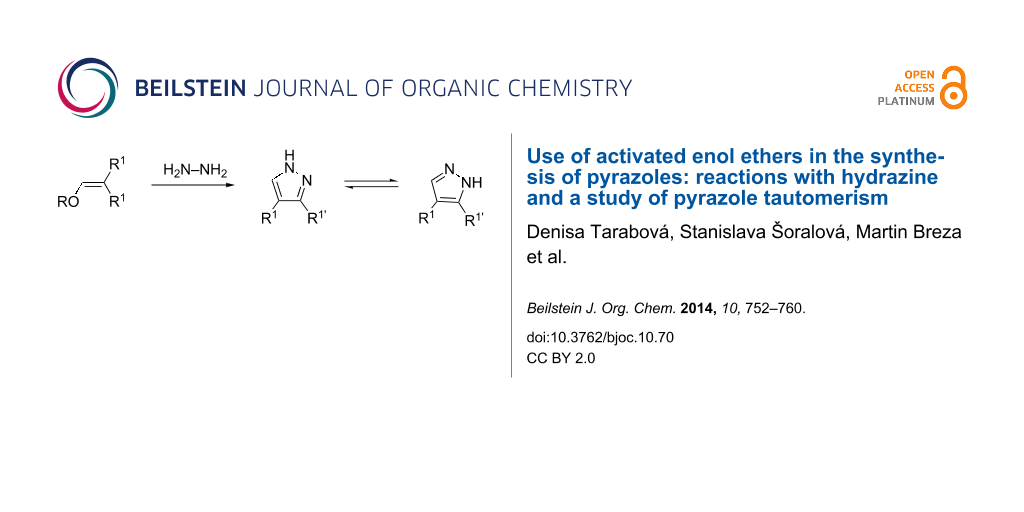
Abstract
Activated enol ethers derived from esters or the dinitrile of malonic acid, or from pentane-2,4-dione were treated with hydrazine hydrate. The structures of the obtained products – pyrazoles 5 – were studied with a focus on tautomerism and supramolecular structure. A reverse addition of the reagents led to the isolation of two novel products, namely bis-enehydrazines 6 with an unsymmetrical arrangement of the formally equivalent subunits.
Graphical Abstract
Introduction
Enol ethers are reactive species frequently used in organic synthesis [1]. They react predominantly with electrophiles. The introduction of one or more strong electron-withdrawing groups at the other end of the double bond from the alkoxy group causes an inversion of electron demand. These activated enol ethers react surprisingly easily with various nucleophiles such as amines, thiols, alcohols or C-anions [2] under the conditions of nucleophilic vinylic substitution [3]. When using bi- or trifunctional nucleophiles, cyclic or bicyclic products are formed. Activated enol ethers thus represent trifunctional electrophiles and are useful building blocks for the introduction of a three carbon fragment into the resulting molecule. Thus, the reaction of enol ethers with hydrazines produces pyrazoles, with amidines pyrimidines, with hydroxylamine isoxazoles, and with anilines quinolines/ones are formed [2].
Electron-accepting groups can include ester, cyano, acetyl, nitro and trifluoroacetyl moieties. Thus, the simplest way to obtain activated enol ethers 3 is the condensation of active methylene components 2 such as malonic acid derivatives (esters, nitrile), (trifluoro)acetoacetic acid derivatives (esters, nitrile), acetylacetone, and nitroacetates with trialkyl orthoformate 1 (Scheme 1). Transesterification can be avoided if 1 bears the same alkyl group as 2. The reaction can be carried out with or without Lewis acid catalysis [2].
Pyrazoles constitute a large group of pharmacologically active compounds and are mostly prepared by reacting hydrazines with suitable 1,3-dielectrophiles. The synthesis of pyrazoles and related compounds can be implemented by four different approaches:
- reaction of (substituted) hydrazines with β-functional compounds or their equivalents (the most frequently used method),
- 1,3-dipolar cycloaddition with diazo compounds as a source of N–N linkage,
- degradation of fused pyrazoles or
- rearrangement of other monocyclic heterocycles under chemical, thermal or photochemical conditions [4].
Upon reaction of hydrazine with symmetrical enol ethers, only a single product is formed, which is capable of annular prototropic tautomerism [5,6]. In contrast, the use of „asymmetric“ enol ethers, that is, R1, R1 in 2 and 3 are not equal, may lead to two different pyrazoles, both of which exhibit tautomerism). However, depending on the different reactivities of the electron-withdrawing groups R1 only a single product is formed (Scheme 2) [7].
Scheme 2: Reaction of enol ethers with hydrazine hydrate.
Scheme 2: Reaction of enol ethers with hydrazine hydrate.
Results and Discussion
In this work we have studied reactions of symmetric enol ethers with hydrazine hydrate according to Scheme 2. The enol ethers ethoxymethylidenemalononitrile (EMMN, 3a), dimethyl methoxymethylidenemalonate (MMMM, 3b), diethyl ethoxymethylidenemalonate (EMME, 3c), and 3-(ethoxymethylidene)pentane-2,4-dione (EMAA, 3d) were employed. Three-component reactions (hydrazine + trialkylorthoformate + active methylidene component) were not studied.
The products resulting from the reaction of a hydrazine hydrate with activated symmetrical enol ethers 3 are shown in Scheme 3.
It is well known that the reaction of hydrazine hydrate with alkoxymethylidenemalononitrile affords 3-amino-1H-pyrazole-4-carbonitrile (5a) [8-13]. Eight tautomeric forms of 5a could theoretically be expected (Figure 1). Five of these tauromeric forms carry an amino substituent, which is denoted by the third character being an A in the numbering of these tautomers and the fourth number giving the position of one hydrogen. Three of the tautomeric forms of 5a are characterized by an exocyclic imino function. This is denoted by the third character I in the numbering and the fourth and fifth numbers giving the positions of two hydrogens.
When dimethyl methoxymethylidenemalonate (3b) is used, methyl 3-hydroxy-1H-pyrazole-4-carboxylate (5b) is formed. Analogously, reaction of diethyl ethoxymethylenemalonate (3c) gives the corresponding ethyl 3-hydroxy-1H-pyrazole-4-carboxylate (5c) [14-18]. The Reaction of enol ethers 3b and 3c with hydrazine hydrate at rt for 10 min gave products of an SNV reaction, 4b and 4c. These then underwent intramolecular cyclization to yield 5b and 5c. Due to oxo–enol tautomerism eight tautomers are theoretically possible (Figure 2). The labeling scheme of the tautomers of 5b uses an E to indicate the (en)ol form and an O to indicate the oxo form.
Figure 2: Tautomers of 5b (R = Me), 5c (R = Et).
Figure 2: Tautomers of 5b (R = Me), 5c (R = Et).
However, more tautomeric forms of the ester species are theoretically possible as the C-4 substituent can also be involved in tautomerism. For instance, additional species, which are possible for ester compounds 5b and 5c are shown in Figure 3, with the last character of the label being an E indicates enol, O denotes oxo, and (Z,E) – HO vs OH/=O
Figure 3: Tautomers of 5b (R = Me), 5c (R = Et).
Figure 3: Tautomers of 5b (R = Me), 5c (R = Et).
Compound 5b was previously prepared by a ring contraction of pyrimidine-2,4-dione [19]. It is known from the literature that after the reaction of ethoxymethylideneacetylacetone (3d) with hydrazine hydrate 1-(3-methyl-1H-pyrazol-4-yl)ethanone (5d) is produced [20]. The latter bears the tautomerizable acetyl group so that the tautomeric characteristics are different from the previous case and similar to that of 5a (Figure 4). Here, Z/E refers to the mutual positions of methyl groups.
Compared to the procedure described by Mitkidou et al. [20], we were able to prepare 5d in one step, in a shorter time (1 h heated under reflux in EtOH) and with a higher yield of 88%. Mitkidou et al. prepared 5d in a two-step synthesis [20] (8 h heated under reflux in toluene 55% yield or 2 h in CHCl3) in 69% yield. Other preparations of 5d were carried out in aqueous media [21-23].
Enol ethers with different alkoxy groups afforded the same products. Reactions were carried out in ethanol or without a solvent. If the ratio enol ether/hydrazine is 1:1, as in the case of the reaction with 3c, the formation of 7-aminopyrazolo[1,5-a]pyrimidine-3,6-dicarbonitrile could be expected as a byproduct [24,25]. When hydrazine hydrochloride is used [14], ethyl 3-ethoxypyrazole-4-carboxylate was obtained in 41% yield and the expected ethyl 3-oxo-2,3-dihydropyrazole-4-carboxylate (5c, oxo form) in 37% yield. The first product was obtained as an oil, whereas compound 5c was afforded as a white powder.
The reversal of the addition by adding hydrazine hydrate dropwise to the stirred enol ether led to the bis-N,N´-product 6a in 60% yield as a colorless crystalline product and 6b (43% yield) as yellowish crystals. This product could not be thermally cyclized into the corresponding pyrazole derivative, presumably due to the reduced nucleophilicity of the hydrazine nitrogen bearing the electron-deficient vinyl substituent (Scheme 4). The structure of 6a was confirmed by spectroscopic analysis, elemental analysis, and X-ray structure analysis (Figure 5).
Scheme 4: Reactions of hydrazine hydrate with dialkyl alkoxymethylidenemalonates.
Scheme 4: Reactions of hydrazine hydrate with dialkyl alkoxymethylidenemalonates.
![[1860-5397-10-70-5]](/bjoc/content/figures/1860-5397-10-70-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Crystal structure and crystal packing of compound 6a.
Figure 5: Crystal structure and crystal packing of compound 6a.
A sample of compound 6a was recrystallized by slow evaporation from chloroform. X-ray diffraction data (Supporting Information File 1, Tables S1–S5) were collected on an Oxford Diffraction Gemini R diffractometer equipped with a Ruby CCD detector and Mo Kα sealed-tube source at rt. Data reduction was performed with Oxford Diffraction CrysAlis RED version 1.171.33.42 software [26]. The crystal structure was solved and refined with SHELXS97 [27].
The molecule consists of the two parts rotated around the N–N bond, with the torsion angle C3–N5–N6–C6 −77.0(2)°, while the N–N bond and adjacent C=C bonds are highly planar with torsion angles 174.09(15)° for C2–C3–N5–N6 and 177.71(16)° for N5–N6–C6–C7. Correspondent bond lengths in the molecule show only minor variations. Three carbonyl bonds in the ester groups display a Z conformation with regard to the carbon atom of the double bond (O5–C8–O6–C14 and O7–C12–O8–C13 to C6 and O3–C10–O4–C11 to C3), while the fourth carbonyl group exhibits an E conformation (O1–C1–O2–C9 to C3). Torsion angles between the carbonyl and double C=C bond are 15.9(3)°, 11.1(3)°, 0.6(3)° for the first three ester groups (C6–C7–C8–O5, C6–C7–C12–O7, C3–C2–C10–O3) and 152.28(17)° for the last one (O1–C1–C2–C3) (Supporting Information File 1, Table S5).
N5–H5A forms an intramolecular hydrogen bond with O3 and an intermolecular hydrogen bond with O4. N6–H6A forms an intramolecular hydrogen bond with O5, while both also form an intermolecular hydrogen bond with O5 from the next molecule as shown in Table S2. These two hydrogen bonds are in a rhomboid arrangement with angles H6A–O5–H6A 86.10(5)° and O5–H6A–O5 93.90(5)°. Selected interatomic distances, bond and torsion angles are presented in Supporting Information File 1, Tables S2, S4 and S5.
NMR measurements
The spectra of compounds 5a–d are characterized by broad to very broad resonance lines, hinting at dynamic processes related to prototropic tautomerism. It was attempted to record spectra in CDCl3 – where a rapid exchange between tautomeric forms was expected – and in DMSO-d6 solution – where we assumed slower interconversion rates due to the acceptor properties of this solvent. However, the solubility in CDCl3 turned out to be very low for the studied compounds, so that only 1H NMR spectra could be obtained from very dilute solutions in this solvent. For all compounds the marked line broadening did not permit the determination of 13C,1H spin coupling constants of the pyrazole C-atoms which, in certain cases, may be employed for a rough estimation of the tautomeric composition [28,29].
The 1H and 13C NMR spectra of aminonitrile 5a showed one set of broad signals. The 13C chemical shifts of the pyrazole C-atoms (δ pyrazole C3(5) 154.1 ppm, δ pyrazole C-4 73.5 ppm, and δ of the quaternary pyrazole C5(3) 140.0 ppm) resemble those of ‘fixed’ N-methyl compound 5-amino-1-methyl-1H-pyrazole-4-carbonitrile (δ C3 139.8 ppm, δ C4 72.1 ppm, δ C5 151.4 ppm). Thus, it can be assumed that isomer 5aA2 provides a marked contribution to the tautomeric mixture. However, NOE measurement between the pyrazole-CH and pyrazole NH hints at the simultaneous presence of species 5aA1, where the protons involved are spatially close. The noticeable contribution of 5al12 is improbable due to the presence of an NH2 group in the 1H NMR spectrum. No isomers with sp3 hybridized ring C-atoms or excocyclic double bonds were detected at all and thus can, at the most, play a marginal role.
Methyl 3-hydroxypyrazole-4-carboxylate (5b) shows a distinct dynamic behavior in DMSO-d6 solution, resulting in broad lines in the 1H and in the 13C NMR spectra. Comparison of the 13C chemical shifts with those of the fixed N-methyl derivatives methyl 3-hydroxy-1-methyl-1H-pyrazole-4-carboxylate and methyl 5-hydroxy-1-methyl-1H-pyrazole-4-carboxylate exhibits a good agreement with the former. Therefore, species 5bE1 can be assumed to have the largest contribution to the overall tautomeric mixture. It is known from the literature that - given such a type of compounds – oxo-forms and forms with exocyclic double bonds do not play an important role. The corresponding ethyl ester 5c exhibits the same characteristics as its methyl congener 5b. As found with 5c dominance of tautomer 5cA1 can also be deduced from an inspection of the 13C NMR spectrum in DMSO-d6 solution. In Supporting Information File 1, Tables S6 and S7 signals for compounds 5a–d in CDCl3 and DMSO-d6 solution are reported whenever measurement was possible.
An interesting feature was observed in the NMR spectra of compound 6a (Figure 6) (IUPAC name: tetramethyl 2,2'-(1,2-hydrazinediyldimethylylidene)dimalonate). In CDCl3 solution a mixture of the symmetrical form A and an unsymmetrical, tautomeric form B was observed (ratio A:B ~ 2:3, Figure 6). The symmetric structure of A can be derived from the fact that in the 1H,15N HMBC spectrum of A there is only one 15N resonance. This resonance consists of residual signals from the incompletely suppressed direct coupling (1J(15N,1H) ~105.9 Hz) and an additional correlation caused by the geminal 2J(N–N–H) coupling. The compound is stabilized by strong intramolecular hydrogen bonds between the NH and the ‘cis’-located ester carbonyl O-atom. This situation gives rise to a sharp, deshielded resonance of NH (δ 10.38 ppm), which is split into a doublet (3J = 11.0 Hz) due to the coupling with the alkene-H (δ 8.05 ppm). The ester moiety involved in the intramolecular hydrogen bond exhibits a marked downfield shift for C=O (δ 168.5 ppm) in comparison to the other ester carbonyl C-atom (δ 164.7 ppm), which is typical for such a situation [30]. The small chemical shift for the quaternary alkene C-atom (δ 93.0 ppm) is characteristic for a polarized C=C bond in this push–pull configuration. One half of the isomer B shows the same characteristics as A , that is, the intramolecular hydrogen bond between NH and the ester C=O. However, the second half of the molecule has two equivalent ester moieties and a C=N bond instead of a C=C bond leading to an sp3 hybridized C-atom (δ 54.8 ppm) between the equivalent methyl ester groups. The two different N-atoms in B have 15N chemical shifts of −48.1 ppm (C=N) and −211.7 ppm (=N–NH) (Figure 6).
Figure 6: Key 1H (red), 13C (black) and 15N (blue) NMR chemical shifts (δ, ppm) in isomers A and B of compound 6a. [DFT data are in square brackets.]
Figure 6: Key 1H (red), 13C (black) and 15N (blue) NMR chemical shifts (δ, ppm) in isomers A and B of compoun...
Quantum-chemical calculations
To support the experimental results were also performed DFT (Density Functional Theory method) calculations by using the B3LYP hybrid functional [31-33] and 6-311++G** basis sets of the Gaussian basis set library [34]. The SCRF theory via the IEFPCM (Integral Equation Formalism Polarizable Continuum Model) model including the effect of the solution was applied to optimize the geometries in (m)ethanol and DMSO. The stability of the obtained structures was proven by vibrational analysis (no imaginary vibrations).
NMR chemical shifts δ (in ppm) were evaluated from the corresponding absolute shieldings σ calculated for vacuum geometry by using the Gauge-Independent Atomic Orbital (GIAO) method [35] at B3LYP/6-311++G** level of theory according to the linear equation
with a = 31.0 ± 0.8 ppm , b = −0.97 ± 0.03 for X = 1H [36], a = 175.7 ± 0.2 ppm , b = −0.963 ± 0.003 for X = 13C [37] and a = −152.0 ± 1.1 ppm , b = −0.946 ± 0.008 for X = 15N [37]. The Gaussian09 [34] software was used for all calculations.
Products 5 are formed during reactions in which the proportions of tautomers can theoretically correspond to the proportion of tautomers by the Boltzmann distribution obtained from theoretical calculations at 293.15 K. However, the calculated proportion of tautomers does not include the effects of the reaction conditions and kinetics factors on the final proportion of individual tautomers in solution.
Total electronic IEFPCM-B3LYP/6-311++G** energies with zero-point corrections E(DFT) and relative energies of tautomers ΔE(DFT) of studied compounds are summarized in Supporting Information File 1, Tables S8–S11. The most stable structural form in the 5a series is the 5aA1 tautomer (56% in ethanol) closely followed by the 5aA2 tautomer (44% in ethanol), which is less stable in both ethanol and DMSO by only 0.6 and 0.4 kJ·mol−1, respectively. The remaining tautomers are probably absent in solution because their energies are more than 85 kJ·mol−1 higher than the 5aA1 tautomer in both solutions (Supporting Information File 1, Table S8). This is in agreement with experimental NMR spectra.
For the 5b series the most stable tautomer is 5bE1 (83% in methanol) followed by 5bE2 (17% in methanol), which is less stable by approximately 4 kJ·mol−1 both in methanol and DMSO. The remaining tautomers are again probably absent in both solutions because their energies are significantly higher in comparison with the most stable 5bE1 tautomer. The tautomers without hydrogens at pyrazole nitrogen atoms (5bE3, 5bE4, 5bE5, 5bO45, 5bE4Z and 5bE4E) exhibit the lowest stability (energy difference is ca 60 kJ mol−1) (Supporting Information File 1, Table S9). This is in agreement with experimental NMR spectra.
As expected, in the case of the 5c series the most stable tautomer is 5cE1 (83% in ethanol) followed by 5cE2 (17% in ethanol), which is less stable by approximately 4 kJ·mol−1 both in ethanol and DMSO, similar to the 5b series. The remaining tautomers are not present in solution and the stability dependence on the presence of hydrogen at pyrazole nitrogen atoms is similar as above (Supporting Information File 1, Table S10). This is in agreement with experimental NMR spectra.
In the 5d series, the most stable tautomer is 5dA2 (70% in ethanol) followed by 5dA1 (30%) which is less stable by 2 kJ·mol−1 both in ethanol and DMSO. The remaining tautomers have no hydrogens at pyrazole nitrogen atoms and their energies are higher by more than 95 kJ·mol−1 higher compared to the two most stable tautomers (Supporting Information File 1, Table S11).
All stable tautomers of the 5a–d series (Supporting Information File 1, Tables S12 and S13) present in solutions have one hydrogen atom at one of the pyrazole nitrogen atoms and, as indicated by the calculated NMR spectra in DMSO, the shift of this hydrogen should be 7.9–8.7 ppm. The single hydrogen at the pyrazole carbons (the same carbon atom in all tautomers of all series) has a shift of 7.2–7.7 ppm and its carbon has a shift of 126.4–143.3 ppm. The calculated shift differences are insufficient to identify the tautomer in solutions and can only be used to exclude some tautomers.
Measured NMR spectra indicate two tautomers of compound 6a in chloroform solutions which differ in a single H atom position within the central >C–C–N–N–C–C< chain (at N or at tertiary C atom). A third tautomer with both H atoms at tertiary C atoms was not detected. DFT geometry optimization confirmed the existence of all three tautomers (Figure 7, Supporting Information File 1, Tables S2–S5 and S14) but because of energy reasons the relative population of the C conformer must be vanishing in agreement with experimental data. This was confirmed by calculated NMR spectra (Supporting Information File 1, Table S15). Nevertheless, DFT data indicate a much lower proportion of the B conformer than implied by the experimental data. In contrast to the A tautomer, the B and C tautomers have planar >C–C–N–N–C–C< chains. X-ray and DFT optimized structures of the A tautomer exhibit significant differences in torsional angles only (see Supporting Information File 1, Tables S2–S5) ascribable to solid state effects. The accuracy of the calculated NMR data is insufficient to distinguish between possible conformers of individual tautomers and so we did not performed a full conformational analysis.
![[1860-5397-10-70-7]](/bjoc/content/figures/1860-5397-10-70-7.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 7: DFT optimized isomers of compound 6a. (Distances are in Å, angles are in degrees.)
Figure 7: DFT optimized isomers of compound 6a. (Distances are in Å, angles are in degrees.)
Conclusion
Activated enol ethers 3 derived from trialkyl orthoformate and active methylidene compounds such as diesters, dinitriles of malonic acid or pentane-2,4-dione are excellent three-carbon synthons. They act as trifunctional electrophiles for the syntheses of tautomeric pyrazoles when reacted with hydrazine thereby obtaining 3,4-disubstituted pyrazoles, namely 3-amino-1H-pyrazole-4-carbonitrile (5a), alkyl 3-hydroxypyrazole-4-carboxylates 5b, 5c or 1-(3-methyl-1H-pyrazol-4-yl)ethanone (5d). The reversed addition of the enol ethers to hydrazine hydrate the formation of bis-enehydrazine 6 product was observed in two tautomeric forms: with symmetrical and unsymmetrical structures of the ethylene substituent. This structure has been established by X-ray analysis. The tautomeric behavior of these materials has been modelled by using DFT quantum-chemical calculations. The most stable structural forms are 5aA1, 5aA2, 5bE1, 5bE2, 5cE1, 5cE2, 5dA1 and 5dA2.
As expected, NH-pyrazoles of type 5 exhibit a pronounced dynamic behavior in solution due to prototropic tautomerism. This results in the broadening of marked lines thereby preventing the identification of individual species. However, a comparison of the chemical shifts in 5 and the corresponding ‘fixed’ N-methyl derivatives reveals the contribution of distinct, individual tautomers to the overall situation. The structure of bis-enehydrazine 6a – unambiguously determined by X-ray analysis – is in accord with the NMR data. Moreover, the presence of a second, asymmetric form of 6a in CDCl3 solution was established by NMR. A tautomer C with an azine (C=N–N=C) structure has not been observed.
Supporting Information
| Supporting Information File 1: Experimental section and Tables S1–S15. | ||
| Format: PDF | Size: 459.8 KB | Download |
References
-
Milata, V.; Rádl, S.; Voltrová, S. Science of Synthesis Product Subclass: Enol Ethers (acyclic, cyclic, i.e. endocyclic C=C-O- unit; Georg Thieme Verlag: Stuttgart, 2008; p 589.
Return to citation in text: [1] -
Milata, V. Aldrichimica Acta 2001, 34, 20.
Return to citation in text: [1] [2] [3] -
Saloň, J.; Milata, V.; Gatial, A.; Prónayová, N.; Leško, J.; Černuchová, P.; Vo-Thanh, G.; Loupy, A. Eur. J. Org. Chem. 2005, 4870. doi:10.1002/ejoc.200500298
Return to citation in text: [1] -
Kornis, G. I. Kirk-Othmer Encyklopedia of Chemical Technology: Pyrazoles, pyrazolines, and pyrazolones; John Wiley and Sons: Hoboken, 2001. doi:10.1002/0471238961.1625180111151814.a01
Return to citation in text: [1] -
Elguero, J.; Marzin, C.; Katritzky, A. R.; Linda, P. The Tautomerism of Heterocycles; Academic Press: New York, 1976; p 655.
Return to citation in text: [1] -
Minkin, V. I.; Garnovskii, A. D.; Elguero, J.; Katritzky, A. R.; Denisko, O. V. Adv. Heterocycl. Chem. 2000, 76, 157. doi:10.1016/S0065-2725(00)76005-3
Return to citation in text: [1] -
Černuchová, P.; Vo-Thanh, G.; Milata, V.; Loupy, A.; Jantová, S.; Theiszová, M. Tetrahedron 2005, 61, 5379. doi:10.1016/j.tet.2005.03.066
Return to citation in text: [1] -
Xu, J.; Liu, H.; Li, G.; He, Y.; Ding, R.; Wang, X.; Feng, M.; Zhang, S.; Chen, Y.; Li, S.; Zhao, M.; Qi, C.; Dang, Y. Bioorg. Med. Chem. Lett. 2011, 21, 4736. doi:10.1016/j.bmcl.2011.06.072
Return to citation in text: [1] -
Reddy, G. J.; Sailaja, S.; Manjula, D.; Rao, K. S.; Khalilullah, M.; Latha, D. Heterocycl. Commun. 2005, 11, 385. doi:10.1515/HC.2005.11.5.385
Return to citation in text: [1] -
Shaikh, A. C.; Chen, C. J. Labelled Compd. Radiopharm. 2008, 51, 72. doi:10.1002/jlcr.1484
Return to citation in text: [1] -
Nagahara, K.; Takagi, K.; Ueda, T. Chem. Pharm. Bull. 1976, 24, 2880. doi:10.1248/cpb.24.2880
Return to citation in text: [1] -
Takagi, K.; Nagahara, K.; Ueda, T. Chem. Pharm. Bull. 1970, 18, 2353. doi:10.1248/cpb.18.2353
Return to citation in text: [1] -
Kim, D. C.; Lee, Y. R.; Yang, B.-S.; Shin, K. J.; Kim, D. J.; Chung, B. Y.; Yoo, K. H. Eur. J. Med. Chem. 2003, 38, 525. doi:10.1016/S0223-5234(03)00065-5
Return to citation in text: [1] -
Guillou, S.; Janin, Y. L. Chem.–Eur. J. 2010, 16, 4669. doi:10.1002/chem.200903442
Return to citation in text: [1] [2] -
Ishimaru, T. Yakugaku Zasshi 1957, 77, 800.
Chem. Abstr. 1957, 17893.
Return to citation in text: [1] -
Ruhemann, S. Ber. Dtsch. Chem. Ges. 1894, 27, 1658. doi:10.1002/cber.189402702104
Return to citation in text: [1] -
Ruhemann, S. Ber. Dtsch. Chem. Ges. 1896, 29, 1016. doi:10.1002/cber.189602901195
Return to citation in text: [1] -
Al-Jallo, H. N.; Al-Khasab, A.; Sallomi, I. G. J. Chem. Soc., Perkin Trans. 1 1972, 1022. doi:10.1039/p19720001022
Return to citation in text: [1] -
Kitade, Y.; Hirota, K.; Maki, Y. J. Chem. Res., Miniprint 1993, 101.
Return to citation in text: [1] -
Mitkidou, S.; Stephanidou-Stephanatou, J.; Stephopoulou, H. J. Heterocycl. Chem. 1993, 30, 441. doi:10.1002/jhet.5570300227
Return to citation in text: [1] [2] [3] -
Menichi, G.; Boutar, M.; Kokel, B.; Takagi, K.; Hubert-Habart, M. J. Heterocycl. Chem. 1986, 23, 275. doi:10.1002/jhet.5570230157
Return to citation in text: [1] -
Nagarajan, K.; Arya, V. P.; Shenoy, S. J. J. Chem. Res., Miniprint 1986, 1401.
Return to citation in text: [1] -
Panizzi, C.; Benati, O. Gazz. Chim. Ital. 1946, 76, 66.
Return to citation in text: [1] -
Elnagdi, M. H.; Sadek, K. U.; Galil, F. M. A.; Hassan, S. M. E. Arch. Pharm. 1988, 321, 851. doi:10.1002/ardp.19883211205
Return to citation in text: [1] -
Nagahara, K.; Kawano, H.; Sasaoka, S.; Ukawa, C.; Hirama, T.; Takada, A.; Cottam, H. B.; Robins, R. K. J. Heterocycl. Chem. 1994, 31, 239. doi:10.1002/jhet.5570310140
Return to citation in text: [1] -
CrysAlisPro, Version 1.171.33.42; Oxford Diffraction Ltd.: Abingdon, Oxford, England, 2009.
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. A 2008, 64, 112. doi:10.1107/S0108767307043930
Return to citation in text: [1] -
Holzer, W.; Schmid, E. J. Heterocycl. Chem. 1995, 32, 1341. doi:10.1002/jhet.5570320440
Return to citation in text: [1] -
Holzer, W.; Krca, I. Heterocycles 2003, 60, 2323. doi:10.3987/COM-03-9857
Return to citation in text: [1] -
Wang, J.-H.; Shen, Y.-Q.; Yu, C.-X.; Si, J.-H. J. Chem. Soc., Perkin Trans. 1 2000, 1455. doi:10.1039/a908958f
Return to citation in text: [1] -
Becke, A. D. Phys. Rev. A 1988, 38, 3098. doi:10.1103/PhysRevA.38.3098
Return to citation in text: [1] -
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785. doi:10.1103/PhysRevB.37.785
Return to citation in text: [1] -
Becke, A. D. J. Chem. Phys. 1993, 98, 5648. doi:10.1063/1.464913
Return to citation in text: [1] -
Gaussian 09; Gaussian, Inc.: Wallingford, CT, 2009.
Return to citation in text: [1] [2] -
Wolinski, K.; Hinton, J. F.; Pulay, P. J. Am. Chem. Soc. 1990, 112, 8251. doi:10.1021/ja00179a005
Return to citation in text: [1] -
Silva, A. M. S.; Sousa, R. M. S.; Jimeno, M. L.; Blanco, F.; Alkorta, I.; Elguero, J. Magn. Reson. Chem. 2008, 46, 859. doi:10.1002/mrc.2272
Return to citation in text: [1] -
Blanco, F.; Alkorta, I.; Elguero, J. Magn. Reson. Chem. 2007, 45, 797. doi:10.1002/mrc.2053
Return to citation in text: [1] [2]
| 1. | Milata, V.; Rádl, S.; Voltrová, S. Science of Synthesis Product Subclass: Enol Ethers (acyclic, cyclic, i.e. endocyclic C=C-O- unit; Georg Thieme Verlag: Stuttgart, 2008; p 589. |
| 21. | Menichi, G.; Boutar, M.; Kokel, B.; Takagi, K.; Hubert-Habart, M. J. Heterocycl. Chem. 1986, 23, 275. doi:10.1002/jhet.5570230157 |
| 22. | Nagarajan, K.; Arya, V. P.; Shenoy, S. J. J. Chem. Res., Miniprint 1986, 1401. |
| 23. | Panizzi, C.; Benati, O. Gazz. Chim. Ital. 1946, 76, 66. |
| 24. | Elnagdi, M. H.; Sadek, K. U.; Galil, F. M. A.; Hassan, S. M. E. Arch. Pharm. 1988, 321, 851. doi:10.1002/ardp.19883211205 |
| 25. | Nagahara, K.; Kawano, H.; Sasaoka, S.; Ukawa, C.; Hirama, T.; Takada, A.; Cottam, H. B.; Robins, R. K. J. Heterocycl. Chem. 1994, 31, 239. doi:10.1002/jhet.5570310140 |
| 3. | Saloň, J.; Milata, V.; Gatial, A.; Prónayová, N.; Leško, J.; Černuchová, P.; Vo-Thanh, G.; Loupy, A. Eur. J. Org. Chem. 2005, 4870. doi:10.1002/ejoc.200500298 |
| 20. | Mitkidou, S.; Stephanidou-Stephanatou, J.; Stephopoulou, H. J. Heterocycl. Chem. 1993, 30, 441. doi:10.1002/jhet.5570300227 |
| 20. | Mitkidou, S.; Stephanidou-Stephanatou, J.; Stephopoulou, H. J. Heterocycl. Chem. 1993, 30, 441. doi:10.1002/jhet.5570300227 |
| 8. | Xu, J.; Liu, H.; Li, G.; He, Y.; Ding, R.; Wang, X.; Feng, M.; Zhang, S.; Chen, Y.; Li, S.; Zhao, M.; Qi, C.; Dang, Y. Bioorg. Med. Chem. Lett. 2011, 21, 4736. doi:10.1016/j.bmcl.2011.06.072 |
| 9. | Reddy, G. J.; Sailaja, S.; Manjula, D.; Rao, K. S.; Khalilullah, M.; Latha, D. Heterocycl. Commun. 2005, 11, 385. doi:10.1515/HC.2005.11.5.385 |
| 10. | Shaikh, A. C.; Chen, C. J. Labelled Compd. Radiopharm. 2008, 51, 72. doi:10.1002/jlcr.1484 |
| 11. | Nagahara, K.; Takagi, K.; Ueda, T. Chem. Pharm. Bull. 1976, 24, 2880. doi:10.1248/cpb.24.2880 |
| 12. | Takagi, K.; Nagahara, K.; Ueda, T. Chem. Pharm. Bull. 1970, 18, 2353. doi:10.1248/cpb.18.2353 |
| 13. | Kim, D. C.; Lee, Y. R.; Yang, B.-S.; Shin, K. J.; Kim, D. J.; Chung, B. Y.; Yoo, K. H. Eur. J. Med. Chem. 2003, 38, 525. doi:10.1016/S0223-5234(03)00065-5 |
| 7. | Černuchová, P.; Vo-Thanh, G.; Milata, V.; Loupy, A.; Jantová, S.; Theiszová, M. Tetrahedron 2005, 61, 5379. doi:10.1016/j.tet.2005.03.066 |
| 20. | Mitkidou, S.; Stephanidou-Stephanatou, J.; Stephopoulou, H. J. Heterocycl. Chem. 1993, 30, 441. doi:10.1002/jhet.5570300227 |
| 5. | Elguero, J.; Marzin, C.; Katritzky, A. R.; Linda, P. The Tautomerism of Heterocycles; Academic Press: New York, 1976; p 655. |
| 6. | Minkin, V. I.; Garnovskii, A. D.; Elguero, J.; Katritzky, A. R.; Denisko, O. V. Adv. Heterocycl. Chem. 2000, 76, 157. doi:10.1016/S0065-2725(00)76005-3 |
| 4. | Kornis, G. I. Kirk-Othmer Encyklopedia of Chemical Technology: Pyrazoles, pyrazolines, and pyrazolones; John Wiley and Sons: Hoboken, 2001. doi:10.1002/0471238961.1625180111151814.a01 |
| 14. | Guillou, S.; Janin, Y. L. Chem.–Eur. J. 2010, 16, 4669. doi:10.1002/chem.200903442 |
| 15. |
Ishimaru, T. Yakugaku Zasshi 1957, 77, 800.
Chem. Abstr. 1957, 17893. |
| 16. | Ruhemann, S. Ber. Dtsch. Chem. Ges. 1894, 27, 1658. doi:10.1002/cber.189402702104 |
| 17. | Ruhemann, S. Ber. Dtsch. Chem. Ges. 1896, 29, 1016. doi:10.1002/cber.189602901195 |
| 18. | Al-Jallo, H. N.; Al-Khasab, A.; Sallomi, I. G. J. Chem. Soc., Perkin Trans. 1 1972, 1022. doi:10.1039/p19720001022 |
| 27. | Sheldrick, G. M. Acta Crystallogr., Sect. A 2008, 64, 112. doi:10.1107/S0108767307043930 |
| 14. | Guillou, S.; Janin, Y. L. Chem.–Eur. J. 2010, 16, 4669. doi:10.1002/chem.200903442 |
| 26. | CrysAlisPro, Version 1.171.33.42; Oxford Diffraction Ltd.: Abingdon, Oxford, England, 2009. |
| 37. | Blanco, F.; Alkorta, I.; Elguero, J. Magn. Reson. Chem. 2007, 45, 797. doi:10.1002/mrc.2053 |
| 37. | Blanco, F.; Alkorta, I.; Elguero, J. Magn. Reson. Chem. 2007, 45, 797. doi:10.1002/mrc.2053 |
| 35. | Wolinski, K.; Hinton, J. F.; Pulay, P. J. Am. Chem. Soc. 1990, 112, 8251. doi:10.1021/ja00179a005 |
| 36. | Silva, A. M. S.; Sousa, R. M. S.; Jimeno, M. L.; Blanco, F.; Alkorta, I.; Elguero, J. Magn. Reson. Chem. 2008, 46, 859. doi:10.1002/mrc.2272 |
| 31. | Becke, A. D. Phys. Rev. A 1988, 38, 3098. doi:10.1103/PhysRevA.38.3098 |
| 32. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785. doi:10.1103/PhysRevB.37.785 |
| 33. | Becke, A. D. J. Chem. Phys. 1993, 98, 5648. doi:10.1063/1.464913 |
| 28. | Holzer, W.; Schmid, E. J. Heterocycl. Chem. 1995, 32, 1341. doi:10.1002/jhet.5570320440 |
| 29. | Holzer, W.; Krca, I. Heterocycles 2003, 60, 2323. doi:10.3987/COM-03-9857 |
| 30. | Wang, J.-H.; Shen, Y.-Q.; Yu, C.-X.; Si, J.-H. J. Chem. Soc., Perkin Trans. 1 2000, 1455. doi:10.1039/a908958f |
© 2014 Tarabová et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)