Abstract
Electrophilic gold(I) catalyst 6 competes with GaCl3 as the catalyst of choice in the synthesis of fluoranthenes by intramolecular hydroarylation of alkynes. The potential of this catalyst for the preparation of polyarenes is illustrated by a synthesis of two functionalized decacyclenes in a one-pot transformation in which three C–C bonds are formed with high efficiency.
Graphical Abstract
Introduction
Electrophilic activation of alkynes in functionalized substrates by gold catalysts allows for the synthesis of complex molecules under mild conditions [1-8]. Alkynes can react in gold-catalyzed Friedel–Crafts-type reactions with arenes to give products resulting from the intermolecular hydroarylation of the alkynes (or alkenylation of the arenes) [9-21]. In addition to gold, the intramolecular version of this reaction was also carried out with Ru(II) [22], Pt(II) [12,22,23], Pt(IV) [24], Ga(III) [25,26], and Hg(II) [27,28] as catalysts.
Electron-rich indoles also react with alkynes in the presence of gold catalysts to form 6–8-membered rings [29-31]. A similar reaction can also be carried out with GaCl3 [32] and Pt(II) [33] as catalysts. In contrast, alkynyl furans react with gold to give phenols by using Au(III), Au(I) [1,2,34-37], or Pt(II) as the catalyst [38,39].
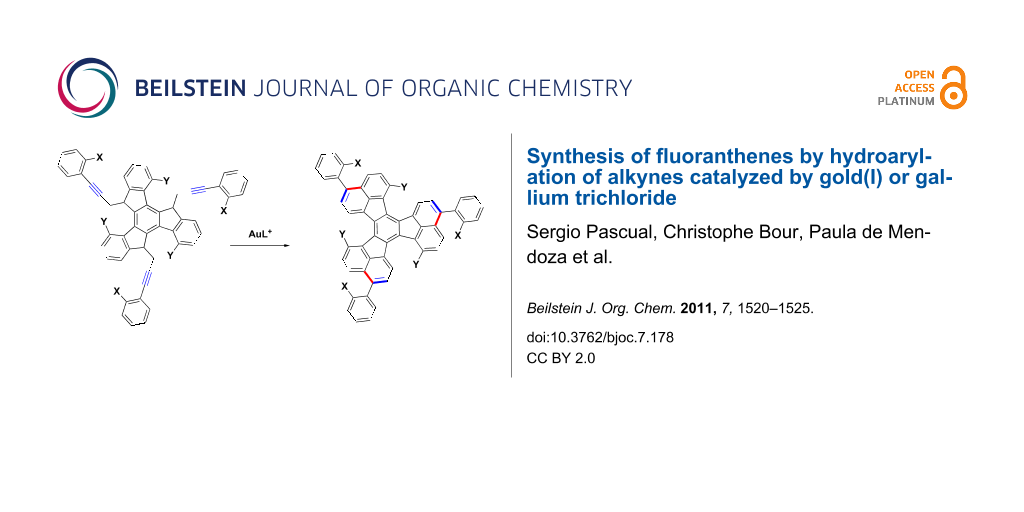
In our efforts towards the synthesis of large polyarenes [40-43], which are related to the fullerenes [44], we used the palladium-catalyzed arylation reaction as the main tool [45-48]. We decided to try the triple hydroarylation of substrates of type 1 to give 3,9,15-triaryldiacenaphtho[1,2-j:1',2'-l]fluoranthenes 2 with X and Y substitutes at strategic positions, which could be activated by palladium in subsequent intramolecular arylations (Scheme 1). Substituted fluoranthenes are of interest since some derivatives have been shown to be useful in light-emitting devices [49-52]. Fluoranthene derivatives have already been synthesized by palladium-catalyzed arylation reactions [53,54]. Strategically halogenated decacyclenes with a substitution pattern similar to that of 2 have been used for the synthesis of circumtrindene by flash vacuum pyrolysis [55]. Here we report the results on the synthesis of large polyarenes 2 and more simple 3-arylfluoranthenes by using gold(I)- or gallium(III)-catalyzed hydroarylation reactions.
Scheme 1: Proposed metal catalyzed annulation for the synthesis of triaryldiacenaphtho[1,2-j:1',2'-l]fluoranthenes 2.
Scheme 1: Proposed metal catalyzed annulation for the synthesis of triaryldiacenaphtho[1,2-j:1',2'-l]fluorant...
Results and Discussion
First, we examined the cyclization of 3 to give 4 or 4' [22,24,26] (Table 1) with cationic gold(I) catalysts 5 [56] and 6 [57] (Figure 1), which have been demonstrated to be amongst the best catalysts in many gold(I)-catalyzed cyclizations [6,58]. No reaction was observed with complex 5 after heating for 5 min at 70 °C in CH2Cl2 under microwave irradiation (Table 1, entry 1), whereas the more electrophilic 6, bearing a less donating phosphite ligand, led almost quantitatively to 4' (Table 1, entry 2). Under these conditions, AuCl3 was not effective as a catalyst (Table 1, entry 3). As previously reported [25,26], GaCl3 is an excellent catalyst for the cyclization of 3 to give 4' (Table 1, entry 4). In all cases the reaction proceeds exclusively though the 6-exo-dig pathway.
Table 1: Hydroarylation of 3 to give dihydronaphthalene 4'.a
|
|
||
| entry | MXn | 4' (yield, %) |
|---|---|---|
| 1 | 5 | —b |
| 2 | 6 | 99 |
| 3 | AuCl3 | —c |
| 4 | GaCl3 | 99 |
a2 mol % catalyst, microwave irradiation, 5 min. b100% 3 was recovered. c87% 3 was recovered.
Figure 1: Cationic gold complexes 5 and 6.
Figure 1: Cationic gold complexes 5 and 6.
The cyclization of 9-(3-phenylprop-2-ynyl)-9H-fluorene (7a) to form 3-phenylfluoranthene (8a) [59] was also examined by using catalysts 5, 6, and GaCl3 (Table 2). Since the initial gold(I)-catalyzed reaction provided a mixture of 3-phenyl-1,10b-dihydrofluoranthene, 3-phenyl-1,2,3,10b-tetrahydrofluoranthene, and 8a, the crude mixtures were treated with excess DDQ in toluene under reflux to provide pure 8a. No reaction or decomposition was observed when the reaction was carried out with gold(I) complex 5 (Table 2, entries 1 and 2). In contrast, the more electrophilic gold(I) complex 6 with phosphite as the ligand led to 8a in 64–70% yield by stirring at room temperature in CH2Cl2 (Table 2, entries 3–5). Satisfactory results were obtained by simply using 1 mol % of 6 (Table 2, entry 5). No reaction was observed with PtCl2 or AuCl3 even after heating in toluene under reflux (Table 2, entries 3–5). Whereas InCl3 led to decomposition of 7a under these conditions (Table 2, entry 6), GaCl3 led to 8a, although satisfactory results were only obtained in toluene at 70 °C (Table 1, entry 10). Interestingly, FeCl3 was also catalytically active, although fluoranthene 8a was only obtained in moderate yields (Table 2, entries 11 and 12). The reaction of 3a with Pd(OAc)2 as catalyst proceeded differently to give known (E)-9-(3-phenylallylidene)-9H-fluorene (9) [60], presumably via the formation of the corresponding allene as an intermediate (Scheme 2).
Table 2: Hydroarylation of 9-(3-phenylprop-2-ynyl)-9H-fluorene (7a) to give 3-phenylfluoranthene (8a).a
|
|
|||||
| entry | MXn (mol %) | solvent | T (ºC) | t (h) | yield (%) |
|---|---|---|---|---|---|
| 1 | 5 (2) | CH2Cl2 | 70b | 0.7 | —c |
| 2 | 5 (5) | toluene | 110 | 1 | —d |
| 3 | 6 (5) | CH2Cl2 | r.t. | 17 | 64 |
| 4 | 6 (2) | CH2Cl2 | r.t. | 16 | 70 |
| 5 | 6 (1) | CH2Cl2 | r.t. | 16 | 70 |
| 6 | PtCl2 (5) | toluene | 110 | 17 | —c |
| 7 | AuCl3 (5) | toluene | 110 | 17 | —c |
| 8 | InCl3 (5) | toluene | 110 | 17 | —d |
| 9 | GaCl3 (2) | CH2Cl2 | r.t. | 26 | 16e |
| 10 | GaCl3 (2) | toluene | 70b | 0.2 | 57 |
| 11 | FeCl3·6H2O (10) | DCEf | r.t. | 40 | 36e |
| 12 | FeCl3·6H2O (5) | DCEf | 70b | 0.2 | 34e |
aCrude reaction mixtures were aromatized by heating in toluene with DDQ (3 equiv) for 12 h. bMicrowave irradiation. cNo reaction. dProduct decomposition. eYield determined by 1H NMR. fDCE = 1,2-dichloroethane.
Scheme 2: Pd(OAc)2-catalyzed isomerization of 7a to form (E)-9-(3-phenylallylidene)-9H-fluorene (9).
Scheme 2: Pd(OAc)2-catalyzed isomerization of 7a to form (E)-9-(3-phenylallylidene)-9H-fluorene (9).
Substrates 7b–j, prepared by alkylation of fluorenyl lithium with the corresponding propargyl bromide or by Sonogashira couplings of 9-(prop-2-ynyl)-9H-fluorene [61], were cyclized by using gold(I) complex 6 or GaCl3 as the catalyst (Table 3). Although both catalysts could be used for the synthesis of 3-arylfluoranthenes 8b–h, better yields were obtained with GaCl3 in toluene at 100 °C. However, in the case of 9-(3-bromoprop-2-yn-1-yl)-9H-fluorene (7i), gold(I) complex 6 gave more satisfactory results (Table 3, compare entries 10 and 11). The reaction proceeded satisfactorily with aryl-substituted substrates bearing either electron-donating (p-Me, o-OMe) or electron-withdrawing (p-Cl, p-Br, p-CN, p-NO2) groups. However, no reaction was observed for n-butyl derivative 7j with 6 or with GaCl3 (Table 3, entries 12 and 13).
Table 3: Hydroarylation of 7b–j to give 3-substituted fluoranthenes 8b–i.a
|
|
|||||||
| entry | fluorene | R | MXn (mol %) | solvent | T (ºC) | t (h) | yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 7b | p-Tol | GaCl3 (5) | toluene | 100b | 0.2 | 45 |
| 2 | 7b | p-Tol | 6 (5) | CH2Cl2 | r.t. | 17 | 28 |
| 3 | 7c | p-ClC6H4 | GaCl3 (5) | toluene | 100b | 0.2 | 71 |
| 4 | 7d | p-NCC6H4 | GaCl3 (2) | toluene | 100b | 0.2 | 88 |
| 5 | 7e | p-O2NC6H4 | GaCl3 (2) | toluene | 70b | 0.2 | 92 |
| 6 | 7f | o-MeOC6H4 | 6 (5) | CH2Cl2 | r.t. | 17 | 17 |
| 7 | 7f | o-MeOC6H4 | GaCl3 (5) | toluene | 100b | 0.2 | 57 |
| 8 | 7g | o-BrC6H4 | GaCl3 (5) | toluene | 100b | 0.2 | 44 |
| 9 | 7h | C6F5 | GaCl3 (5) | toluene | 100b | 2 | 74 |
| 10 | 7i | Br | 6 (5) | CH2Cl2 | r.t. | 20 | 44 |
| 11 | 7i | Br | GaCl3 (5) | toluene | 100b | 0.2 | 21 |
| 12 | 7j | n-Bu | 6 (5) | CH2Cl2 | r.t. | 7 | —c |
| 13 | 7j | n-Bu | GaCl3 (2) | toluene | 70b | 0.2 | —c |
aCrude reaction mixtures were aromatized by heating in toluene with DDQ (3 equiv) for 12 h. bMicrowave irradiation. cNo reaction.
Cyclization of substrate 7k, having an electron-rich aryl group at the alkyne, with catalyst 6 gave 1,10b-dihydrofluoranthene 9 cleanly in quantitative yield (Scheme 3).
Scheme 3: Gold(I)-catalyzed hydroarylation of 7k to give 1,10b-dihydrofluoranthene 9.
Scheme 3: Gold(I)-catalyzed hydroarylation of 7k to give 1,10b-dihydrofluoranthene 9.
Derivatives 1a and 1b were readily prepared by the triple alkylation of the lithium anion of 4,9,14-trimethoxytruxene (Scheme 4) [41,62]. The cyclization reaction was carried out efficiently with gold(I) catalyst 6 (15 mol %) at room temperature in CH2Cl2 to give triaryl substituted diacenaphtho[1,2-j:1',2'-l]fluoranthenes (decacyclenes) 2a and 2b in very good overall yields after aromatization of the crude products with DDQ. Remarkably, this triple hydroarylation occurs efficiently with an average yield per C–C bond formation that is greater than 90%.
Scheme 4: Gold(I)-catalyzed triple hydroarylation of 1a,b to give 2a,b.
Scheme 4: Gold(I)-catalyzed triple hydroarylation of 1a,b to give 2a,b.
Conclusion
Highly electrophilic gold(I) catalyst 6 with a bulky phosphite ligand competes with GaCl3 as the catalyst of choice for the hydroarylation of alkynes. The synthetic potential of this catalyst is illustrated by the synthesis of functionalized triarylated decacyclenes in which three C–C bonds are formed with high efficiency in a one-pot transformation. The reaction is totally compatible with aryl bromides, which do not undergo subsequent arylation reaction due to the inertness of gold(I) catalysts towards oxidative addition reactions under homogeneous conditions [63,64].
Supporting Information
Supporting Information features experimental details and characterization data for new compounds.
| Supporting Information File 1: Experimental details | ||
| Format: PDF | Size: 2.9 MB | Download |
References
-
Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454
Return to citation in text: [1] [2] -
Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180–3211. doi:10.1021/cr000436x
Return to citation in text: [1] [2] -
Zhang, L.; Sun, J.; Kozmin, S. A. Adv. Synth. Catal. 2006, 348, 2271–2296. doi:10.1002/adsc.200600368
Return to citation in text: [1] -
Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. doi:10.1002/anie.200604335
Return to citation in text: [1] -
Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395–403. doi:10.1038/nature05592
Return to citation in text: [1] -
Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326–3350. doi:10.1021/cr0684319
Return to citation in text: [1] [2] -
Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351–3378. doi:10.1021/cr068430g
Return to citation in text: [1] -
Michelet, V.; Toullec, P. Y.; Genêt, J. P. Angew. Chem., Int. Ed. 2008, 47, 4268–4315. doi:10.1002/anie.200701589
Return to citation in text: [1] -
Nevado, C.; Echavarren, A. M. Synthesis 2005, 167–182. doi:10.1055/s-2005-861781
Return to citation in text: [1] -
de Mendoza, P.; Echavarren, A. M. Pure Appl. Chem. 2010, 82, 801–820. doi:10.1351/PAC-CON-09-10-06
Return to citation in text: [1] -
Reetz, M. T.; Sommer, K. Eur. J. Org. Chem. 2003, 3485–3496. doi:10.1002/ejoc.200300260
Return to citation in text: [1] -
Shi, Z.; He, C. J. Org. Chem. 2004, 69, 3669–3671. doi:10.1021/jo0497353
Return to citation in text: [1] [2] -
Li, Z.; Shi, Z.; He, C. J. Organomet. Chem. 2005, 690, 5049–5054. doi:10.1016/j.jorganchem.2005.03.009
Return to citation in text: [1] -
Nevado, C.; Echavarren, A. M. Chem.–Eur. J. 2005, 11, 3155–3164. doi:10.1002/chem.200401069
Return to citation in text: [1] -
Dankwardt, J. W. Tetrahedron Lett. 2001, 42, 5809–5812. doi:10.1016/S0040-4039(01)01146-7
Return to citation in text: [1] -
Fürstner, A.; Mamane, V. J. Org. Chem. 2002, 67, 6264–6267. doi:10.1021/jo025962y
Return to citation in text: [1] -
Mamane, V.; Hannen, P.; Fürstner, A. Chem.–Eur. J. 2004, 10, 4556–4575. doi:10.1002/chem.200400220
Return to citation in text: [1] -
Soriano, E.; Marco-Contelles, J. Organometallics 2006, 25, 4542–4553. doi:10.1021/om0605332
Return to citation in text: [1] -
Seregin, I. V.; Gevorgyan, V. J. Am. Chem. Soc. 2006, 128, 12050–12051. doi:10.1021/ja063278l
Return to citation in text: [1] -
Menon, R. S.; Findlay, A. D.; Bissember, A. C.; Banwell, M. G. J. Org. Chem. 2009, 74, 8901–8903. doi:10.1021/jo902032p
Return to citation in text: [1] -
Jurberg, I. D.; Gagosz, F. J. Organomet. Chem. 2011, 696, 37–41. doi:10.1016/j.jorganchem.2010.06.017
Return to citation in text: [1] -
Chatani, N.; Inoue, H.; Ikeda, T.; Murai, S. J. Org. Chem. 2000, 65, 4913–4918. doi:10.1021/jo000255v
Return to citation in text: [1] [2] [3] -
Fürstner, A.; Kennedy, J. W. J. Chem.–Eur. J. 2006, 12, 7398–7410. doi:10.1002/chem.200600592
Return to citation in text: [1] -
Pastine, S. J.; Youn, S. W.; Sames, D. Org. Lett. 2003, 5, 1055–1058. doi:10.1021/ol034177k
Return to citation in text: [1] [2] -
Inoue, H.; Chatani, N.; Murai, S. J. Org. Chem. 2002, 67, 1414–1417. doi:10.1021/jo016232d
Return to citation in text: [1] [2] -
Li, H. J.; Guillot, R.; Gandon, V. J. Org. Chem. 2010, 75, 8435–8449. doi:10.1021/jo101709n
Return to citation in text: [1] [2] [3] -
Nishizawa, M.; Takao, H.; Yadav, V. K.; Imagawa, H.; Sugihara, T. Org. Lett. 2003, 5, 4563–4565. doi:10.1021/ol035622e
Return to citation in text: [1] -
Nishizawa, M.; Imagawa, H.; Yamamoto, H. Org. Biomol. Chem. 2010, 8, 511–521. doi:10.1039/b920434b
Return to citation in text: [1] -
Ferrer, C.; Echavarren, A. M. Angew. Chem., Int. Ed. 2006, 45, 1105–1109. doi:10.1002/anie.200503484
Return to citation in text: [1] -
Ferrer, C.; Amijs, C. H. M.; Echavarren, A. M. Chem.–Eur. J. 2007, 13, 1358–1373. doi:10.1002/chem.200601324
Return to citation in text: [1] -
Ferrer, C.; Escribano-Cuesta, A.; Echavarren, A. M. Tetrahedron 2009, 65, 9015–9020. doi:10.1016/j.tet.2009.08.067
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Padmavani, B.; Gupta, M. K. Tetrahedron Lett. 2004, 45, 7577–7579. doi:10.1016/j.tetlet.2004.08.126
Return to citation in text: [1] -
Bhuvaneswari, S.; Jeganmohan, M.; Cheng, C.-H. Chem.–Eur. J. 2007, 13, 8285–8293. doi:10.1002/chem.200700589
Return to citation in text: [1] -
Rudolph, M.; McCreery, M. Q.; Frey, W.; Hashmi, A. S. K. Beilstein J. Org. Chem. 2011, 7, 794–801. doi:10.3762/bjoc.7.90
Return to citation in text: [1] -
Hashmi, A. S. K.; Blanco, M. C. Eur. J. Org. Chem. 2006, 4340–4342. doi:10.1002/ejoc.200600546
Return to citation in text: [1] -
Hashmi, A. S. K.; Haufe, P.; Schmid, C.; Nass, A. R.; Frey, W. Chem.–Eur. J. 2006, 12, 5376–5382. doi:10.1002/chem.200600192
Return to citation in text: [1] -
Hashmi, A. S. K.; Yang, M. S. W.; Rominger, F. Angew. Chem., Int. Ed. 2011, 50, 5762–5765. doi:10.1002/anie.201100989
Return to citation in text: [1] -
Martín-Matute, B.; Cárdenas, D. J.; Echavarren, A. M. Angew. Chem., Int. Ed. 2001, 40, 4754–4757. doi:10.1002/1521-3773(20011217)40:24<4754::AID-ANIE4754>3.0.CO;2-9
Return to citation in text: [1] -
Martín-Matute, B.; Nevado, C.; Cárdenas, D. J.; Echavarren, A. M. J. Am. Chem. Soc. 2003, 125, 5757–5766. doi:10.1021/ja029125p
Return to citation in text: [1] -
Gómez-Lor, B.; de Frutos, Ó.; Echavarren, A. M. Chem. Commun. 1999, 2431–2432. doi:10.1039/a906990i
Return to citation in text: [1] -
Gómez-Lor, B.; González-Cantalapiedra, E.; Ruiz, M.; de Frutos, Ó.; Cárdenas, D. J.; Santos, A.; Echavarren, A. M. Chem.–Eur. J. 2004, 10, 2601–2608. doi:10.1002/chem.200306023
Return to citation in text: [1] [2] -
Echavarren, A. M.; Gómez-Lor, B.; González, J. J.; de Frutos, Ó. Synlett 2003, 585–597. doi:10.1055/s-2003-38382
Return to citation in text: [1] -
Pascual, S.; de Mendoza, P.; Echavarren, A. M. Org. Biomol. Chem. 2007, 5, 2727–2734. doi:10.1039/b707940k
Return to citation in text: [1] -
Otero, G.; Biddau, G.; Sánchez-Sánchez, C.; Caillard, R.; López, M. F.; Rogero, C.; Palomares, F. J.; Cabello, N.; Basanta, M. A.; Ortega, J.; Méndez, J.; Echavarren, A. M.; Pérez, R.; Gómez-Lor, B.; Martín-Gago, J. A. Nature 2008, 454, 865–868. doi:10.1038/nature07193
Return to citation in text: [1] -
García-Cuadrado, D.; Braga, A. A. C.; Maseras, F.; Echavarren, A. M. J. Am. Chem. Soc. 2006, 128, 1066–1067. doi:10.1021/ja056165v
Return to citation in text: [1] -
García-Cuadrado, D.; de Mendoza, P.; Braga, A. A. C.; Maseras, F.; Echavarren, A. M. J. Am. Chem. Soc. 2007, 129, 6880–6886. doi:10.1021/ja071034a
Return to citation in text: [1] -
Pascual, S.; de Mendoza, P.; Braga, A. A. C.; Maseras, F.; Echavarren, A. M. Tetrahedron 2008, 64, 6021–6029. doi:10.1016/j.tet.2008.01.056
Return to citation in text: [1] -
Livendahl, M.; Echavarren, A. M. Isr. J. Chem. 2010, 50, 630–651. doi:10.1002/ijch.201000040
Return to citation in text: [1] -
Kim, S.-K.; Park, J.-W. J. Nanosci. Nanotechnol. 2008, 8, 4787–4792. doi:10.1166/jnn.2008.IC69
Return to citation in text: [1] -
Kim, S.-K.; Jaung, J.-Y.; Park, J.-W. Mol. Cryst. Liq. Cryst. 2009, 498, 140–150. doi:10.1080/15421400802615352
Return to citation in text: [1] -
Yan, Q.; Zhou, Y.; Ni, B.-B.; Ma, Y.; Wang, J.; Pei, J.; Cao, Y. J. Org. Chem. 2008, 73, 5328–5339. doi:10.1021/jo800606b
Return to citation in text: [1] -
Chiechi, R. C.; Tseng, R. J.; Marchioni, F.; Yang, Y.; Wudl, F. Adv. Mater. 2006, 18, 325–328. doi:10.1002/adma.200501682
Return to citation in text: [1] -
Wegner, H. A.; Scott, L. T.; de Meijere, A. J. Org. Chem. 2003, 68, 883–887. doi:10.1021/jo020367h
Return to citation in text: [1] -
Quimby, J. M.; Scout, L. T. Adv. Synth. Catal. 2009, 351, 1009–1013. doi:10.1002/adsc.200900018
Return to citation in text: [1] -
Ansems, R. B. M.; Scott, L. T. J. Am. Chem. Soc. 2000, 122, 2719–2724. doi:10.1021/ja993028n
Return to citation in text: [1] -
Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178–6179. doi:10.1021/ja042257t
Return to citation in text: [1] -
Amijs, C. H. M.; López-Carrillo, V.; Raducan, M.; Pérez-Galán, P.; Ferrer, C.; Echavarren, A. M. J. Org. Chem. 2008, 73, 7721–7730. doi:10.1021/jo8014769
Return to citation in text: [1] -
Raducan, M.; Rodríguez-Escrich, C.; Cambeiro, X. C.; Escudero-Adán, E. C.; Pericàs, M. A.; Echavarren, A. M. Chem. Commun. 2011, 47, 4893–4895. doi:10.1039/c1cc10293a
and references therein.
Return to citation in text: [1] -
Berlman, I. B.; Wirth, H. O.; Steingraber, O. J. J. Am. Chem. Soc. 1968, 90, 566–569. doi:10.1021/ja01005a003
Return to citation in text: [1] -
Kuhn, R.; Winterstein, A. Helv. Chim. Acta 1928, 11, 116–122. doi:10.1002/hlca.19280110108
Return to citation in text: [1] -
Gautier, J. A.; Miocque, M.; Moskowitz, H. J. Organomet. Chem. 1964, 1, 212–221. doi:10.1016/S0022-328X(00)85489-3
Return to citation in text: [1] -
Gómez-Lor, B.; de Frutos, Ó.; Ceballos, P. A.; Granier, T.; Echavarren, A. M. Eur. J. Org. Chem. 2001, 2107–2114. doi:10.1002/1099-0690(200106)2001:11<2107::AID-EJOC2107>3.0.CO;2-F
Return to citation in text: [1] -
Lauterbach, T.; Livendahl, M.; Rosellón, A.; Espinet, P.; Echavarren, A. M. Org. Lett. 2010, 12, 3006–3009. doi:10.1021/ol101012n
Return to citation in text: [1] -
Livendahl, M.; Espinet, P.; Echavarren, A. M. Platinum Met. Rev. 2011, 55, 212–214. doi:10.1595/147106711X579128
Return to citation in text: [1]
| 63. | Lauterbach, T.; Livendahl, M.; Rosellón, A.; Espinet, P.; Echavarren, A. M. Org. Lett. 2010, 12, 3006–3009. doi:10.1021/ol101012n |
| 64. | Livendahl, M.; Espinet, P.; Echavarren, A. M. Platinum Met. Rev. 2011, 55, 212–214. doi:10.1595/147106711X579128 |
| 1. | Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454 |
| 2. | Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180–3211. doi:10.1021/cr000436x |
| 3. | Zhang, L.; Sun, J.; Kozmin, S. A. Adv. Synth. Catal. 2006, 348, 2271–2296. doi:10.1002/adsc.200600368 |
| 4. | Fürstner, A.; Davies, P. W. Angew. Chem., Int. Ed. 2007, 46, 3410–3449. doi:10.1002/anie.200604335 |
| 5. | Gorin, D. J.; Toste, F. D. Nature 2007, 446, 395–403. doi:10.1038/nature05592 |
| 6. | Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326–3350. doi:10.1021/cr0684319 |
| 7. | Gorin, D. J.; Sherry, B. D.; Toste, F. D. Chem. Rev. 2008, 108, 3351–3378. doi:10.1021/cr068430g |
| 8. | Michelet, V.; Toullec, P. Y.; Genêt, J. P. Angew. Chem., Int. Ed. 2008, 47, 4268–4315. doi:10.1002/anie.200701589 |
| 24. | Pastine, S. J.; Youn, S. W.; Sames, D. Org. Lett. 2003, 5, 1055–1058. doi:10.1021/ol034177k |
| 45. | García-Cuadrado, D.; Braga, A. A. C.; Maseras, F.; Echavarren, A. M. J. Am. Chem. Soc. 2006, 128, 1066–1067. doi:10.1021/ja056165v |
| 46. | García-Cuadrado, D.; de Mendoza, P.; Braga, A. A. C.; Maseras, F.; Echavarren, A. M. J. Am. Chem. Soc. 2007, 129, 6880–6886. doi:10.1021/ja071034a |
| 47. | Pascual, S.; de Mendoza, P.; Braga, A. A. C.; Maseras, F.; Echavarren, A. M. Tetrahedron 2008, 64, 6021–6029. doi:10.1016/j.tet.2008.01.056 |
| 48. | Livendahl, M.; Echavarren, A. M. Isr. J. Chem. 2010, 50, 630–651. doi:10.1002/ijch.201000040 |
| 12. | Shi, Z.; He, C. J. Org. Chem. 2004, 69, 3669–3671. doi:10.1021/jo0497353 |
| 22. | Chatani, N.; Inoue, H.; Ikeda, T.; Murai, S. J. Org. Chem. 2000, 65, 4913–4918. doi:10.1021/jo000255v |
| 23. | Fürstner, A.; Kennedy, J. W. J. Chem.–Eur. J. 2006, 12, 7398–7410. doi:10.1002/chem.200600592 |
| 49. | Kim, S.-K.; Park, J.-W. J. Nanosci. Nanotechnol. 2008, 8, 4787–4792. doi:10.1166/jnn.2008.IC69 |
| 50. | Kim, S.-K.; Jaung, J.-Y.; Park, J.-W. Mol. Cryst. Liq. Cryst. 2009, 498, 140–150. doi:10.1080/15421400802615352 |
| 51. | Yan, Q.; Zhou, Y.; Ni, B.-B.; Ma, Y.; Wang, J.; Pei, J.; Cao, Y. J. Org. Chem. 2008, 73, 5328–5339. doi:10.1021/jo800606b |
| 52. | Chiechi, R. C.; Tseng, R. J.; Marchioni, F.; Yang, Y.; Wudl, F. Adv. Mater. 2006, 18, 325–328. doi:10.1002/adma.200501682 |
| 22. | Chatani, N.; Inoue, H.; Ikeda, T.; Murai, S. J. Org. Chem. 2000, 65, 4913–4918. doi:10.1021/jo000255v |
| 40. | Gómez-Lor, B.; de Frutos, Ó.; Echavarren, A. M. Chem. Commun. 1999, 2431–2432. doi:10.1039/a906990i |
| 41. | Gómez-Lor, B.; González-Cantalapiedra, E.; Ruiz, M.; de Frutos, Ó.; Cárdenas, D. J.; Santos, A.; Echavarren, A. M. Chem.–Eur. J. 2004, 10, 2601–2608. doi:10.1002/chem.200306023 |
| 42. | Echavarren, A. M.; Gómez-Lor, B.; González, J. J.; de Frutos, Ó. Synlett 2003, 585–597. doi:10.1055/s-2003-38382 |
| 43. | Pascual, S.; de Mendoza, P.; Echavarren, A. M. Org. Biomol. Chem. 2007, 5, 2727–2734. doi:10.1039/b707940k |
| 9. | Nevado, C.; Echavarren, A. M. Synthesis 2005, 167–182. doi:10.1055/s-2005-861781 |
| 10. | de Mendoza, P.; Echavarren, A. M. Pure Appl. Chem. 2010, 82, 801–820. doi:10.1351/PAC-CON-09-10-06 |
| 11. | Reetz, M. T.; Sommer, K. Eur. J. Org. Chem. 2003, 3485–3496. doi:10.1002/ejoc.200300260 |
| 12. | Shi, Z.; He, C. J. Org. Chem. 2004, 69, 3669–3671. doi:10.1021/jo0497353 |
| 13. | Li, Z.; Shi, Z.; He, C. J. Organomet. Chem. 2005, 690, 5049–5054. doi:10.1016/j.jorganchem.2005.03.009 |
| 14. | Nevado, C.; Echavarren, A. M. Chem.–Eur. J. 2005, 11, 3155–3164. doi:10.1002/chem.200401069 |
| 15. | Dankwardt, J. W. Tetrahedron Lett. 2001, 42, 5809–5812. doi:10.1016/S0040-4039(01)01146-7 |
| 16. | Fürstner, A.; Mamane, V. J. Org. Chem. 2002, 67, 6264–6267. doi:10.1021/jo025962y |
| 17. | Mamane, V.; Hannen, P.; Fürstner, A. Chem.–Eur. J. 2004, 10, 4556–4575. doi:10.1002/chem.200400220 |
| 18. | Soriano, E.; Marco-Contelles, J. Organometallics 2006, 25, 4542–4553. doi:10.1021/om0605332 |
| 19. | Seregin, I. V.; Gevorgyan, V. J. Am. Chem. Soc. 2006, 128, 12050–12051. doi:10.1021/ja063278l |
| 20. | Menon, R. S.; Findlay, A. D.; Bissember, A. C.; Banwell, M. G. J. Org. Chem. 2009, 74, 8901–8903. doi:10.1021/jo902032p |
| 21. | Jurberg, I. D.; Gagosz, F. J. Organomet. Chem. 2011, 696, 37–41. doi:10.1016/j.jorganchem.2010.06.017 |
| 44. | Otero, G.; Biddau, G.; Sánchez-Sánchez, C.; Caillard, R.; López, M. F.; Rogero, C.; Palomares, F. J.; Cabello, N.; Basanta, M. A.; Ortega, J.; Méndez, J.; Echavarren, A. M.; Pérez, R.; Gómez-Lor, B.; Martín-Gago, J. A. Nature 2008, 454, 865–868. doi:10.1038/nature07193 |
| 32. | Yadav, J. S.; Reddy, B. V. S.; Padmavani, B.; Gupta, M. K. Tetrahedron Lett. 2004, 45, 7577–7579. doi:10.1016/j.tetlet.2004.08.126 |
| 1. | Hashmi, A. S. K.; Hutchings, G. J. Angew. Chem., Int. Ed. 2006, 45, 7896–7936. doi:10.1002/anie.200602454 |
| 2. | Hashmi, A. S. K. Chem. Rev. 2007, 107, 3180–3211. doi:10.1021/cr000436x |
| 34. | Rudolph, M.; McCreery, M. Q.; Frey, W.; Hashmi, A. S. K. Beilstein J. Org. Chem. 2011, 7, 794–801. doi:10.3762/bjoc.7.90 |
| 35. | Hashmi, A. S. K.; Blanco, M. C. Eur. J. Org. Chem. 2006, 4340–4342. doi:10.1002/ejoc.200600546 |
| 36. | Hashmi, A. S. K.; Haufe, P.; Schmid, C.; Nass, A. R.; Frey, W. Chem.–Eur. J. 2006, 12, 5376–5382. doi:10.1002/chem.200600192 |
| 37. | Hashmi, A. S. K.; Yang, M. S. W.; Rominger, F. Angew. Chem., Int. Ed. 2011, 50, 5762–5765. doi:10.1002/anie.201100989 |
| 29. | Ferrer, C.; Echavarren, A. M. Angew. Chem., Int. Ed. 2006, 45, 1105–1109. doi:10.1002/anie.200503484 |
| 30. | Ferrer, C.; Amijs, C. H. M.; Echavarren, A. M. Chem.–Eur. J. 2007, 13, 1358–1373. doi:10.1002/chem.200601324 |
| 31. | Ferrer, C.; Escribano-Cuesta, A.; Echavarren, A. M. Tetrahedron 2009, 65, 9015–9020. doi:10.1016/j.tet.2009.08.067 |
| 38. | Martín-Matute, B.; Cárdenas, D. J.; Echavarren, A. M. Angew. Chem., Int. Ed. 2001, 40, 4754–4757. doi:10.1002/1521-3773(20011217)40:24<4754::AID-ANIE4754>3.0.CO;2-9 |
| 39. | Martín-Matute, B.; Nevado, C.; Cárdenas, D. J.; Echavarren, A. M. J. Am. Chem. Soc. 2003, 125, 5757–5766. doi:10.1021/ja029125p |
| 27. | Nishizawa, M.; Takao, H.; Yadav, V. K.; Imagawa, H.; Sugihara, T. Org. Lett. 2003, 5, 4563–4565. doi:10.1021/ol035622e |
| 28. | Nishizawa, M.; Imagawa, H.; Yamamoto, H. Org. Biomol. Chem. 2010, 8, 511–521. doi:10.1039/b920434b |
| 25. | Inoue, H.; Chatani, N.; Murai, S. J. Org. Chem. 2002, 67, 1414–1417. doi:10.1021/jo016232d |
| 26. | Li, H. J.; Guillot, R.; Gandon, V. J. Org. Chem. 2010, 75, 8435–8449. doi:10.1021/jo101709n |
| 33. | Bhuvaneswari, S.; Jeganmohan, M.; Cheng, C.-H. Chem.–Eur. J. 2007, 13, 8285–8293. doi:10.1002/chem.200700589 |
| 22. | Chatani, N.; Inoue, H.; Ikeda, T.; Murai, S. J. Org. Chem. 2000, 65, 4913–4918. doi:10.1021/jo000255v |
| 24. | Pastine, S. J.; Youn, S. W.; Sames, D. Org. Lett. 2003, 5, 1055–1058. doi:10.1021/ol034177k |
| 26. | Li, H. J.; Guillot, R.; Gandon, V. J. Org. Chem. 2010, 75, 8435–8449. doi:10.1021/jo101709n |
| 53. | Wegner, H. A.; Scott, L. T.; de Meijere, A. J. Org. Chem. 2003, 68, 883–887. doi:10.1021/jo020367h |
| 54. | Quimby, J. M.; Scout, L. T. Adv. Synth. Catal. 2009, 351, 1009–1013. doi:10.1002/adsc.200900018 |
| 55. | Ansems, R. B. M.; Scott, L. T. J. Am. Chem. Soc. 2000, 122, 2719–2724. doi:10.1021/ja993028n |
| 61. | Gautier, J. A.; Miocque, M.; Moskowitz, H. J. Organomet. Chem. 1964, 1, 212–221. doi:10.1016/S0022-328X(00)85489-3 |
| 41. | Gómez-Lor, B.; González-Cantalapiedra, E.; Ruiz, M.; de Frutos, Ó.; Cárdenas, D. J.; Santos, A.; Echavarren, A. M. Chem.–Eur. J. 2004, 10, 2601–2608. doi:10.1002/chem.200306023 |
| 62. | Gómez-Lor, B.; de Frutos, Ó.; Ceballos, P. A.; Granier, T.; Echavarren, A. M. Eur. J. Org. Chem. 2001, 2107–2114. doi:10.1002/1099-0690(200106)2001:11<2107::AID-EJOC2107>3.0.CO;2-F |
| 59. | Berlman, I. B.; Wirth, H. O.; Steingraber, O. J. J. Am. Chem. Soc. 1968, 90, 566–569. doi:10.1021/ja01005a003 |
| 60. | Kuhn, R.; Winterstein, A. Helv. Chim. Acta 1928, 11, 116–122. doi:10.1002/hlca.19280110108 |
| 6. | Jiménez-Núñez, E.; Echavarren, A. M. Chem. Rev. 2008, 108, 3326–3350. doi:10.1021/cr0684319 |
| 58. |
Raducan, M.; Rodríguez-Escrich, C.; Cambeiro, X. C.; Escudero-Adán, E. C.; Pericàs, M. A.; Echavarren, A. M. Chem. Commun. 2011, 47, 4893–4895. doi:10.1039/c1cc10293a
and references therein. |
| 25. | Inoue, H.; Chatani, N.; Murai, S. J. Org. Chem. 2002, 67, 1414–1417. doi:10.1021/jo016232d |
| 26. | Li, H. J.; Guillot, R.; Gandon, V. J. Org. Chem. 2010, 75, 8435–8449. doi:10.1021/jo101709n |
| 56. | Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178–6179. doi:10.1021/ja042257t |
| 57. | Amijs, C. H. M.; López-Carrillo, V.; Raducan, M.; Pérez-Galán, P.; Ferrer, C.; Echavarren, A. M. J. Org. Chem. 2008, 73, 7721–7730. doi:10.1021/jo8014769 |
© 2011 Pascual et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)