew. Chem., Int. Ed. 2006, 45, 6952–6955.
Return to citation in text: [1] -
You, S.-L.; Dai, L.-X. Angew. Chem. 2006, 118, 5372–5374. doi:10.1002/ange.200601889
Angew. Chem., Int. Ed. 2006, 45, 5246–5248.
Return to citation in text: [1] -
Raluy, E.; Dieguez, M.; Pamies, O. J. Org. Chem. 2007, 72, 2842–2850. doi:10.1021/jo062311j
Return to citation in text: [1] -
Schulz, S. R.; Blechert, S. Angew. Chem. 2007, 119, 4040–4044. doi:10.1002/ange.200604553
Angew. Chem., Int. Ed. 2007, 46, 3966–3970.
Return to citation in text: [1] -
Hayashi, T.; Yamamoto, A.; Hagihara, T.; Ito, Y. Tetrahedron Lett. 1986, 27, 191–194. doi:10.1016/S0040-4039(00)83974-X
Return to citation in text: [1] -
Hayashi, T.; Kanehira, K.; Hagihara, T.; Kumada, M. J. Org. Chem. 1988, 53, 113–120. doi:10.1021/jo00236a023
Return to citation in text: [1] -
Hayashi, T. Pure Appl. Chem. 1988, 60, 7–13. doi:10.1351/pac198860010007
Return to citation in text: [1] -
Sawamura, M.; Ito, Y. Chem. Rev. 1992, 92, 857–871. doi:10.1021/cr00013a005
Return to citation in text: [1] -
Enders, D.; Peters, R.; Lochtman, R.; Raabe, G.; Runsink, J.; Bats, J. W. Eur. J. Org. Chem. 2000, 20, 3399–3426. doi:10.1002/1099-0690(200010)2000:20<3399::AID-EJOC3399>3.0.CO;2-D
Return to citation in text: [1] -
Trost, B. M.; Breit, B.; Peukert, S.; Zambrano, J.; Ziller, J. W. Angew. Chem. 1995, 107, 2577–2579. doi:10.1002/ange.19951072114
Angew. Chem., Int. Ed. 1995, 34, 2386–2388.
Return to citation in text: [1] [2] -
Trost, B. M. Acc. Chem. Res. 1996, 29, 355–364. doi:10.1021/ar9501129
Return to citation in text: [1] -
Trost, B. M.; Heinemann, C.; Ariza, X.; Weigand, S. J. Am. Chem. Soc. 1999, 121, 8667–8668. doi:10.1021/ja991821a
Return to citation in text: [1] -
Trost, B. M.; Ariza, X. J. Am. Chem. Soc. 1999, 121, 10727–10737. doi:10.1021/ja992754n
Return to citation in text: [1] -
Helmchen, G.; Kudis, S.; Sennehenn, P.; Steinhagen, H. Pure Appl. Chem. 1997, 69, 513–519. doi:10.1351/pac199769030513
Return to citation in text: [1] [2] -
Helmchen, G. J. Organomet. Chem. 1999, 576, 203–214. doi:10.1016/S0022-328X(98)01059-6
Return to citation in text: [1] [2] -
Kolmar, M.; Goldfuss, B.; Reggelin, M.; Rominger, F.; Helmchen, G. Chem.–Eur. J. 2001, 7, 4913–4927. doi:10.1002/1521-3765(20011119)7:22<4913::AID-CHEM4913>3.0.CO;2-7
Return to citation in text: [1] [2] -
Kollmar, M.; Steinhagen, H.; Janssen, J. P.; Goldfuss, B.; Malinovskaya, S. A.; Vázques, J.; Rominger, F.; Helmchen, G. Chem.–Eur. J. 2002, 8, 3103–3114. doi:10.1002/1521-3765(20020715)8:14<3103::AID-CHEM3103>3.0.CO;2-C
Return to citation in text: [1] [2] -
Vázquez, J.; Goldfuss, B.; Helmchen, G. J. Organomet. Chem. 2002, 641, 67–70. doi:10.1016/S0022-328X(01)01308-0
Return to citation in text: [1] [2] -
Appleton, T. G.; Clark, H. C.; Manzer, L. E. Coord. Chem. Rev. 1973, 10, 335–422. doi:10.1016/S0010-8545(00)80238-6
Return to citation in text: [1] [2] -
Sprinz, J.; Kiefer, M.; Helmchen, G.; Reggelin, M.; Huttner, G.; Walter, O.; Zsolnai, L. Tetrahedron Lett. 1994, 35, 1523–1526. doi:10.1016/S0040-4039(00)76748-7
Return to citation in text: [1] [2] -
Ward, T. R. Organometallics 1996, 15, 2836–2838. doi:10.1021/om960158l
Return to citation in text: [1] [2] -
Oslob, J. D.; Akermark, B.; Helquist, P.; Norrby, P.-O. Organometallics 1997, 16, 3015–3021. doi:10.1021/om9700371
Return to citation in text: [1] [2] -
Moberg, C.; Bremberg, U.; Hallman, K.; Svensson, M.; Norbby, P.-O.; Hallberg, A.; Larhed, M.; Csöregh, I. Pure Appl. Chem. 1999, 71, 1477–1485. doi:10.1351/pac199971081477
Return to citation in text: [1] [2] -
Hagelin, H.; Akermark, B.; Norrby, P.-O. Organometallics 1999, 18, 2884–2895. doi:10.1021/om990153z
Return to citation in text: [1] [2] -
Hagelin, H.; Svensson, M.; Akermark, B.; Norrby, P.-O. Organometallics 1999, 18, 4574–4583. doi:10.1021/om990228z
Return to citation in text: [1] [2] -
Pedersen, T. M.; Hansen, E.; Louise, K.; Kane, J.; Rein, T.; Helquist, P.; Norrby, P.-O.; Tanner, D. J. Am. Chem. Soc. 2001, 123, 9738–9742. doi:10.1021/ja005809q
Return to citation in text: [1] [2] -
Tu, T.; Zhou, Y.; Hou, X.; Dai, L.; Dong, X.; Yu, Y.; Sun, J. Organometallics 2003, 22, 1255–1265. doi:10.1021/om020706x
Return to citation in text: [1] [2] -
Norrby, P.-O.; Mader, M. M.; Vitale, M.; Prestat, G.; Poli, G. Organometallics 2003, 22, 1849–1855. doi:10.1021/om030066d
Return to citation in text: [1] [2] -
Madec, D.; Prestat, G.; Martini, E.; Fristrup, P.; Poli, G.; Norrby, P.-O. Org. Lett. 2005, 7, 995–998. doi:10.1021/ol047548l
Return to citation in text: [1] [2] -
Fristrup, P.; Jensen, T.; Hoppe, J.; Norrby, P.-O. Chem.–Eur. J. 2006, 12, 5352–5360. doi:10.1002/chem.200600152
Return to citation in text: [1] [2] -
Ahlquist, M.; Fabrizi, G.; Cacchi, S.; Norrby, P.-O. J. Am. Chem. Soc. 2006, 128, 12785–12793. doi:10.1021/ja061543x
Return to citation in text: [1] [2] -
Ahlquist, M.; Norrby, P.-O. Organometallics 2007, 26, 550–553. doi:10.1021/om0604932
Return to citation in text: [1] [2] -
Goldfuss, B.; Kazmeier, U.; Goldfuss, B.; Kazmaier, U. Tetrahedron 2000, 56, 6493–6496. doi:10.1016/S0040-4020(00)00613-X
Return to citation in text: [1] [2] -
Boele, M. D. K.; Kamer, P. C. J.; Lutz, M.; Spek, A. L.; de Vries, J. G.; van Leeuwen, P. W. N. M.; van Strijdonck, G. P. F. Chem.–Eur. J. 2004, 10, 6232–6246. doi:10.1002/chem.200400154
Return to citation in text: [1] -
Goldfuss, B. J. Organomet. Chem. 2006, 691, 4508–4513. doi:10.1016/j.jorganchem.2006.01.061
Return to citation in text: [1] -
Märkl, G.; Lieb, F.; Merz, A. Angew. Chem. 1967, 79, 947–948. doi:10.1002/ange.19670792125
Angew. Chem., Int. Ed. 1967, 6, 458–459.
Return to citation in text: [1] [2] -
Ashe, A. J., III. J. Am. Chem. Soc. 1971, 93, 3293–3295. doi:10.1021/ja00742a038
Return to citation in text: [1] -
Shiotsuka, M.; Tanamachi, T.; Matsuda, Y. Chem. Lett. 1995, 24, 531–533. doi:10.1246/cl.1995.531
Return to citation in text: [1] -
Breit, B.; Winde, R.; Harms, K. J. Chem. Soc., Perkin Trans. 1 1997, 18, 2681–2683. doi:10.1039/a705249i
Return to citation in text: [1] -
Gaussian 03, Revision C.02; Gaussian, Inc.: Wallingford, CT, 2004.
Return to citation in text: [1] -
Ditchfield, R.; Hehre, W. J.; Pople, J. A. J. Chem. Phys. 1971, 54, 724–728. doi:10.1063/1.1674902
Return to citation in text: [1] -
Rassolov, V. A.; Ratner, M. A.; Pople, J. A.; Redfern, P. C.; Curtiss, L. A. J. Comput. Chem. 2001, 22, 976–984. doi:10.1002/jcc.1058
Return to citation in text: [1] -
Becke, A. D. J. Chem. Phys. 1993, 98, 5648–5652. doi:10.1063/1.464913
Return to citation in text: [1] -
Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623–11627. doi:10.1021/j100096a001
Return to citation in text: [1] -
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/PhysRevB.37.785
Return to citation in text: [1] -
Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Chem. Phys. Lett. 1989, 157, 200–206. doi:10.1016/0009-2614(89)87234-3
Return to citation in text: [1] -
Scott, A. P.; Radom, L. J. Phys. Chem. 1996, 100, 16502–16513. doi:10.1021/jp960976r
Return to citation in text: [1]
| 56. | Scott, A. P.; Radom, L. J. Phys. Chem. 1996, 100, 16502–16513. doi:10.1021/jp960976r |
| 1. | Tsuji, J. Acc. Chem. Res. 1969, 2, 144–152. doi:10.1021/ar50017a003 |
| 2. | Trost, B. M.; Fullerton, T. J. J. Am. Chem. Soc. 1973, 95, 292–294. doi:10.1021/ja00782a080 |
| 3. | Trost, B. M.; Van Vranken, D. L. Chem. Rev. 1996, 96, 395–422. doi:10.1021/cr9409804 |
| 4. | Pfaltz, A.; Lautens, M. In Comprehensive Asymmetric Catalysis; Jacobsen, E. N.; Pfaltz, A.; Yamamoto, H., Eds.; Springer: Heidelberg, 1999; Vol. Chapter 24, pp 2–49. |
| 5. | Trost, B. M.; Crawley, M. L. Chem. Rev. 2003, 103, 2921–2944. doi:10.1021/cr020027w |
| 6. | Behenna, D. C.; Stoltz, B. M. J. Am. Chem. Soc. 2004, 126, 15044–15045. doi:10.1021/ja044812x |
| 7. | Trost, B. M.; Xu, J. J. Am. Chem. Soc. 2005, 127, 2846–2847. doi:10.1021/ja043472c |
| 8. | Goldfuss, B.; Löschmann, T.; Kop-Weiershausen, T.; Neudörfl, J.; Rominger, F. Beilstein J. Org. Chem. 2006, 2, No. 7. doi:10.1186/1860-5397-2-7 |
| 9. | Savoia, D.; Alvaro, G.; Di Fabio, R.; Fiorelli, C.; Gualandi, A.; Monari, M.; Piccinelli, F. Adv. Synth. Catal. 2006, 348, 1883–1893. doi:10.1002/adsc.200606109 |
| 10. |
Braun, M.; Meier, T. Angew. Chem. 2006, 118, 7106–7109. doi:10.1002/ange.200602169
Angew. Chem., Int. Ed. 2006, 45, 6952–6955. |
| 11. |
You, S.-L.; Dai, L.-X. Angew. Chem. 2006, 118, 5372–5374. doi:10.1002/ange.200601889
Angew. Chem., Int. Ed. 2006, 45, 5246–5248. |
| 12. | Raluy, E.; Dieguez, M.; Pamies, O. J. Org. Chem. 2007, 72, 2842–2850. doi:10.1021/jo062311j |
| 13. |
Schulz, S. R.; Blechert, S. Angew. Chem. 2007, 119, 4040–4044. doi:10.1002/ange.200604553
Angew. Chem., Int. Ed. 2007, 46, 3966–3970. |
| 28. | Appleton, T. G.; Clark, H. C.; Manzer, L. E. Coord. Chem. Rev. 1973, 10, 335–422. doi:10.1016/S0010-8545(00)80238-6 |
| 50. | Ditchfield, R.; Hehre, W. J.; Pople, J. A. J. Chem. Phys. 1971, 54, 724–728. doi:10.1063/1.1674902 |
| 51. | Rassolov, V. A.; Ratner, M. A.; Pople, J. A.; Redfern, P. C.; Curtiss, L. A. J. Comput. Chem. 2001, 22, 976–984. doi:10.1002/jcc.1058 |
| 23. | Helmchen, G.; Kudis, S.; Sennehenn, P.; Steinhagen, H. Pure Appl. Chem. 1997, 69, 513–519. doi:10.1351/pac199769030513 |
| 24. | Helmchen, G. J. Organomet. Chem. 1999, 576, 203–214. doi:10.1016/S0022-328X(98)01059-6 |
| 25. | Kolmar, M.; Goldfuss, B.; Reggelin, M.; Rominger, F.; Helmchen, G. Chem.–Eur. J. 2001, 7, 4913–4927. doi:10.1002/1521-3765(20011119)7:22<4913::AID-CHEM4913>3.0.CO;2-7 |
| 26. | Kollmar, M.; Steinhagen, H.; Janssen, J. P.; Goldfuss, B.; Malinovskaya, S. A.; Vázques, J.; Rominger, F.; Helmchen, G. Chem.–Eur. J. 2002, 8, 3103–3114. doi:10.1002/1521-3765(20020715)8:14<3103::AID-CHEM3103>3.0.CO;2-C |
| 27. | Vázquez, J.; Goldfuss, B.; Helmchen, G. J. Organomet. Chem. 2002, 641, 67–70. doi:10.1016/S0022-328X(01)01308-0 |
| 52. | Becke, A. D. J. Chem. Phys. 1993, 98, 5648–5652. doi:10.1063/1.464913 |
| 53. | Stephens, P. J.; Devlin, F. J.; Chabalowski, C. F.; Frisch, M. J. J. Phys. Chem. 1994, 98, 11623–11627. doi:10.1021/j100096a001 |
| 54. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785–789. doi:10.1103/PhysRevB.37.785 |
| 55. | Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Chem. Phys. Lett. 1989, 157, 200–206. doi:10.1016/0009-2614(89)87234-3 |
| 19. |
Trost, B. M.; Breit, B.; Peukert, S.; Zambrano, J.; Ziller, J. W. Angew. Chem. 1995, 107, 2577–2579. doi:10.1002/ange.19951072114
Angew. Chem., Int. Ed. 1995, 34, 2386–2388. |
| 20. | Trost, B. M. Acc. Chem. Res. 1996, 29, 355–364. doi:10.1021/ar9501129 |
| 21. | Trost, B. M.; Heinemann, C.; Ariza, X.; Weigand, S. J. Am. Chem. Soc. 1999, 121, 8667–8668. doi:10.1021/ja991821a |
| 22. | Trost, B. M.; Ariza, X. J. Am. Chem. Soc. 1999, 121, 10727–10737. doi:10.1021/ja992754n |
| 44. | Goldfuss, B. J. Organomet. Chem. 2006, 691, 4508–4513. doi:10.1016/j.jorganchem.2006.01.061 |
| 14. | Hayashi, T.; Yamamoto, A.; Hagihara, T.; Ito, Y. Tetrahedron Lett. 1986, 27, 191–194. doi:10.1016/S0040-4039(00)83974-X |
| 15. | Hayashi, T.; Kanehira, K.; Hagihara, T.; Kumada, M. J. Org. Chem. 1988, 53, 113–120. doi:10.1021/jo00236a023 |
| 16. | Hayashi, T. Pure Appl. Chem. 1988, 60, 7–13. doi:10.1351/pac198860010007 |
| 17. | Sawamura, M.; Ito, Y. Chem. Rev. 1992, 92, 857–871. doi:10.1021/cr00013a005 |
| 18. | Enders, D.; Peters, R.; Lochtman, R.; Raabe, G.; Runsink, J.; Bats, J. W. Eur. J. Org. Chem. 2000, 20, 3399–3426. doi:10.1002/1099-0690(200010)2000:20<3399::AID-EJOC3399>3.0.CO;2-D |
| 42. | Goldfuss, B.; Kazmeier, U.; Goldfuss, B.; Kazmaier, U. Tetrahedron 2000, 56, 6493–6496. doi:10.1016/S0040-4020(00)00613-X |
| 43. | Boele, M. D. K.; Kamer, P. C. J.; Lutz, M.; Spek, A. L.; de Vries, J. G.; van Leeuwen, P. W. N. M.; van Strijdonck, G. P. F. Chem.–Eur. J. 2004, 10, 6232–6246. doi:10.1002/chem.200400154 |
| 45. |
Märkl, G.; Lieb, F.; Merz, A. Angew. Chem. 1967, 79, 947–948. doi:10.1002/ange.19670792125
Angew. Chem., Int. Ed. 1967, 6, 458–459. |
| 46. | Ashe, A. J., III. J. Am. Chem. Soc. 1971, 93, 3293–3295. doi:10.1021/ja00742a038 |
| 47. | Shiotsuka, M.; Tanamachi, T.; Matsuda, Y. Chem. Lett. 1995, 24, 531–533. doi:10.1246/cl.1995.531 |
| 48. | Breit, B.; Winde, R.; Harms, K. J. Chem. Soc., Perkin Trans. 1 1997, 18, 2681–2683. doi:10.1039/a705249i |
| 19. |
Trost, B. M.; Breit, B.; Peukert, S.; Zambrano, J.; Ziller, J. W. Angew. Chem. 1995, 107, 2577–2579. doi:10.1002/ange.19951072114
Angew. Chem., Int. Ed. 1995, 34, 2386–2388. |
| 23. | Helmchen, G.; Kudis, S.; Sennehenn, P.; Steinhagen, H. Pure Appl. Chem. 1997, 69, 513–519. doi:10.1351/pac199769030513 |
| 24. | Helmchen, G. J. Organomet. Chem. 1999, 576, 203–214. doi:10.1016/S0022-328X(98)01059-6 |
| 25. | Kolmar, M.; Goldfuss, B.; Reggelin, M.; Rominger, F.; Helmchen, G. Chem.–Eur. J. 2001, 7, 4913–4927. doi:10.1002/1521-3765(20011119)7:22<4913::AID-CHEM4913>3.0.CO;2-7 |
| 26. | Kollmar, M.; Steinhagen, H.; Janssen, J. P.; Goldfuss, B.; Malinovskaya, S. A.; Vázques, J.; Rominger, F.; Helmchen, G. Chem.–Eur. J. 2002, 8, 3103–3114. doi:10.1002/1521-3765(20020715)8:14<3103::AID-CHEM3103>3.0.CO;2-C |
| 27. | Vázquez, J.; Goldfuss, B.; Helmchen, G. J. Organomet. Chem. 2002, 641, 67–70. doi:10.1016/S0022-328X(01)01308-0 |
| 28. | Appleton, T. G.; Clark, H. C.; Manzer, L. E. Coord. Chem. Rev. 1973, 10, 335–422. doi:10.1016/S0010-8545(00)80238-6 |
| 29. | Sprinz, J.; Kiefer, M.; Helmchen, G.; Reggelin, M.; Huttner, G.; Walter, O.; Zsolnai, L. Tetrahedron Lett. 1994, 35, 1523–1526. doi:10.1016/S0040-4039(00)76748-7 |
| 30. | Ward, T. R. Organometallics 1996, 15, 2836–2838. doi:10.1021/om960158l |
| 31. | Oslob, J. D.; Akermark, B.; Helquist, P.; Norrby, P.-O. Organometallics 1997, 16, 3015–3021. doi:10.1021/om9700371 |
| 32. | Moberg, C.; Bremberg, U.; Hallman, K.; Svensson, M.; Norbby, P.-O.; Hallberg, A.; Larhed, M.; Csöregh, I. Pure Appl. Chem. 1999, 71, 1477–1485. doi:10.1351/pac199971081477 |
| 33. | Hagelin, H.; Akermark, B.; Norrby, P.-O. Organometallics 1999, 18, 2884–2895. doi:10.1021/om990153z |
| 34. | Hagelin, H.; Svensson, M.; Akermark, B.; Norrby, P.-O. Organometallics 1999, 18, 4574–4583. doi:10.1021/om990228z |
| 35. | Pedersen, T. M.; Hansen, E.; Louise, K.; Kane, J.; Rein, T.; Helquist, P.; Norrby, P.-O.; Tanner, D. J. Am. Chem. Soc. 2001, 123, 9738–9742. doi:10.1021/ja005809q |
| 36. | Tu, T.; Zhou, Y.; Hou, X.; Dai, L.; Dong, X.; Yu, Y.; Sun, J. Organometallics 2003, 22, 1255–1265. doi:10.1021/om020706x |
| 37. | Norrby, P.-O.; Mader, M. M.; Vitale, M.; Prestat, G.; Poli, G. Organometallics 2003, 22, 1849–1855. doi:10.1021/om030066d |
| 38. | Madec, D.; Prestat, G.; Martini, E.; Fristrup, P.; Poli, G.; Norrby, P.-O. Org. Lett. 2005, 7, 995–998. doi:10.1021/ol047548l |
| 39. | Fristrup, P.; Jensen, T.; Hoppe, J.; Norrby, P.-O. Chem.–Eur. J. 2006, 12, 5352–5360. doi:10.1002/chem.200600152 |
| 40. | Ahlquist, M.; Fabrizi, G.; Cacchi, S.; Norrby, P.-O. J. Am. Chem. Soc. 2006, 128, 12785–12793. doi:10.1021/ja061543x |
| 41. | Ahlquist, M.; Norrby, P.-O. Organometallics 2007, 26, 550–553. doi:10.1021/om0604932 |
| 29. | Sprinz, J.; Kiefer, M.; Helmchen, G.; Reggelin, M.; Huttner, G.; Walter, O.; Zsolnai, L. Tetrahedron Lett. 1994, 35, 1523–1526. doi:10.1016/S0040-4039(00)76748-7 |
| 30. | Ward, T. R. Organometallics 1996, 15, 2836–2838. doi:10.1021/om960158l |
| 31. | Oslob, J. D.; Akermark, B.; Helquist, P.; Norrby, P.-O. Organometallics 1997, 16, 3015–3021. doi:10.1021/om9700371 |
| 32. | Moberg, C.; Bremberg, U.; Hallman, K.; Svensson, M.; Norbby, P.-O.; Hallberg, A.; Larhed, M.; Csöregh, I. Pure Appl. Chem. 1999, 71, 1477–1485. doi:10.1351/pac199971081477 |
| 33. | Hagelin, H.; Akermark, B.; Norrby, P.-O. Organometallics 1999, 18, 2884–2895. doi:10.1021/om990153z |
| 34. | Hagelin, H.; Svensson, M.; Akermark, B.; Norrby, P.-O. Organometallics 1999, 18, 4574–4583. doi:10.1021/om990228z |
| 35. | Pedersen, T. M.; Hansen, E.; Louise, K.; Kane, J.; Rein, T.; Helquist, P.; Norrby, P.-O.; Tanner, D. J. Am. Chem. Soc. 2001, 123, 9738–9742. doi:10.1021/ja005809q |
| 36. | Tu, T.; Zhou, Y.; Hou, X.; Dai, L.; Dong, X.; Yu, Y.; Sun, J. Organometallics 2003, 22, 1255–1265. doi:10.1021/om020706x |
| 37. | Norrby, P.-O.; Mader, M. M.; Vitale, M.; Prestat, G.; Poli, G. Organometallics 2003, 22, 1849–1855. doi:10.1021/om030066d |
| 38. | Madec, D.; Prestat, G.; Martini, E.; Fristrup, P.; Poli, G.; Norrby, P.-O. Org. Lett. 2005, 7, 995–998. doi:10.1021/ol047548l |
| 39. | Fristrup, P.; Jensen, T.; Hoppe, J.; Norrby, P.-O. Chem.–Eur. J. 2006, 12, 5352–5360. doi:10.1002/chem.200600152 |
| 40. | Ahlquist, M.; Fabrizi, G.; Cacchi, S.; Norrby, P.-O. J. Am. Chem. Soc. 2006, 128, 12785–12793. doi:10.1021/ja061543x |
| 41. | Ahlquist, M.; Norrby, P.-O. Organometallics 2007, 26, 550–553. doi:10.1021/om0604932 |
| 42. | Goldfuss, B.; Kazmeier, U.; Goldfuss, B.; Kazmaier, U. Tetrahedron 2000, 56, 6493–6496. doi:10.1016/S0040-4020(00)00613-X |
| 45. |
Märkl, G.; Lieb, F.; Merz, A. Angew. Chem. 1967, 79, 947–948. doi:10.1002/ange.19670792125
Angew. Chem., Int. Ed. 1967, 6, 458–459. |
© 2007 Lange and Goldfuss; licensee Beilstein-Institut
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)