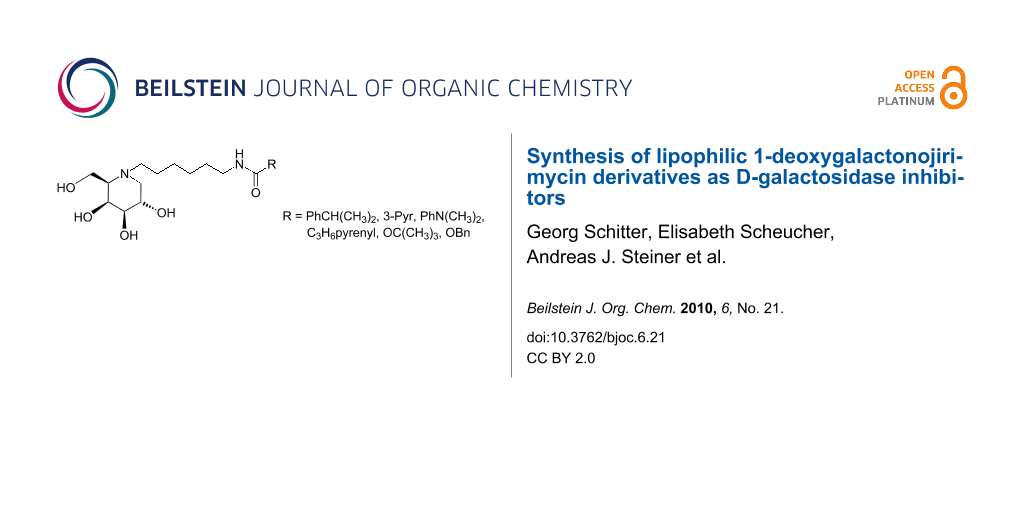
Abstract
N-Alkylation at the ring nitrogen of the D-galactosidase inhibitor 1-deoxygalactonojirimycin with a functionalised C6 alkyl chain followed by modification with different aromatic substituents provided lipophilic 1-deoxygalactonojirimycin derivatives which exhibit inhibitory properties against β-glycosidases from E. coli and Agrobacterium sp. as well as green coffee bean α-galactosidase. In preliminary studies, these compounds also showed potential as chemical chaperones for GM1-gangliosidosis related β-galactosidase mutants.
Graphical Abstract
Introduction
Iminosugars such as compounds 1–4 (Figure 1) have been shown to be potent glycosidase inhibitors and useful tools for the study of glycoside-hydrolysing enzymes. These sugar mimetics have been found to have anti-viral, anti-cancer, anti-diabetes, anti-infective, as well as insect anti-feedant and plant growth regulatory effects. Because of their diverse properties, iminosugars have enjoyed continuous interest since their discovery in the 1960s. Consequently, many different derivatives have been prepared for biological evaluations via a wide range of synthetic approaches and have been used for various medicinal and biomolecular applications [1-9].
Figure 1: Typical representatives of iminosugars.
Figure 1: Typical representatives of iminosugars.
Recently, iminosugars were found to have potential to serve as pharmacological chaperones for the treatment of lysosomal storage diseases in chaperone mediated therapy (CMT) [10]. In contrast to enzyme replacement therapy (ERT), where recombinant enzyme is given to the patient at regular intervals, the iminosugars used for CMT (recently called pharmacological chaperone therapy, PCT) are able to cross the blood brain barrier. This gives the opportunity also to treat types of lysosomal storage diseases involving the central nervous system. Furthermore, CMT is a cost-efficient alternative to ERT. In this context, N-alkylated derivatives of 1-deoxynojirimycin [11] such as 5 and 6 (Figure 2) as well as N-substituted D-glucono-δ-lactams 7 (Figure 2) [12] have been shown to be highly potent pharmacological chaperones for the potential treatment of Gaucher [13] and Pompe [14] diseases by ‘rescuing’ the related mutant enzymes. Both Wong [15] and Overkleeft [16] have shown that a rather large lipophilic substituent such as the adamantyl group (8, Figure 2) attached via an alkyl chain with a chain length from C3 up to C9 to the ring nitrogen of 1-deoxynojirimycin and isofagomine respectively, can increase the interaction with the lysosomal glycosphingolipid glucocerebrosidase. Interestingly, 5-N, 6-X-(N′-alkyliminomethylene)nojirimycin derivatives where X is O, NH or S such as in structure 9 (Figure 2) also have chaperone activity for Gaucher related mutations [17].
Figure 2: N-Modified iminosugars 5–9 as potential pharmacological chaperones.
Figure 2: N-Modified iminosugars 5–9 as potential pharmacological chaperones.
1-Deoxygalactonojirimycin (4) was shown to be a candidate for the treatment of Fabry disease, an X-linked inherited lysosomal storage disorder caused by the deficiency of α-galactosidase A activity resulting in the accumulation of globotriaosylceramide, thereby affecting the lysosomes of vascular endothelial cells. Iminosugar 4 can increase α-galactosidase A levels 1.5 to 28 fold in cultured Fabry patient cell lines (baseline α-Gal A levels range from 0–52%) after incubation for five days, as was observed for 49 different missense mutant forms [18-21]. It can also reduce tissue globotriaosylceramide levels in a mouse model [22].
Suzuki and co-workers found, that N-octyl-4-epi-β-valienamine (10) (Figure 3), a competitive inhibitor of lysosomal β-galactosidase, when orally administered to GM1-gangliosidosis model mice, is able to enter the brain through the blood-brain barrier and thereby enhancing β-galactosidase activity, reduce substrate storage, and clinically improve neurological deterioration [23].
Our studies revealed that 1-deoxy-D-galactonojirimycin-lysine hybrids, when carrying an aromatic substituent, such as a dansyl moiety, in its nature a lipophilic aromatic substituent, are potent D-galactosidase inhibitors and also show activity with human lysosomal β-galactosidase, exhibiting improvements of the enzyme activity in mutant cell lines [24]. In the course of this work, we became interested in the influence of other lipophilic aromatic substituents on the biological activity of such compounds. Different aromatic acid derivatives were prepared by coupling to the free amine at the terminus of the C6 alkyl chain in compound 15, which is anchored to the ring nitrogen of 1-deoxygalactonojirimycin, to yield derivatives 16–19 and 22. The spacer length of six carbon units has been proven suitable for enzyme recognition in previous studies [25] and was kept constant to compare the different aromatic substituents. Additionally, Wong [15] as well as Suzuki [23] have shown from computational studies, that in case of N-substitution on compounds 8b and 10, the iminosugar and carbasugar units respectively, were found to interact with the active site of the corresponding enzymes whereas the alkyl chains were located in the distinctly hydrophobic entrance region to the active site. Thus, for comparison, a lipophilic aliphatic tert-butyl group in compound 20 was included in this study. In addition to the synthetic approaches, the influence of the lipophilic substituents of the new N-modified 1-galactonojirimycin derivatives on their biological interaction with respective glycoside hydrolases are described.
Results and Discussion
The key intermediate for the synthesis of N-modified lipophilic 1-deoxygalactonojirimycin derivatives 16–20 as well as 22 was the 3,4-O-isopropylidene iminosugar 12. Starting from enol ether 10 [26,27], treatment with m-chloroperbenzoic acid gave the 5-O-chlorobenzoic ester via the corresponding 5,6-epoxide. This ester underwent hydrolysis under basic conditions to afford the L-arabino-hexos-5-ulose 11, which was immediately used for the next step after brief silica gel purification. The reductive amination and N-deprotection of 11 was carried out under an atmosphere of H2 with benzylamine in methanol and Pd/C as catalyst to produce 3,4-O-isopropylidene-1-deoxy-D-galactonojirimycin (12) in an overall yield of 75% (Scheme 1).
Scheme 1: Three-step-synthesis of partially protected 1-deoxy-D-galactonojirimycin derivative 12 from 10 via L-arabino-hexos-5-ulose 11.
Scheme 1: Three-step-synthesis of partially protected 1-deoxy-D-galactonojirimycin derivative 12 from 10 via ...
Compound 12 underwent N-substitution upon treatment with 1-O-tosyl-6-N-(tert-butoxycarbonyl)-6-aminohexanol (13) [28] in DMF to give the 1-deoxygalactonojirimycin derivative 14 in 64% yield. The isopropylidene and tert-butoxycarbonyl protecting groups were simultaneously removed under standard conditions to afford the desired free amine 15 [25], the key building block for further modifications (Scheme 2).
Scheme 2: Synthesis of N-(6-aminohexyl)-1-deoxygalactonojirimycin (15) from 12 via 14.
Scheme 2: Synthesis of N-(6-aminohexyl)-1-deoxygalactonojirimycin (15) from 12 via 14.
The chemoselective acylation of the free amine 15 was conducted with three different benzoic acid derivatives in order to investigate the influence of the potential basicity of an additional nitrogen at the aromatic substituent. For the synthesis of compound 16, 4-isopropylbenzoic acid was reacted with the primary amine under amide coupling conditions with O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoro-borate (TBTU) as the coupling reagent in DMF and triethylamine. Likewise, nicotinic acid under the same conditions gave compound 17 in 20% yield. Reaction of 15 with 4-(dimethylamino)benzoyl chloride in DMF and triethylamine afforded derivative 18 in 22% yield (Scheme 3). These unusually low yields for the standard coupling reactions were due to the formation of very polar side products as well as material losses during column chromatography.
Scheme 3: Synthesis of lipophilic 1-deoxy-D-galactonojirimycin derivatives 16–18 by chemoselective acylation of 15.
Scheme 3: Synthesis of lipophilic 1-deoxy-D-galactonojirimycin derivatives 16–18 by chemoselective acylation ...
For increased lipophilicity as well as for analytical purposes, compound 15 was also coupled to 1-pyrenebutyric acid with TBTU in DMF in the presence of triethylamine to give compound 19 in 56% yield. The pyrenyl substituent was chosen because of its potential to serve as diagnostic tool. Conventional BOC-protection of amine 15 gave derivative 20 in 81% yield (Scheme 4).
Scheme 4: Synthesis of compounds 19 as well as 20 from primary amine 15.
Scheme 4: Synthesis of compounds 19 as well as 20 from primary amine 15.
Aromatic derivative 22 was synthesised from 4 [29-42] (conveniently obtained by deprotection of compound 12 under acidic conditions), in 77% yield by ring nitrogen alkylation with tosylate 21 (Scheme 5) [43].
Inhibition constants of the compounds synthesised are presented in Table 1. The 1-deoxygalactonojirimycin analogues were tested as inhibitors of Agrobacterium sp. β-glucosidase/galactosidase and E. coli β-galactosidase as well as green coffee bean α-galactosidase (Table 1). All new compounds inhibited the Agrobacterium sp. enzyme better than the parent iminosugar 4. The pyrenyl substituted compound 19 with an extended aromatic system turned out to be the most active inhibitor with a Ki value of 60 nM against Agrobacterium sp. β-glucosidase/galactosidase and 0.25 µM against E. coli β-galactosidase. However, the toxicity of this compound clearly requires further evaluation. In general, N-substitution does not dramatically affect the inhibitory properties of the derivatives against β-galactosidase from E. coli, with Ki values distributed in the range of the parent iminosugar 4. No particular trend could be observed in a comparison of compounds 16–19 as regards the presence or absence of the additional nitrogen at the aromatic substituent. Iminosugars 16–20, as well as 22, were less active than the parent compound with α-galactosidase from green coffee beans. However, the Ki values are still in the low µM range and thus, suitable for use as chemical chaperones. Gratifyingly, compounds 20 and 22 exhibited IC50 values of 10.9 µM (Ki = 2.0 µM) and 3.26 µM (Ki = 0.72 µM), respectively, with human lysosomal β-galactosidase.
Table 1: Inhibitory activities of compounds 16–20 and 22 with β-glycosidases from Agrobacterium sp. (β-glu/gal Abg), E. coli (β-gal E. coli) as well as with the α-galactosidase from green coffee beans (α-gal GCB).
| Compound |
Ki [µM]
β-glu/gal Abg |
Ki [µM]
β-gal E. coli |
Ki [µM]
α-gal GCB |
|---|---|---|---|
| 4 | 100 | 13 | 0.013a |
| 16 | 13 | 1.3 | 2.6 |
| 17 | 1.5 | 0.83 | 2.2 |
| 18 | 3.8 | 1.1 | 0.49 |
| 19 | 0.06 | 0.25 | 7.0 |
| 20 | 3.0 | 2.4 | 4.3 |
| 22 | 6.0 | 0.4 | 2.2 |
aSee reference [7].
In preliminary studies compounds 17 as well as 22 served as chemical chaperone and increased the enzyme activity of a β-galactosidase mutant feline fibroblast cell line up to 5.5 fold when applied at a concentration of 100 µM. Compound 18 was a significantly better chemical chaperone for this mutant increasing the relative enzyme activity 4.8 fold at a concentration of 2 µM.
Conclusion
We have synthesised new 1-deoxygalactonojirimycin derivatives 16–20, as well as 22, which feature a C6 chain anchored to the ring nitrogen. Different lipophilic aromatic and aliphatic substituents at the N-alkyl chain were introduced resulting in an interesting Ki-value profile against β-galactosidases from Abg and E. coli, respectively, as well as with α-galactosidase from green coffee beans. The Ki values with human lysosomal β-galactosidase and preliminary data for chaperone activity in a cat fibroblast model of the new compounds suggest that such iminosugar derivatives have interesting potential in the chaperone-mediated therapy of lysosomal storage diseases, such as, for example, GM1 gangliosidosis as well as Morquio B disease, possibly also Fabry’s disease. Further bio-medicinal evaluation and toxicity studies are currently in progress.
Supporting Information
| Supporting Information File 1: Full experimental details and characterisation data | ||
| Format: PDF | Size: 120.7 KB | Download |
References
-
Winchester, B. G. Tetrahedron: Asymmetry 2009, 20, 645–651. doi:10.1016/j.tetasy.2009.02.048
Return to citation in text: [1] -
Compain, P.; Martin, O. Iminosugars: From Synthesis to Therapeutic Applications; Wiley-VCH: Weinheim, 2008.
Return to citation in text: [1] -
Martin, O. R.; Compain, P. Curr. Top. Med. Chem. 2003, 3, 541–560. doi:10.2174/1568026033452474
Return to citation in text: [1] -
Asano, N.; Nash, R. J.; Molyneux, R. J.; Fleet, G. W. J. Tetrahedron: Asymmetry 2000, 11, 1645–1680. doi:10.1016/S0957-4166(00)00113-0
Return to citation in text: [1] -
Wrodnigg, T. M. From Lianas to glycobiology tools: Twenty-five years of 2,5-dideoxy-2,5-imino-D-mannitol. In Timely Research Perspectives in Carbohydrate Chemistry; Schmid, W.; Stütz, A. E., Eds.; Springer: Vienna, AUT., 2002; pp 393–426.
Return to citation in text: [1] -
Heightman, T. D.; Vasella, A. T. Angew. Chem., Int. Ed. 1999, 38, 750–770. doi:10.1002/(SICI)1521-3773(19990315)38:6<750::AID-ANIE750>3.0.CO;2-6
Return to citation in text: [1] -
Stütz, A. E. Iminosugars as Glycosidase Inhibitors; Wiley-VCH: Weinheim, 1999.
Return to citation in text: [1] [2] -
Bols, M. Acc. Chem. Res. 1998, 31, 1–8. doi:10.1021/ar970058r
Return to citation in text: [1] -
Legler, G. Adv. Carbohydr. Chem. Biochem. 1990, 48, 319–384. doi:10.1016/S0065-2318(08)60034-7
Return to citation in text: [1] -
Parenti, G. EMBO Mol. Med. 2009, 1, 268–278. doi:10.1002/emmm.200900036
Return to citation in text: [1] -
Yu, L.; Ikeda, K.; Kato, A.; Adachi, I.; Godin, G.; Compain, P.; Martin, O.; Asano, N. Bioorg. Med. Chem. 2006, 14, 7736–7744. doi:10.1016/j.bmc.2006.08.003
Return to citation in text: [1] -
Wang, G. N.; Reinkensmeier, G.; Zhang, S.-W.; Zhou, J.; Zhang, L. R.; Zhang, L.-H.; Butters, T. D.; Ye, X. S. J. Med. Chem. 2009, 52, 3146–3149. doi:10.1021/jm801506m
Return to citation in text: [1] -
Sanchez-Olle, G.; Duque, J.; Egido-Gabas, M.; Casa, J.; Lluch, M.; Chabas, A.; Grinberg, D.; Vilageliu, L. Blood Cells, Mol., Dis. 2009, 42, 159–166. doi:10.1016/j.bcmd.2008.11.002
Return to citation in text: [1] -
Porto, C.; Cardone, M.; Fontana, F.; Rossi, F.; Tuzzi, M. R.; Tarallo, A.; Barone, M. V.; Andria, G.; Parenti, G. Mol. Ther. 2009, 17, 964–971. doi:10.1038/mt.2009.53
Return to citation in text: [1] -
Yu, Z.; Sawkar, A. R.; Whalen, L. J.; Wong, C.-H.; Kelly, J. W. J. Med. Chem. 2007, 50, 94–100. doi:10.1021/jm060677i
Return to citation in text: [1] [2] -
Wennekes, T.; van den Berg, R. J. B. H. N.; Donker, W.; van der Marel, G. A.; Strijland, A.; Aerts, J. M. F. G.; Overkleeft, H. S. J. Org. Chem. 2007, 72, 1088–1097. doi:10.1021/jo061280p
Return to citation in text: [1] -
Luan, Z.; Higaki, K.; Aguilar-Moncayo, M.; Ninomiya, H.; Ohno, K.; García-Moreno, M. I.; Ortiz Mellet, C.; García Fernandez, J. M.; Suzuki, Y. ChemBioChem 2009, 10, 2780–2792. doi:10.1002/cbic.200900442
Return to citation in text: [1] -
Fan, J. Q.; Ishii, S. FEBS J. 2007, 274, 4962–4971. doi:10.1111/j.1742-4658.2007.06041.x
Return to citation in text: [1] -
Hamanaka, R.; Shinohara, T.; Yano, S.; Nakamura, M.; Yasuda, A.; Yokoyama, S.; Fan, J. Q.; Kawasaki, K.; Watanabe, M.; Ishii, S. Biochim. Biophys. Acta 2008, 1782, 408–413.
Return to citation in text: [1] -
Benjamin, E. R.; Flanagan, J. J.; Schilling, A.; Chang, H. H.; Agarwal, L.; Katz, E.; Wu, X.; Pine, C.; Wustman, B.; Desnick, R. J.; Lockhart, D. J.; Valenzano, K. J. J. Inherited Metab. Dis. 2009, 32, 424–440. doi:10.1007/s10545-009-1077-0
Return to citation in text: [1] -
Park, J. Y.; Kim, G. H.; Kim, S. S.; Ko, J. M.; Lee, J. J.; Yoo, H. W. Exp. Mol. Med. 2009, 41, 1–7. doi:10.3858/emm.2009.41.1.001
Return to citation in text: [1] -
Khanna, R.; Soska, R.; Lun, Y.; Feng, J.; Frascella, M.; Young, B.; Brignol, N.; Pellegrina, L.; Sitaraman, S. A.; Desnick, R. J.; Benjamin, E. R.; Lockhart, D. J.; Valenzyno, K. J. Mol. Ther. 2010, 18, 23–33. doi:10.1038/mt.2009.220
Return to citation in text: [1] -
Suzuki, Y.; Ogawa, S.; Sakakibara, Y. Perspect. Med. Chem. 2009, 3, 7–17.
Return to citation in text: [1] [2] -
Steiner, A. J.; Schitter, G.; Stütz, A. E.; Wrodnigg, T. M.; Tarling, C. A.; Withers, S. G.; Fantur, K.; Mahuran, D.; Paschke, E.; Tropak, M. Bioorg. Med. Chem. 2008, 16, 10216–10220. doi:10.1016/j.bmc.2008.10.054
Return to citation in text: [1] -
Greimel, P.; Häusler, H.; Lundt, I.; Rupitz, K.; Stütz, A. E.; Tarling, C. A.; Withers, S. G.; Wrodnigg, T. M. Bioorg. Med. Chem. Lett. 2006, 16, 2067–2070. doi:10.1016/j.bmcl.2006.01.095
Return to citation in text: [1] [2] -
Garegg, P. J.; Samuelsson, B. J. Chem. Soc., Perkin Trans. 1 1980, 2866–2869. doi:10.1039/P19800002866
Return to citation in text: [1] -
Helferich, B.; Himmen, E. Ber. Dtsch. Chem. Ges. B 1929, 62, 2139–2141. doi:10.1002/cber.19290620842
Return to citation in text: [1] -
Hansen, J. B.; Buchardt, O. J. Chem. Soc., Chem. Commun. 1983, 4, 162–164. doi:10.1039/C39830000162
Return to citation in text: [1] -
Wennekes, T.; Lang, B.; Leeman, M.; van der Marel, G. A.; Smits, E.; Weber, M.; van Wiltenburg, J.; Wolberg, M.; Aerts, J. M. F. G.; Ovekleeft, H. S. Org. Process Res. Dev. 2008, 12, 414–423. doi:10.1021/op700295x
Return to citation in text: [1] -
Takahata, H.; Banda, Y.; Ouchi, H.; Nemoto, H. Org. Lett. 2003, 5, 2527–2529. doi:10.1021/ol034886y
Return to citation in text: [1] -
Shilvock, J. P.; Nash, R. J.; Watson, A. A.; Winters, A. L.; Butters, T. D.; Dwek, R. A.; Winkler, D. A.; Fleet, G. W. J. J. Chem. Soc., Perkin Trans. 1 1999, 2747–2754. doi:10.1039/a904145a
Return to citation in text: [1] -
Asano, K.; Hakogi, T.; Iwama, S.; Katsumura, S. Chem. Commun. 1999, 41–42. doi:10.1039/a807532h
Return to citation in text: [1] -
Barili, P. L.; Berti, G.; Catelani, G.; D’Andrea, F.; de Rensis, F.; Puccioni, L. Tetrahedron 1997, 53, 3407–3416. doi:10.1016/S0040-4020(97)00063-X
Return to citation in text: [1] -
Johnson, C. R.; Golebiowski, A.; Sundram, H.; Miller, M. W.; Dwaihy, R. L. Tetrahedron Lett. 1995, 36, 653–654. doi:10.1016/0040-4039(94)02343-A
Return to citation in text: [1] -
Chida, N.; Tanikawa, T.; Tobe, T.; Ogawa, S. J. Chem. Soc., Chem. Commun. 1994, 1247–1248. doi:10.1039/C39940001247
Return to citation in text: [1] -
Furneaux, R.; Tyler, P. C.; Whitehouse, L. A. Tetrahedron Lett. 1993, 34, 3609–3612. doi:10.1016/S0040-4039(00)73649-5
Return to citation in text: [1] -
Lees, W. J.; Whitsides, G. M. Bioorg. Chem. 1992, 20, 173–179. doi:10.1016/0045-2068(92)90037-4
Return to citation in text: [1] -
Liu, K. K. C.; Kajimoto, T.; Chen, L.; Zhong, Z.; Ichikawa, Y.; Wong, C. H. J. Org. Chem. 1991, 56, 6280–6289. doi:10.1021/jo00022a013
Return to citation in text: [1] -
Aoyagi, S.; Fujimaki, S.; Yamazaki, N.; Kibayashi, C. J. Org. Chem. 1991, 56, 815–819. doi:10.1021/jo00002a057
Return to citation in text: [1] -
Heiker, F. R.; Schueller, A. M. Carbohydr. Res. 1990, 203, 314–318. doi:10.1016/0008-6215(90)80031-W
Return to citation in text: [1] -
Bernotas, R. C.; Pezzone, M. A.; Ganem, B. Carbohydr. Res. 1987, 167, 305–311. doi:10.1016/0008-6215(87)80289-6
Return to citation in text: [1] -
Legler, G.; Pohl, S. Carbohydr. Res. 1986, 155, 119–129. doi:10.1016/S0008-6215(00)90138-1
Return to citation in text: [1] -
Krivickas, S. J.; Tamanini, E.; Todd, M. H.; Watkinson, M. J. Org. Chem. 2007, 72, 8280–8289. doi:10.1021/jo071175v
Return to citation in text: [1]
| 25. | Greimel, P.; Häusler, H.; Lundt, I.; Rupitz, K.; Stütz, A. E.; Tarling, C. A.; Withers, S. G.; Wrodnigg, T. M. Bioorg. Med. Chem. Lett. 2006, 16, 2067–2070. doi:10.1016/j.bmcl.2006.01.095 |
| 26. | Garegg, P. J.; Samuelsson, B. J. Chem. Soc., Perkin Trans. 1 1980, 2866–2869. doi:10.1039/P19800002866 |
| 27. | Helferich, B.; Himmen, E. Ber. Dtsch. Chem. Ges. B 1929, 62, 2139–2141. doi:10.1002/cber.19290620842 |
| 28. | Hansen, J. B.; Buchardt, O. J. Chem. Soc., Chem. Commun. 1983, 4, 162–164. doi:10.1039/C39830000162 |
| 1. | Winchester, B. G. Tetrahedron: Asymmetry 2009, 20, 645–651. doi:10.1016/j.tetasy.2009.02.048 |
| 2. | Compain, P.; Martin, O. Iminosugars: From Synthesis to Therapeutic Applications; Wiley-VCH: Weinheim, 2008. |
| 3. | Martin, O. R.; Compain, P. Curr. Top. Med. Chem. 2003, 3, 541–560. doi:10.2174/1568026033452474 |
| 4. | Asano, N.; Nash, R. J.; Molyneux, R. J.; Fleet, G. W. J. Tetrahedron: Asymmetry 2000, 11, 1645–1680. doi:10.1016/S0957-4166(00)00113-0 |
| 5. | Wrodnigg, T. M. From Lianas to glycobiology tools: Twenty-five years of 2,5-dideoxy-2,5-imino-D-mannitol. In Timely Research Perspectives in Carbohydrate Chemistry; Schmid, W.; Stütz, A. E., Eds.; Springer: Vienna, AUT., 2002; pp 393–426. |
| 6. | Heightman, T. D.; Vasella, A. T. Angew. Chem., Int. Ed. 1999, 38, 750–770. doi:10.1002/(SICI)1521-3773(19990315)38:6<750::AID-ANIE750>3.0.CO;2-6 |
| 7. | Stütz, A. E. Iminosugars as Glycosidase Inhibitors; Wiley-VCH: Weinheim, 1999. |
| 8. | Bols, M. Acc. Chem. Res. 1998, 31, 1–8. doi:10.1021/ar970058r |
| 9. | Legler, G. Adv. Carbohydr. Chem. Biochem. 1990, 48, 319–384. doi:10.1016/S0065-2318(08)60034-7 |
| 13. | Sanchez-Olle, G.; Duque, J.; Egido-Gabas, M.; Casa, J.; Lluch, M.; Chabas, A.; Grinberg, D.; Vilageliu, L. Blood Cells, Mol., Dis. 2009, 42, 159–166. doi:10.1016/j.bcmd.2008.11.002 |
| 15. | Yu, Z.; Sawkar, A. R.; Whalen, L. J.; Wong, C.-H.; Kelly, J. W. J. Med. Chem. 2007, 50, 94–100. doi:10.1021/jm060677i |
| 12. | Wang, G. N.; Reinkensmeier, G.; Zhang, S.-W.; Zhou, J.; Zhang, L. R.; Zhang, L.-H.; Butters, T. D.; Ye, X. S. J. Med. Chem. 2009, 52, 3146–3149. doi:10.1021/jm801506m |
| 11. | Yu, L.; Ikeda, K.; Kato, A.; Adachi, I.; Godin, G.; Compain, P.; Martin, O.; Asano, N. Bioorg. Med. Chem. 2006, 14, 7736–7744. doi:10.1016/j.bmc.2006.08.003 |
| 24. | Steiner, A. J.; Schitter, G.; Stütz, A. E.; Wrodnigg, T. M.; Tarling, C. A.; Withers, S. G.; Fantur, K.; Mahuran, D.; Paschke, E.; Tropak, M. Bioorg. Med. Chem. 2008, 16, 10216–10220. doi:10.1016/j.bmc.2008.10.054 |
| 25. | Greimel, P.; Häusler, H.; Lundt, I.; Rupitz, K.; Stütz, A. E.; Tarling, C. A.; Withers, S. G.; Wrodnigg, T. M. Bioorg. Med. Chem. Lett. 2006, 16, 2067–2070. doi:10.1016/j.bmcl.2006.01.095 |
| 17. | Luan, Z.; Higaki, K.; Aguilar-Moncayo, M.; Ninomiya, H.; Ohno, K.; García-Moreno, M. I.; Ortiz Mellet, C.; García Fernandez, J. M.; Suzuki, Y. ChemBioChem 2009, 10, 2780–2792. doi:10.1002/cbic.200900442 |
| 22. | Khanna, R.; Soska, R.; Lun, Y.; Feng, J.; Frascella, M.; Young, B.; Brignol, N.; Pellegrina, L.; Sitaraman, S. A.; Desnick, R. J.; Benjamin, E. R.; Lockhart, D. J.; Valenzyno, K. J. Mol. Ther. 2010, 18, 23–33. doi:10.1038/mt.2009.220 |
| 16. | Wennekes, T.; van den Berg, R. J. B. H. N.; Donker, W.; van der Marel, G. A.; Strijland, A.; Aerts, J. M. F. G.; Overkleeft, H. S. J. Org. Chem. 2007, 72, 1088–1097. doi:10.1021/jo061280p |
| 15. | Yu, Z.; Sawkar, A. R.; Whalen, L. J.; Wong, C.-H.; Kelly, J. W. J. Med. Chem. 2007, 50, 94–100. doi:10.1021/jm060677i |
| 29. | Wennekes, T.; Lang, B.; Leeman, M.; van der Marel, G. A.; Smits, E.; Weber, M.; van Wiltenburg, J.; Wolberg, M.; Aerts, J. M. F. G.; Ovekleeft, H. S. Org. Process Res. Dev. 2008, 12, 414–423. doi:10.1021/op700295x |
| 30. | Takahata, H.; Banda, Y.; Ouchi, H.; Nemoto, H. Org. Lett. 2003, 5, 2527–2529. doi:10.1021/ol034886y |
| 31. | Shilvock, J. P.; Nash, R. J.; Watson, A. A.; Winters, A. L.; Butters, T. D.; Dwek, R. A.; Winkler, D. A.; Fleet, G. W. J. J. Chem. Soc., Perkin Trans. 1 1999, 2747–2754. doi:10.1039/a904145a |
| 32. | Asano, K.; Hakogi, T.; Iwama, S.; Katsumura, S. Chem. Commun. 1999, 41–42. doi:10.1039/a807532h |
| 33. | Barili, P. L.; Berti, G.; Catelani, G.; D’Andrea, F.; de Rensis, F.; Puccioni, L. Tetrahedron 1997, 53, 3407–3416. doi:10.1016/S0040-4020(97)00063-X |
| 34. | Johnson, C. R.; Golebiowski, A.; Sundram, H.; Miller, M. W.; Dwaihy, R. L. Tetrahedron Lett. 1995, 36, 653–654. doi:10.1016/0040-4039(94)02343-A |
| 35. | Chida, N.; Tanikawa, T.; Tobe, T.; Ogawa, S. J. Chem. Soc., Chem. Commun. 1994, 1247–1248. doi:10.1039/C39940001247 |
| 36. | Furneaux, R.; Tyler, P. C.; Whitehouse, L. A. Tetrahedron Lett. 1993, 34, 3609–3612. doi:10.1016/S0040-4039(00)73649-5 |
| 37. | Lees, W. J.; Whitsides, G. M. Bioorg. Chem. 1992, 20, 173–179. doi:10.1016/0045-2068(92)90037-4 |
| 38. | Liu, K. K. C.; Kajimoto, T.; Chen, L.; Zhong, Z.; Ichikawa, Y.; Wong, C. H. J. Org. Chem. 1991, 56, 6280–6289. doi:10.1021/jo00022a013 |
| 39. | Aoyagi, S.; Fujimaki, S.; Yamazaki, N.; Kibayashi, C. J. Org. Chem. 1991, 56, 815–819. doi:10.1021/jo00002a057 |
| 40. | Heiker, F. R.; Schueller, A. M. Carbohydr. Res. 1990, 203, 314–318. doi:10.1016/0008-6215(90)80031-W |
| 41. | Bernotas, R. C.; Pezzone, M. A.; Ganem, B. Carbohydr. Res. 1987, 167, 305–311. doi:10.1016/0008-6215(87)80289-6 |
| 42. | Legler, G.; Pohl, S. Carbohydr. Res. 1986, 155, 119–129. doi:10.1016/S0008-6215(00)90138-1 |
| 14. | Porto, C.; Cardone, M.; Fontana, F.; Rossi, F.; Tuzzi, M. R.; Tarallo, A.; Barone, M. V.; Andria, G.; Parenti, G. Mol. Ther. 2009, 17, 964–971. doi:10.1038/mt.2009.53 |
| 18. | Fan, J. Q.; Ishii, S. FEBS J. 2007, 274, 4962–4971. doi:10.1111/j.1742-4658.2007.06041.x |
| 19. | Hamanaka, R.; Shinohara, T.; Yano, S.; Nakamura, M.; Yasuda, A.; Yokoyama, S.; Fan, J. Q.; Kawasaki, K.; Watanabe, M.; Ishii, S. Biochim. Biophys. Acta 2008, 1782, 408–413. |
| 20. | Benjamin, E. R.; Flanagan, J. J.; Schilling, A.; Chang, H. H.; Agarwal, L.; Katz, E.; Wu, X.; Pine, C.; Wustman, B.; Desnick, R. J.; Lockhart, D. J.; Valenzano, K. J. J. Inherited Metab. Dis. 2009, 32, 424–440. doi:10.1007/s10545-009-1077-0 |
| 21. | Park, J. Y.; Kim, G. H.; Kim, S. S.; Ko, J. M.; Lee, J. J.; Yoo, H. W. Exp. Mol. Med. 2009, 41, 1–7. doi:10.3858/emm.2009.41.1.001 |
| 43. | Krivickas, S. J.; Tamanini, E.; Todd, M. H.; Watkinson, M. J. Org. Chem. 2007, 72, 8280–8289. doi:10.1021/jo071175v |
© 2010 Schitter et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)