Abstract
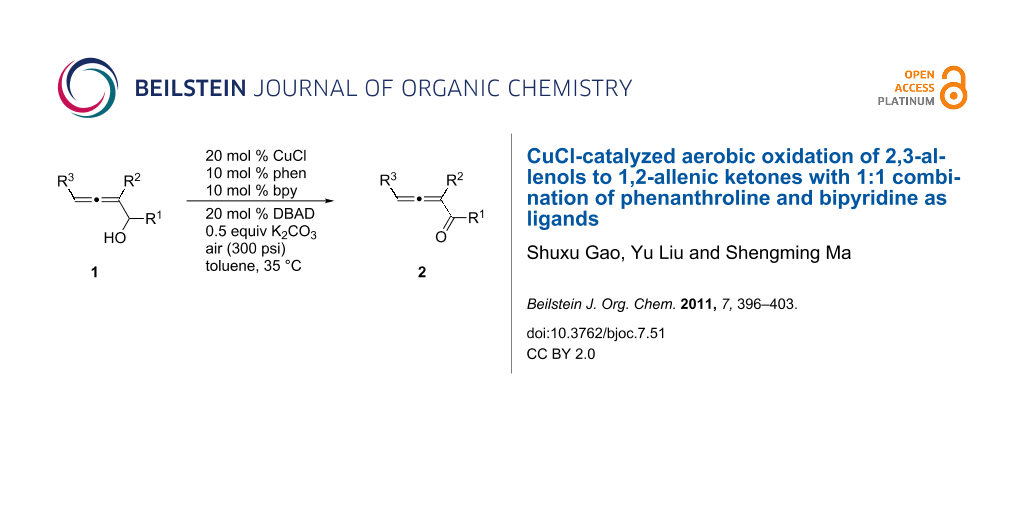
A protocol has been developed to prepare 1,2-allenyl ketones using molecular oxygen in air or pure oxygen as the oxidant from 2,3-allenylic alcohols with moderate to good yields under mild conditions. In this reaction CuCl (20 mol %) with 1,10-phenanthroline (10 mol %) and bipyridine (10 mol %) was used as the catalyst. It is interesting to observe that the use of the mixed ligands is important for the higher yields of this transformation: With the monoligand approach developed by Markó et al., the yields are relatively lower.
Graphical Abstract
Introduction
The oxidation of alcohols is one of many fundamental reactions in organic synthesis [1,2]. Usually, stoichiometric oxidants such as MnO2 [3], PCC [4], PDC [4], etc. were employed for this type of transformation. However, the cost and the byproducts derived from these reagents cause economic and environmental problems [5]. In the past decades, much attention has been paid to catalytic oxidation of alcohols using molecular oxygen as the oxidant with Pd [6-10], Cu [11-13], Ru [14,15] as the catalysts.
1,2-Allenic ketones have become very useful in organic synthesis [16-33]. Current methods for the oxidation of allenic alcohols to ketones include oxidation with MnO2 [30,34,35], Swern oxidation [17,24] or Dess–Martin oxidation [16,17,24,25,28,31-33]: Catalytic aerobic oxidation has not so far been reported. Due to the synthetic potential of 1,2-allenyl ketones, it is desirable to develop an aerobic oxidation protocol for 2,3-allenols. In this paper we wish to report the CuCl-catalyzed aerobic oxidation of 2,3-allenols by applying a mixed ligand approach using copper as the catalyst [12,13].
Results and Discussion
After screening the Pd- [6-10] and Ru-catalyzed [14,15] protocols without success, we began a study of the oxidation of 2-hexyl-1-phenylbuta-2,3-diene-1-ol (1a) with O2 based on the pioneering study of oxidation of normal simple alcohols by Markó et al. [12]. Under the original conditions [12], the expected allenylic ketone 2a was obtained in 59% yield when CuCl and 1,10-phenanthroline were used (Table 1, entry 1). A series of bases and solvents were then screened for the oxidation of 1a. The results are summarized in Table 1 and Table 2. We found that (1) K2CO3 is the most effective base (Table 1, entry 1) and that organic bases such as NEt3 and DBU are generally ineffective (Table 1, entries 6 and 7); (2) toluene is the best solvent (Table 2).
Table 1: Screening of bases for the CuCl-catalyzed oxidation of 1aa.
|
|
|||
| entry | base | time (h) | yield of 2a (%)b |
|---|---|---|---|
| 1 | K2CO3 | 40 | 59c,d |
| 2 | Na2CO3 | 48 | 22e |
| 3 | Cs2CO3 | 35 | 27d,f,g |
| 4 | KHCO3 | 45 | 15h |
| 5 | KOHi | 48 | 39j |
| 6 | NEt3 | 45 | NRk |
| 7 | DBU | 45 | NRl |
aThe reaction was carried out using 0.3 mmol of 1a, 20 mol % of CuCl, 20 mol % of phen, 20 mol % of DBAD, and 2.0 equiv of base in 3 mL of toluene under 1 atm of oxygen unless otherwise stated. b1H NMR yield using CH2Br2 as the internal standard. c1.0 equiv K2CO3 was used. dIsolated yield. e50% of 1a was recovered as determined by 1H NMR analysis. f15 mol % of catalyst was used. g32% of 1a was recovered by column chromatography. h53% of 1a was recovered as determined by 1H NMR analysis. i100 mg of 3 Å MS and 20 mol % of KOH was used. j28% of 1a was recovered as determined by 1H NMR analysis. k70% of 1a was recovered as determined by 1H NMR analysis. l72% of 1a was recovered by column chromatography.
Table 2: Screening of solvents for the CuCl-catalyzed oxidation of 1aa.
|
|
|||
| entry | solvent | time (h) | yield of 2a (%) |
|---|---|---|---|
| 1 | toluene | 40 | 59b |
| 2 | CH3CN | 48 | NRc |
| 3 | DCE | 47 | 31d |
| 4 | CHCl3 | 47 | 20e |
| 5 | DMF | 47 | NRf |
aThe reaction was carried out using 0.3 mmol of 1a, 20 mol % of CuCl, 20 mol % of phen, 20 mol % of DBAD, and 1.0 equiv of K2CO3 in 3 mL of solvent under 1 atm of oxygen. bIsolated yield. c64% of 1a was recovered as determined by 1H NMR analysis. d1H NMR yield using CH2Br2 as the internal standard. e78% of 1a was recovered as determined by 1H NMR analysis. f76% of 1a was recovered as determined by 1H NMR analysis.
In order to improve the yield further, we examined the effect of ligands. When 2,2'-bipyridine, which has a weaker coordinating ability, was used [36], the yield of 2a was lower (Table 3, entry 2). With 4,7-diphenyl-1,10-phenanthroline the yield was slightly improved to 66% (Table 3, entry 3). These experimental results obviously indicated that the CuCl-catalyzed oxidation of allenic alcohol was influenced by the coordinating ability of nitrogen ligands. Consequently, we carried out the reaction with a mixture of a stronger coordinating ligand together with a relatively weaker coordinating ligand. Indeed, it was interesting to observe that when 4,7-diphenyl-1,10-phenanthroline and 2,2'-bipyridine were mixed in the ratio of 1:1 [37,38], the isolated yield was improved to 82% (Table 3, entry 6). The yield with 1,10-phenanthroline and 2,2'-bipyridine (1:1) was 83% (Table 3, entry 10). However, 4,7-diphenyl-1,10-phenanthroline is relatively expensive (1 g, $ 94, Aldrich), so the cheaper 1,10-phenanthroline (5 g, $ 26.4, Aldrich) was used for further study. The effect of ratio of 1,10-phenanthroline vs 2,2'-bipyridine on the yield was also studied: A ratio of 1:1 proved to be the best (Table 3, entries 7–12). This may be explained by considering that the coordination of 2,2'-bipyridine is important for the formation of the catalytically active species and may be easily replaced with that of the alcohol. We also tried N-methylimidazole (NMI), which was used in oxidation of primary aliphatic alcohols reported by Markó et al. [13], however, both the turnover and yield were low (Table 3, entry 14). Both 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline and iPr-Pybox were ineffective in this reaction (Table 3, entries 4 and 5).
Table 3: Screening for different nitrogen ligands in the CuCl-catalyzed oxidation of 1aa.
|
|
||||
| entry | ligand 1 (mol %) | ligand 2 (mol %) | time (h) | yield of 2a (%)b |
|---|---|---|---|---|
| 1 |
|
— | 40 | 61 |
| 2 |
|
— | 46 | 43c |
| 3 |
|
— | 14.5 | 66 |
| 4 |
|
— | 45 | NRd |
| 5 |
|
— | 24 | NRe |
| 6 |
(10) |
L-B (10) | 45.5 | 82 |
| 7 | L-A (17.5) | L-B (2.5) | 40 | 79 |
| 8 | L-A (15.0) | L-B (5.0) | 40 | 65 |
| 9 | L-A (12.5) | L-B (7.5) | 40 | 82 |
| 10 | L-A (10.0) | L-B (10.0) | 42 | 83 |
| 11 | L-A (7.5) | L-B (12.5) | 42 | 78 |
| 12 | L-A (5.0) | L-B (15.0) | 40 | 73 |
| 13 | L-A (2.5) | L-B (17.5) | 40 | 68 |
| 14f | L-A (5.0) | NMI (7.0) | 35 | 17 |
aThe reaction was carried out using 0.3 mmol of 1a, 20 mol % of CuCl, 20 mol % of nitrogen ligand, 20 mol % of DBAD, and 1.0 equiv of K2CO3 in 3 mL of toluene under 1 atm of oxygen. b1H NMR yields determined by 300 MHz, 1H NMR analysis using CH2Br2 as the internal standard. c52% of 1a was recovered as determined by 1H NMR analysis. d87% of 1a was recovered as determined by 1H NMR analysis. e82% of 1a was recovered as determined by 1H NMR analysis. fThe reaction was carried out using 0.5 mmol of 1a, 5 mol % of CuCl, 5 mol % of t-BuOK, 5 mol % of DBAD and the indicated ligands in 5 mL of C6H5F at 70 °C under 1 atm of oxygen. 52% of 1a was recovered as determined by 1H NMR analysis.
Some other Cu(I) catalysts, such as CuBr, CuI, and CuCN were also investigated, but no higher yield was achieved (Table 4). Further studies led to the observation that air (300 psi, 35 °C (oil bath)) could be used instead of pure oxygen (1 atm, 15–24 °C) to shorten the reaction time from 40 to 10 hours and the yield was similar (86%) (Table 5, entry 1). Thus, 20 mol % of CuCl, 10 mol % of 1,10-phenanthroline, 10 mol % of 2,2'-bipyridine and 50 mol % of K2CO3 in toluene with air (300 psi, 35 °C) as the oxidant were defined as the standard conditions.
Table 5: The CuCl-catalyzed oxidation of allenic alcohols using air as the oxidanta.
|
|
|||||
| entry | substrate | time (h) | yield (%)b | ||
|---|---|---|---|---|---|
| R1 | R2 | R3 | |||
| 1 | Ph | n-C6H13 | H (1a) | 10 | 86 (2a) |
| 2 | p-EtC6H4 | n-Pr | H (1b) | 10 | 83 (2b) |
| 3 | p-BrC6H4 | n-Pr | H (1c) | 6 | 80 (2c) |
| 4 | p-ClC6H4 | n-C6H13 | H (1d) | 6 | 78 (2d) |
| 5 | p-O2NC6H4 | n-C6H13 | H (1e) | 6 | 63 (2e) |
| 6 | 3-furanyl | n-C6H13 | H (1f) | 11 | 61 (2f) |
| 7 | 3-thienyl | n-C5H11 | H (1g) | 8.5 | 73 (2g) |
| 8 | 1-naphthyl | Me | H (1h) | 11 | 74 (2h) |
| 9 | Ph | allyl | H (1i) | 11 | 75 (2i) |
| 10 | Ph | Bu | n-C5H11 (1j) | 11 | 91 (2j) |
aThe reaction was carried out using 0.3 mmol of 1, 20 mol % of CuCl, 10 mol % of phen, 10 mol % of bpy, 20 mol % of DBAD, and 0.5 equiv of K2CO3 in 3 mL of toluene, air (300 psi, 35 °C (oil bath)). bIsolated yields.
Under the standard conditions a series of 1-aryl-2,3-allenols were oxidized to the corresponding 1,2-allenic aryl ketones: A para-nitro group led to a 63% yield of 2e (Table 5, entry 5); heteroaryl groups such as furanyl and thienyl were also tolerated under the reaction conditions, affording the corresponding allenic ketones 2f and 2g in 61% and 73% yields, respectively (Table 5, entries 6 and 7), whilst the reaction of 1-naphthyl-substituted 1h afforded 2h in 74% yield (Table 5, entry 8). Tri-substituted allenic alcohol 1j was also oxidized to the corresponding allenic ketone 2j in 91% yield (Table 5, entry 10).
The reaction may be easily carried out on a 1 g scale: the oxidation of allenol 1k afforded the corresponding allenic ketone 2k in 74% yield in 12 hours with just 10 mol % of CuCl and 5 mol % each of 1,10-phenanthroline and 2,2'-bipyridine (Scheme 1).
Scheme 1: 1 gram scale reaction of allenol 1k.
Scheme 1: 1 gram scale reaction of allenol 1k.
When the reaction of 1-alkyl-substituted-2,3-allenols oxidation was conducted under 1 atm of oxygen at 60 °C, 81% of conversion was observed and the corresponding allenic ketones 2l and 2m were obtained in 58% and 60% isolated yields (72% and 74% based on the starting material consumed), respectively (Scheme 2). As a comparison, it should be noted that when 1l was oxidized with air (300 psi, 60 °C), the allenic ketone 2l was formed in 43% 1H NMR yield with 73% conversion of 1l within 10 hours.
Scheme 2: The oxidation of 1l and 1m under 1 atm of oxygen.
Scheme 2: The oxidation of 1l and 1m under 1 atm of oxygen.
Conclusion
In conclusion, we have developed a method for the aerobic oxidation of 2,3-allenols, which uses molecular oxygen in air or pure oxygen as the oxidant. In this reaction, CuCl with a 1:1 ratio of 1,10-phenanthroline and bipyridine was used as the catalyst to provide the best results. A series of 1,2-allenic ketones were obtained in moderate to good yields under mild conditions. Compared to the traditional monoligand approach, allenols are obviously unique demanding a mixed ligands approach for better yields probably as a consequence of the coordinating ability of the allene moiety. Further study in this area is being pursued in this laboratory.
Experimental
General experimental methods for starting materials
The starting allenols 1a–e, 1i, 1k, 1l, 1m were prepared via the reaction of propargyl bromides and corresponding aldehydes in the presence of SnCl2 and NaI in DMF [39,40]; allenols 1f [41], 1g [42], 1h [43], and 1j [44] were prepared as reported. These starting allenols were purified by flash chromatography before use.
General experimental procedure for the aerobic oxidation of allenic alcohols
2-Hexyl-1-phenylbuta-2,3-dien-1-one (2a)
Typical procedure: 1,10-phenanthroline (5.5 mg, 0.03 mmol), 2,2'-bipyridine (4.7 mg, 0.03 mmol), CuCl (5.9 mg, 0.06 mmol), K2CO3 (20.6 mg, 0.15 mmol), and 1.5 mL of dry toluene were added successively into an oven dried reaction vessel (sealed with a stopper to isolate the contents from atmospheric moisture). The resulting mixture was stirred at rt for 0.5 h. Then the stopper was removed to add DBAD (13.7 mg, 0.06 mmol), 2-hexyl-1-phenylbuta-2,3-dien-1-ol (69.6 mg, 0.3 mmol), and 1.5 mL of dry toluene. The reaction vessel was then transferred to an autoclave, which was charged with air to a pressure of 300 psi, and stirred at 35 °C (oil bath). After 10 h, the pressure was carefully released in the hood, the mixture filtered through a short column of silica gel (100–140 mesh) and washed with diethyl ether. Evaporation of the solvent and flash chromatography on silica gel (eluent: petroleum ether/diethyl ether = 30:1) afforded 2a (59.3 mg, 86%): liquid; 1H NMR (300 MHz , CDCl3) δ 7.76 (d, J = 7.2 Hz, 2H), 7.49 (t, J = 7.4 Hz, 1H), 7.39 (t, J = 7.2 Hz, 2H), 5.04 (t, J = 2.7 Hz, 2H), 2.46–2.35 (m, 2H), 1.58–1.45 (m, 2H), 1.45–1.20 (m, 6H), 0.89 (t, J = 6.6 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 217.0, 194.9, 138.3, 131.9, 129.0, 127.8, 106.9, 79.3, 31.6, 28.9, 27.8, 22.6, 14.0 ppm; MS (m/z) 228 (M+, 7.25), 105 (100); IR (neat) 2927, 2857, 1933, 1653, 1599, 1450, 1312, 1273 cm−1; HRMS-EI (m/z) calcd for C16H20O+ [M+]: 228.1514; found: 228.1517.
2-Propyl-1-(4-ethylphenyl)buta-2,3-dien-1-one (2b)
The reaction of 1,10-phenanthroline (5.3 mg, 0.03 mmol), 2,2'-bipyridine (4.6 mg, 0.03 mmol), CuCl (5.9 mg, 0.06 mmol), K2CO3 (20.8 mg, 0.15 mmol), dry toluene (1.5 mL), DBAD (13.7 mg, 0.06 mmol), and 2-propyl-1-(4-ethylphenyl)buta-2,3-dien-1-ol (64.7 mg, 0.3 mmol)/dry toluene (1.5 mL) afforded 2b (53.0 mg, 83%): liquid; 1H NMR (300 MHz, CDCl3) δ 7.73 (d, J = 8.1 Hz, 2H), 7.22 (d, J = 8.1 Hz, 2H), 5.05 (t, J = 2.9 Hz, 2H), 2.69 (q, J = 7.6 Hz, 2H), 2.44–2.32 (m, 2H), 1.63–1.45 (m, 2H), 1.25 (t, J = 7.5 Hz, 3H), 0.99 (t, J = 7.4 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 216.5, 194.4, 148.8, 135.8, 129.3, 127.3, 106.5, 79.1, 30.1, 28.8, 21.1, 15.1, 13.7 ppm; MS (m/z) 214 (M+, 1.75), 133 (100); IR (neat) 2964, 2931, 2872, 1933, 1650, 1607, 1458, 1414, 1273, 1182, 1058 cm−1; HRMS-EI (m/z) calcd for C15H18O+ [M+]: 214.1358; found: 214.1360.
2-Propyl-1-(4-bromophenyl)buta-2,3-dien-1-one (2c)
The reaction of 1,10-phenanthroline (5.4 mg, 0.03 mmol), 2,2'-bipyridine (4.6 mg, 0.03 mmol), CuCl (5.9 mg, 0.06 mmol), K2CO3 (20.6 mg, 0.15 mmol), dry toluene (1.5 mL), DBAD (13.8 mg, 0.06 mmol), and 2-propyl-1-(4-bromophenyl)buta-2,3-dien-1-ol (80.3 mg, 0.3 mmol)/dry toluene (1.5 mL) afforded 2c (64.1 mg, 80%): liquid; 1H NMR (300 MHz, CDCl3) δ 7.62 (d, J = 8.7 Hz, 2H), 7.52 (d, J = 8.4 Hz, 2H), 5.07 (t, J = 2.9 Hz, 2H), 2.41–2.30 (m, 2H), 1.60–1.45 (m, 2H), 0.97 (t, J = 7.5 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 217.0, 193.7, 137.0, 131.1, 130.5, 126.7, 106.7, 79.7, 29.7, 21.1, 13.7 ppm; MS (m/z) 266 (M+ (81Br), 1.68), 264 (M+ (79Br), 1.76), 185 (100); IR (neat) 2961, 2929, 2870, 1931, 1653, 1585, 1458, 1391, 1270, 1071, 1010 cm−1; HRMS-EI (m/z) calcd for C13H13O81Br+ [M+]: 266.0129; found: 266.0136.
2-Hexyl-1-(4-chlorophenyl)buta-2,3-dien-1-one (2d)
The reaction of 1,10-phenanthroline (5.5 mg, 0.03 mmol), 2,2'-bipyridine (4.8 mg, 0.03 mmol), CuCl (6.0 mg, 0.06 mmol), K2CO3 (20.9 mg, 0.15 mmol), dry toluene (1.5 mL), DBAD (13.8 mg, 0.06 mmol), and 2-hexyl-1-(4-chlorophenyl)buta-2,3-dien-1-ol (79.6 mg, 0.3 mmol)/dry toluene (1.5 mL) afforded 2d (62.0 mg, 78%): liquid; 1H NMR (300 MHz, CDCl3) δ 7.70 (d, J = 8.4 Hz, 2H), 7.35 (d, J = 8.4 Hz, 2H), 5.06 (t, J = 2.6 Hz, 2H), 2.43–2.32 (m, 2H), 1.55–1.42 (m, 2H), 1.43–1.18 (m, 6H), 0.88 (t, J = 6.3 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 217.0, 193.6, 138.2, 136.6, 130.4, 128.1, 106.9, 79.6, 31.6, 28.9, 27.7, 22.5, 14.0 ppm; MS (m/z) 264 (M+ (37Cl), 0.76), 262 (M+ (35Cl), 2.08), 139 (100); IR (neat) 2927, 2857, 1931, 1654, 1590, 1460, 1397, 1274, 1091 cm−1; HRMS-EI (m/z) calcd for C16H19O35Cl+ [M+]: 262.1124; found: 262.1130.
2-Hexyl-1-(4'-nitrophenyl)buta-2,3-dien-1-one (2e)
The reaction of 1,10-phenanthroline (5.5 mg, 0.03 mmol), 2,2'-bipyridine (4.7 mg, 0.03 mmol), CuCl (5.9 mg, 0.06 mmol), K2CO3 (20.8 mg, 0.15 mmol), dry toluene (1.5 mL), DBAD (13.9 mg, 0.06 mmol), and 2-hexyl-1-(4-nitrophenyl)buta-2,3-dien-1-ol (82.7 mg, 0.3 mmol)/dry toluene (1.5 mL) afforded 2e (51.4 mg, 63%) (eluent: petroleum ether/diethyl ether = 20:1): liquid; 1H NMR (300 MHz, CDCl3) δ 8.24 (d, J = 8.7 Hz, 2H), 7.84 (d, J = 8.7 Hz, 2H), 5.12 (t, J = 2.9 Hz, 2H), 2.44–2.33 (m, 2H), 1.57–1.44 (m, 2H), 1.44–1.20 (m, 6H), 0.89 (t, J = 6.5 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 218.0, 193.4, 149.4, 143.6, 129.7, 123.1, 107.6, 80.4, 31.5, 28.8, 27.7, 27.3, 22.5, 14.0 ppm; MS (m/z) 273 (M+, 2.56), 150 (100); IR (neat) 2927, 2857, 1930, 1660, 1602, 1526, 1461, 1349, 1272, 1104, 1011 cm−1; HRMS-EI (m/z) calcd for C16H19NO3+ [M+]: 273.1365; found: 273.1367.
2-Hexyl-1-(3-furanyl)buta-2,3-dien-1-one (2f)
The reaction of 1,10-phenanthroline (5.4 mg, 0.03 mmol), 2,2'-bipyridine (4.8 mg, 0.03 mmol), CuCl (6.2 mg, 0.06 mmol), K2CO3 (21.3 mg, 0.15 mmol), dry toluene (1.5 mL), DBAD (13.6 mg, 0.06 mmol), and 2-hexyl-1-(3-furanyl)buta-2,3-dien-1-ol (66.2 mg, 0.3 mmol)/dry toluene (1.5 mL) afforded 2f (40.4 mg, 61%): liquid; 1H NMR (300 MHz, CDCl3) δ 8.10 (s, 1H), 7.37 (s, 1H), 6.81 (d, J = 1.2 Hz, 1H), 5.21 (t, J = 2.9 Hz, 2H), 2.39–2.27 (m, 2 H), 1.52–1.38 (m, 2H), 1.38–1.18 (m, 6H), 0.87 (t, J = 6.5 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 215.8, 186.4, 147.4, 143.0, 126.7, 110.0, 108.1, 80.0, 31.6, 28.9, 27.8, 27.6, 22.6, 14.1 ppm; MS (m/z) 218 (M+, 3.49), 95 (100); IR (neat) 2956, 2928, 2857, 1933, 1724, 1645, 1561, 1509, 1458, 1379, 1311, 1163, 1077, 1009 cm−1; HRMS-EI (m/z) calcd for C14H18O2+ [M+]: 218.1307; found: 218.1305.
2-Pentyl-1-(3-thienyl)buta-2,3-dien-1-one (2g)
The reaction of 1,10-phenanthroline (5.5 mg, 0.03 mmol), 2,2'-bipyridine (4.8 mg, 0.03 mmol), CuCl (5.9 mg, 0.06 mmol), K2CO3 (20.9 mg, 0.15 mmol), dry toluene (1.5 mL), DBAD (13.7 mg, 0.06 mmol), and 2-pentyl-1-(3-thienyl)buta-2,3-dien-1-ol (66.4 mg, 0.3 mmol)/dry toluene (1.5 mL) afforded 2g (48.3 mg, 73%) (eluent: petroleum ether/diethyl ether = 50:1): liquid; 1H NMR (300 MHz, CDCl3) δ 8.07 (d, J = 1.8 Hz, 1H) 7.53 (d, J = 4.8 Hz, 1H), 7.25 (dd, J1 = 4.8 Hz, J2 = 3.3 Hz, 1H), 5.16 (d, J = 2.7 Hz, 2H), 2.41–2.32 (m, 2H), 1.56–1.42 (m, 2H), 1.42–1.24 (m, 4H), 0.90 (t, J = 6.6 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 216.0, 187.1, 141.5, 132.2, 128.2, 125.1, 107.6, 79.6, 31.4, 27.9, 27.5, 22.4, 14.0 ppm; MS (m/z) 220 (M+, 3.02), 111 (100); IR (neat) 2956, 2927, 2861, 1933, 1641, 1511, 1460, 1411, 1260, 1082 cm−1; HRMS-EI (m/z) calcd for C13H16OS+ [M+]: 220.0922; found: 220.0922.
2-Methyl-1-(1-naphthyl)buta-2,3-dien-1-one (2h)
The reaction of 1,10-phenanthroline (5.5 mg, 0.03 mmol), 2,2'-bipyridine (4.8 mg, 0.03 mmol), CuCl (6.2 mg, 0.06 mmol), K2CO3 (21.4 mg, 0.15 mmol), dry toluene (1.5 mL), DBAD (13.5 mg, 0.06 mmol), and 2-methyl-1-naphthylbuta-2,3-dien-1-ol (63.6 mg, 0.3 mmol)/dry toluene (1.5 mL) afforded 2h (47.2 mg, 74%, an unknown substance could not be separated via column chromatography and the purity of 2h is 95%, which was determined by 1H NMR with mesitylene as the internal standard): liquid; 1H NMR (300 MHz, CDCl3) δ 8.11–8.03 (m, 1H), 7.95–7.81 (m, 2H), 7.61–7.39 (m, 4H), 4.80 (q, J = 2.8 Hz, 2H), 2.11 (t, J = 2.7 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 218.6, 197.5, 136.8, 133.5, 130.6, 130.4, 128.2, 126.9, 126.5, 126.1, 125.3, 123.9, 104.8, 78.2, 13.8 ppm; MS (m/z) 208 (M+, 63.16), 155 (100); IR (neat) 3059, 1957, 1930, 1650, 1508, 1285, 1251, 1204, 1155, 1080, 1059 cm−1; HRMS-EI (m/z) calcd for C15H12O [M+]: 208.0888; found: 208.0887.
2-Allyl-1-phenylbuta-2,3-dien-1-one (2i)
The reaction of 1,10-phenanthroline (5.4 mg, 0.03 mmol), 2,2'-bipyridine (4.6 mg, 0.03 mmol), CuCl (6.1 mg, 0.06 mmol), K2CO3 (21.5 mg, 0.15 mmol), dry toluene (1.5 mL), DBAD (13.7 mg, 0.06 mmol), and 2-allyl-1-phenylbuta-2,3-dien-1-ol (55.1 mg, 0.3 mmol)/dry toluene (1.5 mL) afforded 2i (41.1 mg, 75%) (eluent: petroleum ether/diethyl ether = 40:1): liquid; 1H NMR (300 MHz, CDCl3) δ 7.78 (d, J = 7.8 Hz, 2H), 7.50 (t, J = 7.2 Hz, 1H), 7.39 (t, J = 7.5 Hz, 2H), 5.98–5.82 (m, 1H), 5.22–5.04 (m, 4H), 3.20–3.14 (m, 2H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 217.2, 194.1, 138.0, 134.9, 132.1, 129.0, 127.8, 116.4, 105.3, 79.8, 32.5 ppm; MS (m/z) 184 (M+, 2.53), 105 (100); IR (neat) 3081, 3062, 2982, 1956, 1931, 1651, 1598, 1578, 1447, 1422, 1316, 1272 cm−1; HRMS-EI (m/z) calcd for C13H12O [M+]: 184.0888; found: 184.0889.
2-Butyl-1-phenylnona-2,3-dien-1-one (2j)
The reaction of 1,10-phenanthroline (5.6 mg, 0.03 mmol), 2,2'-bipyridine (4.9 mg, 0.03 mmol), CuCl (6.2 mg, 0.06 mmol), K2CO3 (21.5 mg, 0.15 mmol), dry toluene (1.5 mL), DBAD (14.1 mg, 0.06 mmol), and 2-butyl-1-phenylnona-2,3-dien-1-ol (82.2 mg, 0.3 mmol)/dry toluene (1.5 mL) afforded 2j [43] (74.8 mg, 91%): liquid; 1H NMR (300 MHz, CDCl3) 7.71 (d, J = 7.8 Hz, 2H), 7.50–7.42 (m, 1H), 7.40–7.32 (m, 2H), 5.36 (t, J = 7.2 Hz, 1H), 2.48–2.30 (m, 2H), 2.16–1.96 (m, 2H), 1.55–1.11 (m, 10H), 0.93 (t, J = 6.9 Hz, 3H), 0.84 (t, J = 7.2 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 213.3, 195.7, 138.9, 131.5, 128.8, 127.6, 107.4, 95.0, 31.1, 30.2, 28.6, 28.4, 27.8, 22.31, 22.29, 13.89, 13.86 ppm.
2-Propyl-1-phenylbuta-2,3-dien-1-one (2k)
The reaction of 1,10-phenanthroline (49.1 mg, 0.27 mmol), 2,2'-bipyridine (42.6 mg, 0.27 mmol), CuCl (54.2 mg, 0.54 mmol), K2CO3 (373.8 mg, 2.7 mmol), dry toluene (9 mL), DBAD (124.4 mg, 0.54 mmol), and 2-propyl-1-phenylbuta-2,3-dien-1-ol (1.0141 g, 5.4 mmol)/dry toluene (9 mL) afforded 2k (0.7512 g, 74%): liquid; 1H NMR (300 MHz, CDCl3) δ 7.76 (d, J = 7.5 Hz, 2H), 7.50 (t, J = 7.4 Hz, 1H), 7.39 (t, J = 7.5 Hz, 2H), 5.05 (s, 2H), 2.39 (t, J = 7.2 Hz, 2H), 1.62–1.46 (m, 2H), 0.99 (t, J = 7.5 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 217.1, 194.9, 138.3, 131.9, 129.0, 127.8, 106.7, 79.3, 29.9, 21.1, 13.7 ppm; MS (m/z) 186 (M+, 6.46), 105 (100); IR (neat) 2961, 2932, 2872, 1933, 1651, 1598, 1578, 1447, 1315, 1271 cm−1; HRMS-EI (m/z) calcd for C13H14O+ [M+]: 186.1045; found: 186.1045.
General experimental procedure for the oxidation of allenic alcohols with pure oxygen
3-Hexylocta-1,2-dien-4-one (2l)
Typical procedure: 1,10-phenanthroline (6.9 mg, 0.0375 mmol), 2,2'-bipyridine (5.8 mg, 0.0375 mmol), CuCl (5.9 mg, 0.06 mmol), and K2CO3 (20.9 mg, 0.15 mmol) were added sequentially to an oven dried Schlenk tube, which was purged with air and refilled with oxygen (twice). Then 1.5 mL of dry toluene was added, the resulting mixture was stirred at rt for 0.5 h which was followed by the sequential addition of DBAD (14.0 mg, 0.06 mmol), 2-hexyl-1-butylbuta-2,3-dien-1-ol (63.8 mg, 0.3 mmol) and 1.5 mL of dry toluene. After stirring at 60 °C for 24 h, the reaction mixture was filtered through silica gel (100–140 mesh) and washed with diethyl ether. Evaporation of the solvent and flash chromatography on silica gel (eluent: petroleum ether/ether = 50:1) afforded 2l (37.1 mg, 58%) (conv. = 81%, yield = 72% (based on the alcohol consumed)): liquid; 1H NMR (300 MHz, CDCl3) δ 5.14 (d, J = 2.7 Hz, 2H), 2.62 (t, J = 7.4 Hz, 2H), 2.18–2.09 (m, 2H), 1.60–1.47 (m, 2H), 1.42–1.18 (m, 10H), 0.92–0.78 (m, 6H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 216.2, 201.3, 108.5, 79.3, 38.9, 31.6, 28.8, 27.8, 27.2, 26.2, 22.5, 22.3, 14.0, 13.8 ppm; MS (m/z) 208 (M+, 1.00), 85 (100); IR (neat) 2958, 2929, 2862, 1934, 1679, 1461, 1174 cm−1; HRMS-EI (m/z) calcd for C14H24O+ [M+]: 208.1827; found: 208.1828.
4-Pentyl-1-phenylhexa-4,5-dien-3-one (2m)
The reaction of 1,10-phenanthroline (6.9 mg, 0.0375 mmol), 2,2'-bipyridine (5.9 mg, 0.0375 mmol), CuCl (6.1 mg, 0.06 mmol), K2CO3 (21.4 mg, 0.15 mmol), dry toluene (1.5 mL), DBAD (13.9 mg, 0.06 mmol), and 2-pentyl-1-(phenylethyl)buta-2,3-dien-1-ol (72.7 mg, 0.3 mmol)/dry toluene (1.5 mL) afforded 2m (43.8 mg, 60%) (conv. = 81%, yield = 74% (based on the alcohol consumed)): liquid; 1H NMR (300 MHz, CDCl3) δ 7.31–7.22 (m, 2H), 7.22–7.13 (m, 3H), 5.13 (s, 2 H), 3.02–2.85 (m, 4H), 2.22–2.10 (m, 2H), 1.45–1.20 (m, 6H), 0.88 (t, J = 6.5 Hz, 3H) ppm; 13C NMR (75.4 MHz, CDCl3) δ 216.3, 200.1, 141.3, 128.3, 126.0, 108.6, 79.7, 40.9, 31.4, 30.9, 27.4, 26.1, 22.4, 14.0 ppm; MS (m/z) 242 (M+, 0.87), 105 (100); IR (neat) 2956, 2928, 2861, 1933, 1678, 1496, 1456, 1171, 1100 cm−1; Anal. calcd for C17H22O: C, 84.25; H, 9.15. found: C, 84.16; H, 9.50.
Supporting Information
| Supporting Information File 1: 1H and 13C NMR spectra of products prepared. | ||
| Format: PDF | Size: 3.5 MB | Download |
References
-
Sheldon, R. A.; Kochi, J. K. Metal-Catalyzed Oxidations of Organic Compounds; Academic Press: New York, 1981.
Return to citation in text: [1] -
Trost, B. M.; Fleming, I.; Ley, S. V. Comprehensive Organic Synthesis; Pergamon: Oxford, 1991; Vol. 7.
Return to citation in text: [1] -
Fatiadi, A. J. Synthesis 1976, 65. doi:10.1055/s-1976-23961
Return to citation in text: [1] -
Cainelli, G.; Cardillo, G. Chromium Oxidations in Organic Chemistry; Springer: Berlin, 1984.
Return to citation in text: [1] [2] -
Anastas, P.; Warner, J. Green Cemistry: Theory and Practice; Oxford University Press: Oxford, 1998.
Return to citation in text: [1] -
Blackburn, T. F.; Schwartz, J. J. Chem. Soc., Chem. Commun. 1977, 157. doi:10.1039/C39770000157
Return to citation in text: [1] [2] -
Peterson, K. P.; Larock, R. C. J. Org. Chem. 1998, 63, 3185. doi:10.1021/jo971268k
Return to citation in text: [1] [2] -
Nishimura, T.; Onoue, T.; Ohe, K.; Uemura, S. J. Org. Chem. 1999, 64, 6750. doi:10.1021/jo9906734
Return to citation in text: [1] [2] -
ten Brink, G. J.; Arends, I. W. C. E.; Sheldon, R. A. Science 2000, 287, 1636. doi:10.1126/science.287.5458.1636
Return to citation in text: [1] [2] -
Muzart, J. Tetrahedron 2003, 59, 5789. doi:10.1016/S0040-4020(03)00866-4
for a review.
Return to citation in text: [1] [2] -
Semmelhack, M. F.; Schmid, C. R.; Cortés, D. A.; Chou, C. S. J. Am. Chem. Soc. 1984, 106, 3374. doi:10.1021/ja00323a064
Return to citation in text: [1] -
Markó, I. E.; Giles, P. R.; Tsukazaki, M.; Brown, S. M.; Urch, C. J. Science 1996, 274, 2044. doi:10.1126/science.274.5295.2044
Return to citation in text: [1] [2] [3] [4] -
Markó, I. E.; Gautier, A.; Dumeunier, R.; Doda, K.; Philippart, F.; Brown, S. M.; Urch, C. J. Angew. Chem., Int. Ed. 2004, 43, 1588. doi:10.1002/anie.200353458
Return to citation in text: [1] [2] [3] -
Sheldon, R. A.; Arends, I. W. C. E.; ten Brink, G. J.; Dijksman, A. Acc. Chem. Res. 2002, 35, 774. doi:10.1021/ar010075n
Return to citation in text: [1] [2] -
Zhan, B.; Thompson, A. Tetrahedron 2004, 60, 2917. doi:10.1016/j.tet.2004.01.043
Return to citation in text: [1] [2] -
Marshall, J. A.; Robinson, E. D. J. Org. Chem. 1990, 55, 3450. doi:10.1021/jo00298a012
Return to citation in text: [1] [2] -
Mashall, J. A.; Wang, X. J. Org. Chem. 1991, 56, 960. doi:10.1021/jo00003a013
Return to citation in text: [1] [2] [3] -
Hashmi, A. S. K. Angew. Chem., Int. Ed. 1995, 34, 1581. doi:10.1002/anie.199515811
Return to citation in text: [1] -
Hashmi, A. S. K.; Schwarz, L.; Choi, J. H.; Frost, T. M. Angew. Chem., Int. Ed. 2000, 39, 2285. doi:10.1002/1521-3773(20000703)39:13<2285::AID-ANIE2285>3.0.CO;2-F
Return to citation in text: [1] -
Ma, S.; Zhang, J. Chem. Commun. 2000, 117. doi:10.1039/a908627g
Return to citation in text: [1] -
Ma, S.; Yin, S.; Li, L.; Tao, F. Org. Lett. 2002, 4, 505. doi:10.1021/ol0170859
Return to citation in text: [1] -
Ma, S.; Yu, Z. Angew. Chem., Int. Ed. 2002, 41, 1775. doi:10.1002/1521-3773(20020517)41:10<1775::AID-ANIE1775>3.0.CO;2-8
Return to citation in text: [1] -
Ma, S.; Zhang, J.; Lu, L. Chem.–Eur. J. 2003, 9, 2447. doi:10.1002/chem.200305341
Return to citation in text: [1] -
Ma, S.; Yu, S.; Yin, S. J. Org. Chem. 2003, 68, 8996. doi:10.1021/jo034633i
Return to citation in text: [1] [2] [3] -
Ma, S.; Yu, Z. Chem.–Eur. J. 2004, 10, 2078. doi:10.1002/chem.200305341
Return to citation in text: [1] [2] -
Ma, S.; Yu, S. Org. Lett. 2005, 7, 5063. doi:10.1021/ol052073z
Return to citation in text: [1] -
Ma, S.; Gu, Z.; Yu, Z. J. Org. Chem. 2005, 70, 6291. doi:10.1021/jo0507441
Return to citation in text: [1] -
Sromek, A. W.; Rubina, M.; Gevorgyan, V. J. Am. Chem. Soc. 2005, 127, 10500. doi:10.1021/ja053290y
Return to citation in text: [1] [2] -
Dudnik, A. S.; Gevorgyan, V. Angew. Chem., Int. Ed. 2007, 46, 5195. doi:10.1002/anie.200701128
Return to citation in text: [1] -
Marx, V. M.; Burnell, D. J. Org. Lett. 2009, 11, 1229. doi:10.1021/ol900029d
Return to citation in text: [1] [2] -
Alcaide, B.; Almendros, P.; Martínez del Campo, T. Eur. J. Org. Chem. 2007, 2844. doi:10.1002/ejoc.200700128
Return to citation in text: [1] [2] -
Sampath, M.; Loh, T.-P. Chem. Commun. 2009, 1568. doi:10.1039/b819959k
Return to citation in text: [1] [2] -
Zhao, J.; Loh, T.-P. Angew. Chem., Int. Ed. 2009, 48, 7232. doi:10.1002/anie.200902471
Return to citation in text: [1] [2] -
Russell, S. W.; Weedon, B. C. L. J. Chem. Soc. D 1969, 85. doi:10.1039/C29690000085
Return to citation in text: [1] -
Landor, P. D.; Landor, S. R.; Mukasa, S. J. Chem. Soc. D 1971, 1638. doi:10.1039/C29710001638
Return to citation in text: [1] -
Rannulu, N. S.; Rodgers, M. T. J. Phys. Chem. A 2007, 111, 3465. doi:10.1021/jp066903h
Return to citation in text: [1] -
Reetz, M. T.; Li, X. Angew. Chem., Int. Ed. 2005, 44, 2962. doi:10.1002/anie.200462613
Return to citation in text: [1] -
Reetz, M. T. Angew. Chem., Int. Ed. 2008, 47, 2556. doi:10.1002/anie.200704327
Return to citation in text: [1] -
Li, J.; Zhou, C.; Fu, C.; Ma, S. Tetrahedron 2009, 65, 3695. doi:10.1016/j.tet.2009.02.061
Return to citation in text: [1] -
Mukaiyama, T.; Harada, T. Chem. Lett. 1981, 10, 621. doi:10.1246/cl.1981.621
Return to citation in text: [1] -
Alcaide, B.; Almendros, P.; Aragoncillo, C.; Redondo, M. C. Eur. J. Org. Chem. 2005, 98. doi:10.1002/ejoc.200400527
Return to citation in text: [1] -
Yang, Y.; Shen, Z.; Loh, T.-P. Org. Lett. 2009, 11, 1209. doi:10.1021/ol8027362
Return to citation in text: [1] -
Isaac, M. B.; Chan, T. H. J. Chem. Soc., Chem. Commun. 1995, 1003. doi:10.1039/C39950001003
Return to citation in text: [1] [2] -
Ma, S.; Yu, S.; Yin, S. J. Org. Chem. 2003, 68, 8996. doi:10.1021/jo034633i
Return to citation in text: [1]
| 1. | Sheldon, R. A.; Kochi, J. K. Metal-Catalyzed Oxidations of Organic Compounds; Academic Press: New York, 1981. |
| 2. | Trost, B. M.; Fleming, I.; Ley, S. V. Comprehensive Organic Synthesis; Pergamon: Oxford, 1991; Vol. 7. |
| 5. | Anastas, P.; Warner, J. Green Cemistry: Theory and Practice; Oxford University Press: Oxford, 1998. |
| 14. | Sheldon, R. A.; Arends, I. W. C. E.; ten Brink, G. J.; Dijksman, A. Acc. Chem. Res. 2002, 35, 774. doi:10.1021/ar010075n |
| 15. | Zhan, B.; Thompson, A. Tetrahedron 2004, 60, 2917. doi:10.1016/j.tet.2004.01.043 |
| 4. | Cainelli, G.; Cardillo, G. Chromium Oxidations in Organic Chemistry; Springer: Berlin, 1984. |
| 12. | Markó, I. E.; Giles, P. R.; Tsukazaki, M.; Brown, S. M.; Urch, C. J. Science 1996, 274, 2044. doi:10.1126/science.274.5295.2044 |
| 4. | Cainelli, G.; Cardillo, G. Chromium Oxidations in Organic Chemistry; Springer: Berlin, 1984. |
| 12. | Markó, I. E.; Giles, P. R.; Tsukazaki, M.; Brown, S. M.; Urch, C. J. Science 1996, 274, 2044. doi:10.1126/science.274.5295.2044 |
| 13. | Markó, I. E.; Gautier, A.; Dumeunier, R.; Doda, K.; Philippart, F.; Brown, S. M.; Urch, C. J. Angew. Chem., Int. Ed. 2004, 43, 1588. doi:10.1002/anie.200353458 |
| 6. | Blackburn, T. F.; Schwartz, J. J. Chem. Soc., Chem. Commun. 1977, 157. doi:10.1039/C39770000157 |
| 7. | Peterson, K. P.; Larock, R. C. J. Org. Chem. 1998, 63, 3185. doi:10.1021/jo971268k |
| 8. | Nishimura, T.; Onoue, T.; Ohe, K.; Uemura, S. J. Org. Chem. 1999, 64, 6750. doi:10.1021/jo9906734 |
| 9. | ten Brink, G. J.; Arends, I. W. C. E.; Sheldon, R. A. Science 2000, 287, 1636. doi:10.1126/science.287.5458.1636 |
| 10. |
Muzart, J. Tetrahedron 2003, 59, 5789. doi:10.1016/S0040-4020(03)00866-4
for a review. |
| 16. | Marshall, J. A.; Robinson, E. D. J. Org. Chem. 1990, 55, 3450. doi:10.1021/jo00298a012 |
| 17. | Mashall, J. A.; Wang, X. J. Org. Chem. 1991, 56, 960. doi:10.1021/jo00003a013 |
| 18. | Hashmi, A. S. K. Angew. Chem., Int. Ed. 1995, 34, 1581. doi:10.1002/anie.199515811 |
| 19. | Hashmi, A. S. K.; Schwarz, L.; Choi, J. H.; Frost, T. M. Angew. Chem., Int. Ed. 2000, 39, 2285. doi:10.1002/1521-3773(20000703)39:13<2285::AID-ANIE2285>3.0.CO;2-F |
| 20. | Ma, S.; Zhang, J. Chem. Commun. 2000, 117. doi:10.1039/a908627g |
| 21. | Ma, S.; Yin, S.; Li, L.; Tao, F. Org. Lett. 2002, 4, 505. doi:10.1021/ol0170859 |
| 22. | Ma, S.; Yu, Z. Angew. Chem., Int. Ed. 2002, 41, 1775. doi:10.1002/1521-3773(20020517)41:10<1775::AID-ANIE1775>3.0.CO;2-8 |
| 23. | Ma, S.; Zhang, J.; Lu, L. Chem.–Eur. J. 2003, 9, 2447. doi:10.1002/chem.200305341 |
| 24. | Ma, S.; Yu, S.; Yin, S. J. Org. Chem. 2003, 68, 8996. doi:10.1021/jo034633i |
| 25. | Ma, S.; Yu, Z. Chem.–Eur. J. 2004, 10, 2078. doi:10.1002/chem.200305341 |
| 26. | Ma, S.; Yu, S. Org. Lett. 2005, 7, 5063. doi:10.1021/ol052073z |
| 27. | Ma, S.; Gu, Z.; Yu, Z. J. Org. Chem. 2005, 70, 6291. doi:10.1021/jo0507441 |
| 28. | Sromek, A. W.; Rubina, M.; Gevorgyan, V. J. Am. Chem. Soc. 2005, 127, 10500. doi:10.1021/ja053290y |
| 29. | Dudnik, A. S.; Gevorgyan, V. Angew. Chem., Int. Ed. 2007, 46, 5195. doi:10.1002/anie.200701128 |
| 30. | Marx, V. M.; Burnell, D. J. Org. Lett. 2009, 11, 1229. doi:10.1021/ol900029d |
| 31. | Alcaide, B.; Almendros, P.; Martínez del Campo, T. Eur. J. Org. Chem. 2007, 2844. doi:10.1002/ejoc.200700128 |
| 32. | Sampath, M.; Loh, T.-P. Chem. Commun. 2009, 1568. doi:10.1039/b819959k |
| 33. | Zhao, J.; Loh, T.-P. Angew. Chem., Int. Ed. 2009, 48, 7232. doi:10.1002/anie.200902471 |
| 17. | Mashall, J. A.; Wang, X. J. Org. Chem. 1991, 56, 960. doi:10.1021/jo00003a013 |
| 24. | Ma, S.; Yu, S.; Yin, S. J. Org. Chem. 2003, 68, 8996. doi:10.1021/jo034633i |
| 14. | Sheldon, R. A.; Arends, I. W. C. E.; ten Brink, G. J.; Dijksman, A. Acc. Chem. Res. 2002, 35, 774. doi:10.1021/ar010075n |
| 15. | Zhan, B.; Thompson, A. Tetrahedron 2004, 60, 2917. doi:10.1016/j.tet.2004.01.043 |
| 16. | Marshall, J. A.; Robinson, E. D. J. Org. Chem. 1990, 55, 3450. doi:10.1021/jo00298a012 |
| 17. | Mashall, J. A.; Wang, X. J. Org. Chem. 1991, 56, 960. doi:10.1021/jo00003a013 |
| 24. | Ma, S.; Yu, S.; Yin, S. J. Org. Chem. 2003, 68, 8996. doi:10.1021/jo034633i |
| 25. | Ma, S.; Yu, Z. Chem.–Eur. J. 2004, 10, 2078. doi:10.1002/chem.200305341 |
| 28. | Sromek, A. W.; Rubina, M.; Gevorgyan, V. J. Am. Chem. Soc. 2005, 127, 10500. doi:10.1021/ja053290y |
| 31. | Alcaide, B.; Almendros, P.; Martínez del Campo, T. Eur. J. Org. Chem. 2007, 2844. doi:10.1002/ejoc.200700128 |
| 32. | Sampath, M.; Loh, T.-P. Chem. Commun. 2009, 1568. doi:10.1039/b819959k |
| 33. | Zhao, J.; Loh, T.-P. Angew. Chem., Int. Ed. 2009, 48, 7232. doi:10.1002/anie.200902471 |
| 11. | Semmelhack, M. F.; Schmid, C. R.; Cortés, D. A.; Chou, C. S. J. Am. Chem. Soc. 1984, 106, 3374. doi:10.1021/ja00323a064 |
| 12. | Markó, I. E.; Giles, P. R.; Tsukazaki, M.; Brown, S. M.; Urch, C. J. Science 1996, 274, 2044. doi:10.1126/science.274.5295.2044 |
| 13. | Markó, I. E.; Gautier, A.; Dumeunier, R.; Doda, K.; Philippart, F.; Brown, S. M.; Urch, C. J. Angew. Chem., Int. Ed. 2004, 43, 1588. doi:10.1002/anie.200353458 |
| 6. | Blackburn, T. F.; Schwartz, J. J. Chem. Soc., Chem. Commun. 1977, 157. doi:10.1039/C39770000157 |
| 7. | Peterson, K. P.; Larock, R. C. J. Org. Chem. 1998, 63, 3185. doi:10.1021/jo971268k |
| 8. | Nishimura, T.; Onoue, T.; Ohe, K.; Uemura, S. J. Org. Chem. 1999, 64, 6750. doi:10.1021/jo9906734 |
| 9. | ten Brink, G. J.; Arends, I. W. C. E.; Sheldon, R. A. Science 2000, 287, 1636. doi:10.1126/science.287.5458.1636 |
| 10. |
Muzart, J. Tetrahedron 2003, 59, 5789. doi:10.1016/S0040-4020(03)00866-4
for a review. |
| 30. | Marx, V. M.; Burnell, D. J. Org. Lett. 2009, 11, 1229. doi:10.1021/ol900029d |
| 34. | Russell, S. W.; Weedon, B. C. L. J. Chem. Soc. D 1969, 85. doi:10.1039/C29690000085 |
| 35. | Landor, P. D.; Landor, S. R.; Mukasa, S. J. Chem. Soc. D 1971, 1638. doi:10.1039/C29710001638 |
| 37. | Reetz, M. T.; Li, X. Angew. Chem., Int. Ed. 2005, 44, 2962. doi:10.1002/anie.200462613 |
| 38. | Reetz, M. T. Angew. Chem., Int. Ed. 2008, 47, 2556. doi:10.1002/anie.200704327 |
| 12. | Markó, I. E.; Giles, P. R.; Tsukazaki, M.; Brown, S. M.; Urch, C. J. Science 1996, 274, 2044. doi:10.1126/science.274.5295.2044 |
| 36. | Rannulu, N. S.; Rodgers, M. T. J. Phys. Chem. A 2007, 111, 3465. doi:10.1021/jp066903h |
| 43. | Isaac, M. B.; Chan, T. H. J. Chem. Soc., Chem. Commun. 1995, 1003. doi:10.1039/C39950001003 |
| 43. | Isaac, M. B.; Chan, T. H. J. Chem. Soc., Chem. Commun. 1995, 1003. doi:10.1039/C39950001003 |
| 41. | Alcaide, B.; Almendros, P.; Aragoncillo, C.; Redondo, M. C. Eur. J. Org. Chem. 2005, 98. doi:10.1002/ejoc.200400527 |
| 42. | Yang, Y.; Shen, Z.; Loh, T.-P. Org. Lett. 2009, 11, 1209. doi:10.1021/ol8027362 |
| 13. | Markó, I. E.; Gautier, A.; Dumeunier, R.; Doda, K.; Philippart, F.; Brown, S. M.; Urch, C. J. Angew. Chem., Int. Ed. 2004, 43, 1588. doi:10.1002/anie.200353458 |
| 39. | Li, J.; Zhou, C.; Fu, C.; Ma, S. Tetrahedron 2009, 65, 3695. doi:10.1016/j.tet.2009.02.061 |
| 40. | Mukaiyama, T.; Harada, T. Chem. Lett. 1981, 10, 621. doi:10.1246/cl.1981.621 |
© 2011 Gao et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)