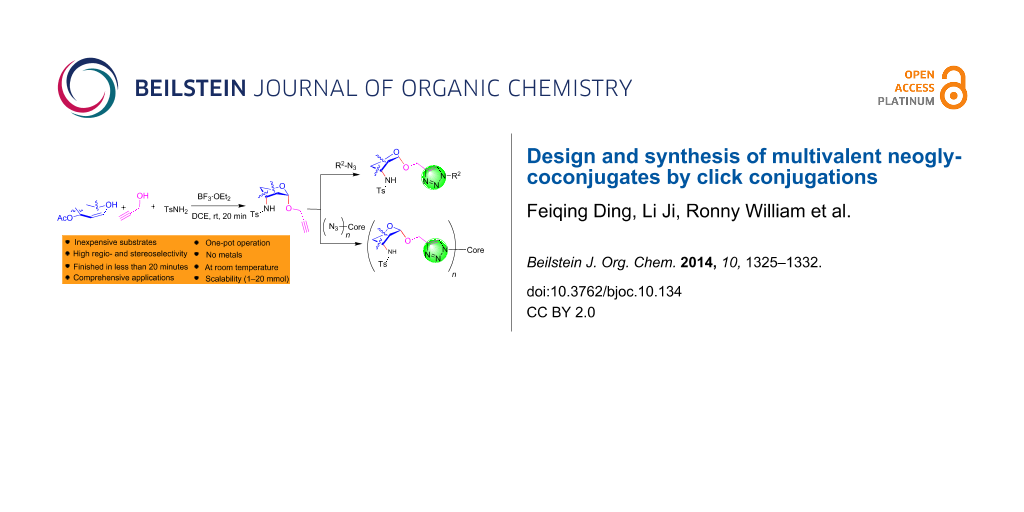
Abstract
A highly stereoselective BF3∙OEt2-promoted tandem hydroamination/glycosylation on glycal scaffolds has been developed to form propargyl 3-tosylamino-2,3-dideoxysugars in a one-pot manner. Subsequent construction of multivalent 3-tosylamino-2,3-dideoxyneoglycoconjugates with potential biochemical applications was presented herein involving click conjugations as the key reaction step. The copper-catalyzed regioselective click reaction was tremendously accelerated with assistance of microwave irradiation.
Graphical Abstract
Introduction
Oligosaccharides and glycopeptides are the key constituents of the cellular membrane and extracellular matrix, and play a pivotal role in various key cellular events such as cell–cell recognition, host–pathogen or host–symbiont interactions, molecular recognition of antibodies and metastasis [1-5]. The construction of a 1,4-disubstituted-1,2,3-triazole unit via a copper(I)-catalyzed modern version of the Huisgen-type azide–alkyne cycloaddition [6-10] has been considered to be a powerful ligation method for glycoconjugation [11-16]. In addition to the simplicity of this reaction and the ease of purification, 1,4-disubstituted-1,2,3-triazoles, the regiospecific product of this reaction, exhibit similarities to the ubiquitous amide moiety found in nature. However, unlike amides, the triazole moiety proved to be robust and resistant to chemical and enzymatic cleavage [17-20]. Moreover, the inertness of both azide and alkyne groups towards a majority of functional groups connected to the core of a variety of biomolecules also renders the click reaction particularly suitable for covalently linking bioactive molecular entities [21,22]. For example, the click strategy is especially versatile for the effective construction of complex glycosylated structures such as clusters, dendrimers, polymers, peptides and macrocycles. In all the cases the triazole ring plays a crucial role in combining divergent units together to establish a complex molecular architecture [23-31].
The α-GalNAc-linked glycopeptides, α-N-glycosidically linked to the polypeptide chain through the amido nitrogen of an asparagine residue at the N-terminal [32], were found to be the most important semi-synthetic glycoconjugates, usually modified from their naturally occurring parent precursors [33-39]. Over the years, many structural analogues of this class of antibiotics have been synthesized. In addition, triazoles are considered as peptidic linkage surrogates. Surprisingly, despite the enormous research interests associated with their synthesis, only a few examples of oligosaccharides and glycopeptides mimics have so far been prepared by a click chemistry strategy [40-48]. Most recently, we developed a strategy for the synthesis of 3-amino-2,3-dideoxysugars using a regio- and stereoselective tandem hydroamination/glycosylation of the glycal shown in Figure 1 [49-53]. Extending the synthetic utility of this protocol, herein, we wish to report the synthetic modification of α-GalNAc-linked glycopeptides to 3-tosylamino-2,3-dideoxyneoglycoconjugates via click conjugations (Figure 2).
Figure 1: Our reported strategy for quick access to 3-amino-2,3-dideoxysugars via regio- and stereoselective tandem hydroamination/glycosylation of glycals.
Figure 1: Our reported strategy for quick access to 3-amino-2,3-dideoxysugars via regio- and stereoselective ...
Figure 2: Synthetic modification of α-GalNAc linked glycopeptides to 3-tosylamino-2,3-dideoxyneoglycoconjugates via click conjugation.
Figure 2: Synthetic modification of α-GalNAc linked glycopeptides to 3-tosylamino-2,3-dideoxyneoglycoconjugat...
Given the success in using “click chemistry” in the glycosylation reactions, we aspired to apply the highly efficient triazole formation employing an azide 3 and a suitable alkyne appended to the 3-amino-2,3-dideoxysugars moiety 2 (Figure 3). In continuation of our previous work, herein we report a direct and reliable synthetic approach to multivalent 3-tosylamino-2,3-dideoxyneoglyco conjugates 4 with potential biochemical applications involving click conjugations as the key reaction step (Figure 3).
Figure 3: Our proposal for access to 3-tosylamino-2,3-dideoxyneoglycoconjugates via tandem hydroamination/glycosylation of glycals followed by click conjugations.
Figure 3: Our proposal for access to 3-tosylamino-2,3-dideoxyneoglycoconjugates via tandem hydroamination/gly...
Results and Discussion
Primarily, we successfully synthesized propargyl 3-p-toluenesulfonamido-4,6-di-O-acetyl-2,3-dideoxy-α-D-allohexopyranoside (2a) in gram scale via BF3∙OEt2-promoted one-pot three-component α-selective tandem hydroamination/glycosylation reaction (Scheme 1). In fact, when 3,4,6-tri-O-acetyl-D-glucal (1a), propargyl alcohol and p-toluenesulfonamide were subjected to a one-pot reaction in the presence of 2.2 equiv of BF3∙OEt2 in DCE at room temperature for 20 min, the desired aminoglycoside 2a was obtained in good yield with exclusive α-stereoselectivity [50]. Later, a systematic screening was executed using 3-tosylamino-2,3-dideoxysugar 2a and benzyl azide (3a) as our model system under varied conditions of catalysts, additives, solvents and reaction temperatures (Table 1). The initial evaluation involved no catalyst and additives at 100 °C and DMF, MeCN/H2O 3:1 or MeOH as the solvent system, which resulted in unsuccessful reactions (Table 1, entries 1–3). However, a trace amount of the desired product was detected in the presence of 10 mol % of copper(I) iodide (Table 1, entry 4). The combination of CuSO4·5H2O (10 mol %) and sodium ascorbate (10 mol %) was found to be a suitable catalyst leading regiospecifically to the 1,4-disubstituted-1,2,3-triazole 4a with moderate yield of 46% in t-BuOH/H2O 1:1 after 20 hours at 70 °C (Table 1, entry 5). The yield was further improved to 97% by employing DMF as solvent in a shorter period of 12 hours (Table 1, entry 6). Encouraged by these results, we attempted to improve the assemblies and to shorten the reaction times further; reactions were subjected to microwave irradiation, which is best known to accelerate transition metal-catalyzed homogeneous reactions [54]. Microwave-assisted organic reactions are rapidly becoming recognized as a valuable tool for facilitating a wide variety of organic transformations [55,56]. Finally, we found that the rate of conversion accelerated dramatically when microwave irradiation was used under 70 °C. To our delight under microwave conditions and in DMF with addition of 1 mol % of CuSO4·5H2O and 10 mol % of sodium ascorbate, a quantative yield of desired 3-tosylamino-2,3-dideoxyneoglycoconjugate 4a was obtained in 15 min (Table 1, entry 7).
Scheme 1: Synthesis of propargyl 3-tosylamino-2,3-dideoxy-α-D-allohexopyranoside (2a).
Scheme 1: Synthesis of propargyl 3-tosylamino-2,3-dideoxy-α-D-allohexopyranoside (2a).
Table 1: Optimization for synthesis of 3-tosylamino-2,3-dideoxyneoglycoconjugate 4a.
|
|
|||||
| Entry | Catalyst (mol %) | Solvent | Temperature (°C) | Time (h) | Yield (mol %)a |
|---|---|---|---|---|---|
| 1 | none | DMF | 100 | 20 | NRb |
| 2 | none | MeCN/H2O | 100 | 20 | NRb |
| 3 | none | MeOH | 100 | 20 | NRb |
| 4 | CuI (10) | THF | 60 | 12 | trace |
| 5 | CuSO4·5H2O (1) | t-BuOH/H2O | 70 | 20 | 46 |
| 6 | CuSO4·5H2O (1) | DMF | 70 | 12 | 97 |
| 7 | CuSO4·5H2O (1) | DMF | 70c | 0.25 | 98 |
aIsolated yield after purification. bNR = no reaction. cAssisted by microwave irradiation, 200 W.
Next, the required α-propargyl 3-tosylamino-2,3-dideoxyglycosides 2 were synthesized by BF3∙OEt2-promoted one-pot three-component tandem hydroamination/glycosylation reaction on a glycal scaffold including tri-O-acetyl-D-glucal (1a), tri-O-acetyl-D-allal (1b), tri-O-acetyl-D-galactal (1c), di-O-acetyl-D-rhamnal (1d), hexa-O-acetyl-D-maltal (1e). Accordingly, a series of α-propargyl 3-tosylamino-2,3-dideoxyglycosides 2a–2d were obtained exclusively with α-stereoselectivity in good yields (Table 2, entries 1–5).
Table 2: One-pot synthesis of α-propargyl 3-tosylamino-2,3-dideoxyglycosides 2.
|
|
|||
| Entry | 1 | 2 | Yield (%)a |
|---|---|---|---|
| 1 |
|
|
86 |
| 1a | 2a | ||
| 2 |
|
|
84 |
| 1b | 2a | ||
| 3 |
|
|
81 |
| 1c | 2b | ||
| 4 |
|
|
74 |
| 1d | 2c | ||
| 5 |
|
|
67 |
| 1e | 2d | ||
aIsolated yields after purification.
With pure α-propargyl 3-tosylamino-2,3-dideoxyglycosides and the optimized conditions in hand, we focused on performing a Huisgen cycloaddition reaction. The scope and generality of this method to prepare 3-tosylamino-2,3-dideoxyneoglycoconjugates 4 with the assistance of copper sulfate and sodium ascorbate was examined extensively. A range of α-alkyne-3-tosylamino-2,3-dideoxysugars and azides with various substituent groups (R2) were screened and the summarized results are shown in Table 3. Overall, the yields obtained were from good to excellent while preserving the anomeric selectivity and regioselectivity. In general, the analogous reaction of a set of azides with different substituent groups (3a–3e) with α-propargyl 3-tosylamino-2,3-dideoxy glycosides 2 afforded the corresponding 3-tosylamino-2,3-dideoxyneoglycoconjugates (4a–4h) in good to excellent yields with exclusive anomeric selectivity (Table 3, entries 1–8). This encouraging result prompted us to apply these conditions to alkyne 2a and a series of azido-linked monosaccharides 3f, 3g and 3h as well as the propargyl disaccharide 2d with α-GlaNAc azido 3g which were also obtained in good yields and selectivities (Table 3, entries 9–13). Subsequently, to shorten the reaction times, we subjected all the click conjugations to microwave irradiation. All the reactions were completed in considerably shorter reaction times of less than 30 min for the Huisgen cycloaddition of alkenes and azides catalyzed by copper sulfate and sodium ascorbate, affording the corresponding products in good to excellent yields in each case (Table 3, method B). This result showed that the synthesis of 3-tosylamino-2,3-dideoxyneoglycoconjugates via copper-catalyzed Huisgen cycloaddition is highly efficient under microwave irradiation.
Table 3: Scope for synthesis of 3-tosylamino-2,3-dideoxyneoglycoconjugates.
|
|
|||||
| Entry | 2 | 3 | 4 | Yield (%)a | |
|---|---|---|---|---|---|
| Ab | Bc | ||||
| 1 | 2a | 3a |
|
97 | 98 |
| 4a | |||||
| 2 | 2b | 3a |
|
89 | 93 |
| 4b | |||||
| 3 | 2c | 3a |
|
74 | 81 |
| 4c | |||||
| 4 | 2d | 3a |
|
71 | 78 |
| 4d | |||||
| 5 | 2a |
|
|
82 | 85 |
| 3b | 4e | ||||
| 6 | 2a |
|
|
91 | 92 |
| 3c | 4f | ||||
| 7 | 2a |
|
|
86 | 89 |
| 3d | 4g | ||||
| 8 | 2a |
|
|
87 | 92 |
| 3e | 4h | ||||
| 9 | 2a |
|
|
76 | 80 |
| 3f | 4i | ||||
| 10 | 2a |
|
|
93 | 95 |
| 3g | 4j | ||||
| 11 | 2d | 3g |
|
80 | 82 |
| 4k | |||||
| 12 | 2a |
|
|
72 | 78 |
| 3h | 4l | ||||
aIsolated yields after purification. b70 °C under conventional heating, 12 hours. c70 °C under microwave irradiation, 200 W, 15 minutes.
In carbohydrate recognition events, higher multivalent interactions are absolutely essential as the monovalent affinities of carbohydrate monosaccharides are comparatively low and weak. To enhance this multivalent effect, thereby increasing the binding efficiencies of carbohydrates with the coupling counterparts, there has been a constant development of new glycoconjugates such as glycodendrimers [57]. Hence, as continuation of previous encouraging results, we have further designed the use of noncarbohydrate diazide 5a in the cycloaddition reaction with the α-propargyl 3-tosylamino-2,3-dideoxyalloside 2a and α-propargyl 3-tosylamino-2,3,6-trideoxy-α-L-ribohexopyranoside 2c (Scheme 2) to obtain divalent 3-tosylamino-2,3-dideoxyneoglycoconjugates 6a and 6b in 83% and 61% yield respectively. The synthesis of trivalent 3-tosylamino-2,3-dideoxyneoglycoconjugates 6c was also feasible by using triazide 5b in 66% yield (Scheme 3). Interestingly, for all the reactions under microwave irradiation, reaction times were reduced to 15 minutes. As such, this novel synthetic protocol provides a straightforward access to a wide range of 3-tosylamino-2,3-dideoxyneoglycoconjugate derivatives which may find numerous biochemical applications [40-48].
Scheme 2: Synthesis of divalent 3-tosylamino-2,3-dideoxyneoglycoconjugates 6a and 6b.
Scheme 2: Synthesis of divalent 3-tosylamino-2,3-dideoxyneoglycoconjugates 6a and 6b.
Scheme 3: Synthesis of trivalent 3-tosylamino-2,3-dideoxyneoglycoconjugate 6c.
Scheme 3: Synthesis of trivalent 3-tosylamino-2,3-dideoxyneoglycoconjugate 6c.
Conclusion
In conclusion, it has been established that the construction of well-defined multivalent, anomerically pure 3-amino-2,3-dideoxyneoglycoconjugate architectures was successfully achieved by using cycloaddition reactions of alkynes and azides. It is expected that this strategy will find extensive applications in glycoscience, because triazole-linked glycoconjugates can exhibit very interesting biological properties, offering a convenient access toward oligosaccharides, glycopeptide mimics, or multivalent carbohydrate systems [40-48]. Their further application in molcecular biosystems is currently underway and the results will be reported in due course.
Supporting Information
| Supporting Information File 1: Experimental, analytical data and 1H NMR and 13C NMR spectra for all new compounds. | ||
| Format: PDF | Size: 3.8 MB | Download |
References
-
Driguez, H.; Thiem, J. Glycoscience; Springer: Berlin, 1999; Vol. 1–2.
Return to citation in text: [1] -
Ernst, B.; Hart, G.; Sinaý, P. Carbohydrates in Chemistry and Biology; Wiley, 2000; Vol. 1–4. doi:10.1002/9783527618255
Return to citation in text: [1] -
Wang, P. G.; Bertozzi, C. R. Glycochemistry: Principles, Synthesis, and Applications; Marcel Dekker: New York, NY, 2001.
Return to citation in text: [1] -
Kiessling, L. L.; Gestwicki, J. E.; Strong, L. E. Angew. Chem., Int. Ed. 2006, 45, 2348. doi:10.1002/anie.200502794
Return to citation in text: [1] -
Gruner, S. A. W.; Locardi, E.; Lohof, E.; Kessler, H. Chem. Rev. 2002, 102, 491. doi:10.1021/cr0004409
Return to citation in text: [1] -
Huisgen, R. 1,3-Dipolar cycloaddition – Introduction, survey, mechanism. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley, 1984; Vol. 1, pp 1–176.
Return to citation in text: [1] -
Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952. doi:10.1021/cr0783479
Return to citation in text: [1] -
Rostovtsev, V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
Return to citation in text: [1] -
Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5
Return to citation in text: [1] -
Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057. doi:10.1021/jo011148j
Return to citation in text: [1] -
Davis, B. G. J. Chem. Soc., Perkin Trans. 1 1999, 3215. doi:10.1039/A809773I
Return to citation in text: [1] -
Turnbull, W. B.; Stoodart, J. F. Rev. Mol. Biotechnol. 2002, 90, 231. doi:10.1016/S1389-0352(01)00062-9
Return to citation in text: [1] -
Roy, R.; Baek, M.-G. Rev. Mol. Biotechnol. 2002, 90, 291. doi:10.1016/S1389-0352(01)00065-4
Return to citation in text: [1] -
Bezouška, K. Rev. Mol. Biotechnol. 2002, 90, 269. doi:10.1016/S1389-0352(01)00064-2
Return to citation in text: [1] -
Dedola, S.; Nepogodiev, S. A.; Field, R. A. Org. Biomol. Chem. 2007, 5, 1006. doi:10.1039/b618048p
Return to citation in text: [1] -
Dondoni, A. Chem.–Asian J. 2007, 2, 700. doi:10.1002/asia.200700015
Return to citation in text: [1] -
Patani, G. A.; LaVoie, E. J. Chem. Rev. 1996, 96, 3147. doi:10.1021/cr950066q
Return to citation in text: [1] -
Tron, G. C.; Pirali, T.; Billington, R. A.; Canonico, P. L.; Sorba, G.; Genazzani, A. A. Med. Res. Rev. 2008, 28, 278. doi:10.1002/med.20107
Return to citation in text: [1] -
Wilkinson, B. L.; Bornaghi, L. F.; Poulsen, S.-A.; Houston, T. A. Tetrahedron 2006, 62, 8115. doi:10.1016/j.tet.2006.06.001
Return to citation in text: [1] -
Jung, K.-H.; Schmidt, R. R. Glycosyltransferase Inhibitors. In Carbohydrate-Based Drug Discovery; Wong, C.-H., Ed.; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, 2003; pp 609 ff.
Return to citation in text: [1] -
Kiick, K. L.; Saxon, E.; Tirrell, D. A.; Bertozzi, C. R. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 19. doi:10.1073/pnas.012583299
Return to citation in text: [1] -
Jewett, J. C.; Bertozzi, C. R. Chem. Soc. Rev. 2010, 39, 1272. doi:10.1039/b901970g
Return to citation in text: [1] -
Kolb, H. C.; Sharpless, K. B. Drug Discovery Today 2003, 8, 1128. doi:10.1016/S1359-6446(03)02933-7
Return to citation in text: [1] -
Whiting, M.; Muldoon, J.; Lin, Y.-C.; Silverman, S. M.; Lindstrom, W.; Olson, A. J.; Kolb, H. C.; Finn, M. G.; Sharpless, K. B.; Elder, J. H.; Fokin, V. V. Angew. Chem., Int. Ed. 2006, 45, 1435. doi:10.1002/anie.200502161
Return to citation in text: [1] -
Oh, K.; Guan, Z. Chem. Commun. 2006, 3069. doi:10.1039/b606185k
Return to citation in text: [1] -
Bock, V. D.; Speijer, D.; Hiemstra, H.; van Maarseveen, J. H. Org. Biomol. Chem. 2007, 5, 971. doi:10.1039/b616751a
Return to citation in text: [1] -
Angell, Y. L.; Burgess, K. Chem. Soc. Rev. 2007, 36, 1674. doi:10.1039/b701444a
Return to citation in text: [1] -
Nagarajan, S.; Das, T. M. Carbohydr. Res. 2009, 344, 1028. doi:10.1016/j.carres.2009.03.009
Return to citation in text: [1] -
Nagarajan, S.; Arjun, P.; Raaman, N.; Das, T. M. Carbohydr. Res. 2010, 345, 1988. doi:10.1016/j.carres.2010.07.016
Return to citation in text: [1] -
Prasad, V.; Kale, R. R.; Kumar, V.; Tiwari, V. K. Curr. Org. Synth. 2010, 7, 506. doi:10.2174/157017910792246063
Return to citation in text: [1] -
Pandey, V. P.; Bisht, S. S.; Mishra, M.; Kumar, A.; Siddiqi, M. I.; Verma, A.; Mittal, M.; Sane, S. A.; Gupta, S.; Tripathi, R. P. Eur. J. Med. Chem. 2010, 45, 2381. doi:10.1016/j.ejmech.2010.02.018
Return to citation in text: [1] -
Shibata, S.; Takeda, T.; Natori, Y. J. Biol. Chem. 1988, 263, 12483.
Return to citation in text: [1] -
Pajk, S.; Garvas, M.; Štrancar, J.; Pečar, S. Org. Biomol. Chem. 2011, 9, 4150. doi:10.1039/c0ob01173h
Return to citation in text: [1] -
Kumar, G. D. K.; Baskaran, S. J. Org. Chem. 2005, 70, 4520. doi:10.1021/jo0502697
Return to citation in text: [1] -
Yan, R.-B.; Yang, F.; Wu, Y.; Zhang, L.-H.; Ye, X.-S. Tetrahedron Lett. 2005, 46, 8993. doi:10.1016/j.tetlet.2005.10.103
Return to citation in text: [1] -
Alix, A.; Chassaing, S.; Pale, P.; Sommer, J. Tetrahedron 2008, 64, 8922. doi:10.1016/j.tet.2008.06.086
Return to citation in text: [1] -
Wilkinson, B. L.; Stone, R. S.; Capicciotti, C. J.; Thaysen-Andersen, M.; Matthews, J. M.; Packer, N. H.; Ben, R. N.; Payne, R. J. Angew. Chem., Int. Ed. 2012, 51, 3606. doi:10.1002/anie.201108682
Return to citation in text: [1] -
Liu, S.; Wang, W.; von Moos, E.; Jackman, J.; Mealing, G.; Monette, R.; Ben, R. N. Biomacromolecules 2007, 8, 1456. doi:10.1021/bm061044o
Return to citation in text: [1] -
Bouvet, V. R.; Ben, R. N. In Antifreeze Glycoprotein Analogues: Synthesis. In Vitro Testing and Applications; Roy, R., Ed.; American Chemical Society, Oxford University Press: Washington, D.C., 2004; p 151.
Return to citation in text: [1] -
Santoyo-González, F.; Hernández-Mateo, F. Top. Heterocycl. Chem. 2007, 7, 133. doi:10.1007/7081_2007_050
Return to citation in text: [1] [2] [3] -
Chen, Q.; Yang, F.; Du, Y. Carbohydr. Res. 2005, 340, 2476. doi:10.1016/j.carres.2005.08.013
Return to citation in text: [1] [2] [3] -
Gouin, S. G.; Bultel, L.; Falentin, C.; Kovensky, J. Eur. J. Org. Chem. 2007, 1160. doi:10.1002/ejoc.200600814
Return to citation in text: [1] [2] [3] -
Hotha, S.; Kashyap, S. J. Org. Chem. 2006, 71, 364. doi:10.1021/jo051731q
Return to citation in text: [1] [2] [3] -
Ortega-Muñoz, M.; Lopez-Jaramillo, J.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Adv. Synth. Catal. 2006, 348, 2410. doi:10.1002/adsc.200600254
Return to citation in text: [1] [2] [3] -
Touaibia, M.; Wellens, A.; Shiao, T. C.; Wang, Q.; Sirois, S.; Bouckaert, J.; Roy, R. ChemMedChem 2007, 2, 1190. doi:10.1002/cmdc.200700063
Return to citation in text: [1] [2] [3] -
Guo, Z.; Lei, A.; Zhang, Y.; Xu, Q.; Xue, X.; Zhang, F.; Liang, X. Chem. Commun. 2007, 2491. doi:10.1039/b701831b
Return to citation in text: [1] [2] [3] -
Nepogodiev, S. A.; Dedola, S.; Marmuse, L.; de Oliveira, M. T.; Field, R. A. Carbohydr. Res. 2007, 342, 529. doi:10.1016/j.carres.2006.09.026
Return to citation in text: [1] [2] [3] -
Pietrzik, N.; Schips, C.; Ziegler, T. Synthesis 2008, 519. doi:10.1055/s-2008-1032150
Return to citation in text: [1] [2] [3] -
Ding, F.; William, R.; Wang, F.; Ma, J.; Ji, L.; Liu, X.-W. Org. Lett. 2011, 13, 652. doi:10.1021/ol102891t
Return to citation in text: [1] -
Ding, F. Q.; William, R.; Wang, S.; Gorityala, B. K.; Liu, X.-W. Org. Biomol. Chem. 2011, 9, 3929. doi:10.1039/c1ob05068k
Return to citation in text: [1] [2] -
Ding, F.; William, R.; Cai, S.; Ma, J.; Liu, X.-W. J. Org. Chem. 2012, 77, 5245. doi:10.1021/jo300791v
Return to citation in text: [1] -
Ding, F.; William, R.; Liu, X.-W. J. Org. Chem. 2013, 78, 1293. doi:10.1021/jo302619b
Return to citation in text: [1] -
Ding, F.; Cai, S.; William, R.; Liu, X.-W. RSC Adv. 2013, 3, 13594. doi:10.1039/C3RA40595H
Return to citation in text: [1] -
Pérez-Balderas, F.; Ortega-Muñoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; Calvo-Flores, F. G.; Calvo-Asin, J. A.; Isac-García, J.; Santoyo-González, F. Org. Lett. 2003, 5, 1951. doi:10.1021/ol034534r
Return to citation in text: [1] -
Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Tetrahedron 2001, 57, 9225. doi:10.1016/S0040-4020(01)00906-1
Return to citation in text: [1] -
Kappe, C. O.; Pieber, B.; Dallinger, D. Angew. Chem., Int. Ed. 2013, 52, 1088. doi:10.1002/anie.201204103
Return to citation in text: [1] -
Chabre, Y. M.; Roy, R. Curr. Top. Med. Chem. 2008, 8, 1237. doi:10.2174/156802608785848987
Return to citation in text: [1]
| 1. | Driguez, H.; Thiem, J. Glycoscience; Springer: Berlin, 1999; Vol. 1–2. |
| 2. | Ernst, B.; Hart, G.; Sinaý, P. Carbohydrates in Chemistry and Biology; Wiley, 2000; Vol. 1–4. doi:10.1002/9783527618255 |
| 3. | Wang, P. G.; Bertozzi, C. R. Glycochemistry: Principles, Synthesis, and Applications; Marcel Dekker: New York, NY, 2001. |
| 4. | Kiessling, L. L.; Gestwicki, J. E.; Strong, L. E. Angew. Chem., Int. Ed. 2006, 45, 2348. doi:10.1002/anie.200502794 |
| 5. | Gruner, S. A. W.; Locardi, E.; Lohof, E.; Kessler, H. Chem. Rev. 2002, 102, 491. doi:10.1021/cr0004409 |
| 21. | Kiick, K. L.; Saxon, E.; Tirrell, D. A.; Bertozzi, C. R. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 19. doi:10.1073/pnas.012583299 |
| 22. | Jewett, J. C.; Bertozzi, C. R. Chem. Soc. Rev. 2010, 39, 1272. doi:10.1039/b901970g |
| 40. | Santoyo-González, F.; Hernández-Mateo, F. Top. Heterocycl. Chem. 2007, 7, 133. doi:10.1007/7081_2007_050 |
| 41. | Chen, Q.; Yang, F.; Du, Y. Carbohydr. Res. 2005, 340, 2476. doi:10.1016/j.carres.2005.08.013 |
| 42. | Gouin, S. G.; Bultel, L.; Falentin, C.; Kovensky, J. Eur. J. Org. Chem. 2007, 1160. doi:10.1002/ejoc.200600814 |
| 43. | Hotha, S.; Kashyap, S. J. Org. Chem. 2006, 71, 364. doi:10.1021/jo051731q |
| 44. | Ortega-Muñoz, M.; Lopez-Jaramillo, J.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Adv. Synth. Catal. 2006, 348, 2410. doi:10.1002/adsc.200600254 |
| 45. | Touaibia, M.; Wellens, A.; Shiao, T. C.; Wang, Q.; Sirois, S.; Bouckaert, J.; Roy, R. ChemMedChem 2007, 2, 1190. doi:10.1002/cmdc.200700063 |
| 46. | Guo, Z.; Lei, A.; Zhang, Y.; Xu, Q.; Xue, X.; Zhang, F.; Liang, X. Chem. Commun. 2007, 2491. doi:10.1039/b701831b |
| 47. | Nepogodiev, S. A.; Dedola, S.; Marmuse, L.; de Oliveira, M. T.; Field, R. A. Carbohydr. Res. 2007, 342, 529. doi:10.1016/j.carres.2006.09.026 |
| 48. | Pietrzik, N.; Schips, C.; Ziegler, T. Synthesis 2008, 519. doi:10.1055/s-2008-1032150 |
| 17. | Patani, G. A.; LaVoie, E. J. Chem. Rev. 1996, 96, 3147. doi:10.1021/cr950066q |
| 18. | Tron, G. C.; Pirali, T.; Billington, R. A.; Canonico, P. L.; Sorba, G.; Genazzani, A. A. Med. Res. Rev. 2008, 28, 278. doi:10.1002/med.20107 |
| 19. | Wilkinson, B. L.; Bornaghi, L. F.; Poulsen, S.-A.; Houston, T. A. Tetrahedron 2006, 62, 8115. doi:10.1016/j.tet.2006.06.001 |
| 20. | Jung, K.-H.; Schmidt, R. R. Glycosyltransferase Inhibitors. In Carbohydrate-Based Drug Discovery; Wong, C.-H., Ed.; Wiley-VCH Verlag GmbH & Co KGaA: Weinheim, 2003; pp 609 ff. |
| 40. | Santoyo-González, F.; Hernández-Mateo, F. Top. Heterocycl. Chem. 2007, 7, 133. doi:10.1007/7081_2007_050 |
| 41. | Chen, Q.; Yang, F.; Du, Y. Carbohydr. Res. 2005, 340, 2476. doi:10.1016/j.carres.2005.08.013 |
| 42. | Gouin, S. G.; Bultel, L.; Falentin, C.; Kovensky, J. Eur. J. Org. Chem. 2007, 1160. doi:10.1002/ejoc.200600814 |
| 43. | Hotha, S.; Kashyap, S. J. Org. Chem. 2006, 71, 364. doi:10.1021/jo051731q |
| 44. | Ortega-Muñoz, M.; Lopez-Jaramillo, J.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Adv. Synth. Catal. 2006, 348, 2410. doi:10.1002/adsc.200600254 |
| 45. | Touaibia, M.; Wellens, A.; Shiao, T. C.; Wang, Q.; Sirois, S.; Bouckaert, J.; Roy, R. ChemMedChem 2007, 2, 1190. doi:10.1002/cmdc.200700063 |
| 46. | Guo, Z.; Lei, A.; Zhang, Y.; Xu, Q.; Xue, X.; Zhang, F.; Liang, X. Chem. Commun. 2007, 2491. doi:10.1039/b701831b |
| 47. | Nepogodiev, S. A.; Dedola, S.; Marmuse, L.; de Oliveira, M. T.; Field, R. A. Carbohydr. Res. 2007, 342, 529. doi:10.1016/j.carres.2006.09.026 |
| 48. | Pietrzik, N.; Schips, C.; Ziegler, T. Synthesis 2008, 519. doi:10.1055/s-2008-1032150 |
| 11. | Davis, B. G. J. Chem. Soc., Perkin Trans. 1 1999, 3215. doi:10.1039/A809773I |
| 12. | Turnbull, W. B.; Stoodart, J. F. Rev. Mol. Biotechnol. 2002, 90, 231. doi:10.1016/S1389-0352(01)00062-9 |
| 13. | Roy, R.; Baek, M.-G. Rev. Mol. Biotechnol. 2002, 90, 291. doi:10.1016/S1389-0352(01)00065-4 |
| 14. | Bezouška, K. Rev. Mol. Biotechnol. 2002, 90, 269. doi:10.1016/S1389-0352(01)00064-2 |
| 15. | Dedola, S.; Nepogodiev, S. A.; Field, R. A. Org. Biomol. Chem. 2007, 5, 1006. doi:10.1039/b618048p |
| 16. | Dondoni, A. Chem.–Asian J. 2007, 2, 700. doi:10.1002/asia.200700015 |
| 55. | Lidström, P.; Tierney, J.; Wathey, B.; Westman, J. Tetrahedron 2001, 57, 9225. doi:10.1016/S0040-4020(01)00906-1 |
| 56. | Kappe, C. O.; Pieber, B.; Dallinger, D. Angew. Chem., Int. Ed. 2013, 52, 1088. doi:10.1002/anie.201204103 |
| 6. | Huisgen, R. 1,3-Dipolar cycloaddition – Introduction, survey, mechanism. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley, 1984; Vol. 1, pp 1–176. |
| 7. | Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952. doi:10.1021/cr0783479 |
| 8. | Rostovtsev, V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 |
| 9. | Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004. doi:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5 |
| 10. | Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057. doi:10.1021/jo011148j |
| 57. | Chabre, Y. M.; Roy, R. Curr. Top. Med. Chem. 2008, 8, 1237. doi:10.2174/156802608785848987 |
| 40. | Santoyo-González, F.; Hernández-Mateo, F. Top. Heterocycl. Chem. 2007, 7, 133. doi:10.1007/7081_2007_050 |
| 41. | Chen, Q.; Yang, F.; Du, Y. Carbohydr. Res. 2005, 340, 2476. doi:10.1016/j.carres.2005.08.013 |
| 42. | Gouin, S. G.; Bultel, L.; Falentin, C.; Kovensky, J. Eur. J. Org. Chem. 2007, 1160. doi:10.1002/ejoc.200600814 |
| 43. | Hotha, S.; Kashyap, S. J. Org. Chem. 2006, 71, 364. doi:10.1021/jo051731q |
| 44. | Ortega-Muñoz, M.; Lopez-Jaramillo, J.; Hernandez-Mateo, F.; Santoyo-Gonzalez, F. Adv. Synth. Catal. 2006, 348, 2410. doi:10.1002/adsc.200600254 |
| 45. | Touaibia, M.; Wellens, A.; Shiao, T. C.; Wang, Q.; Sirois, S.; Bouckaert, J.; Roy, R. ChemMedChem 2007, 2, 1190. doi:10.1002/cmdc.200700063 |
| 46. | Guo, Z.; Lei, A.; Zhang, Y.; Xu, Q.; Xue, X.; Zhang, F.; Liang, X. Chem. Commun. 2007, 2491. doi:10.1039/b701831b |
| 47. | Nepogodiev, S. A.; Dedola, S.; Marmuse, L.; de Oliveira, M. T.; Field, R. A. Carbohydr. Res. 2007, 342, 529. doi:10.1016/j.carres.2006.09.026 |
| 48. | Pietrzik, N.; Schips, C.; Ziegler, T. Synthesis 2008, 519. doi:10.1055/s-2008-1032150 |
| 50. | Ding, F. Q.; William, R.; Wang, S.; Gorityala, B. K.; Liu, X.-W. Org. Biomol. Chem. 2011, 9, 3929. doi:10.1039/c1ob05068k |
| 33. | Pajk, S.; Garvas, M.; Štrancar, J.; Pečar, S. Org. Biomol. Chem. 2011, 9, 4150. doi:10.1039/c0ob01173h |
| 34. | Kumar, G. D. K.; Baskaran, S. J. Org. Chem. 2005, 70, 4520. doi:10.1021/jo0502697 |
| 35. | Yan, R.-B.; Yang, F.; Wu, Y.; Zhang, L.-H.; Ye, X.-S. Tetrahedron Lett. 2005, 46, 8993. doi:10.1016/j.tetlet.2005.10.103 |
| 36. | Alix, A.; Chassaing, S.; Pale, P.; Sommer, J. Tetrahedron 2008, 64, 8922. doi:10.1016/j.tet.2008.06.086 |
| 37. | Wilkinson, B. L.; Stone, R. S.; Capicciotti, C. J.; Thaysen-Andersen, M.; Matthews, J. M.; Packer, N. H.; Ben, R. N.; Payne, R. J. Angew. Chem., Int. Ed. 2012, 51, 3606. doi:10.1002/anie.201108682 |
| 38. | Liu, S.; Wang, W.; von Moos, E.; Jackman, J.; Mealing, G.; Monette, R.; Ben, R. N. Biomacromolecules 2007, 8, 1456. doi:10.1021/bm061044o |
| 39. | Bouvet, V. R.; Ben, R. N. In Antifreeze Glycoprotein Analogues: Synthesis. In Vitro Testing and Applications; Roy, R., Ed.; American Chemical Society, Oxford University Press: Washington, D.C., 2004; p 151. |
| 54. | Pérez-Balderas, F.; Ortega-Muñoz, M.; Morales-Sanfrutos, J.; Hernández-Mateo, F.; Calvo-Flores, F. G.; Calvo-Asin, J. A.; Isac-García, J.; Santoyo-González, F. Org. Lett. 2003, 5, 1951. doi:10.1021/ol034534r |
| 23. | Kolb, H. C.; Sharpless, K. B. Drug Discovery Today 2003, 8, 1128. doi:10.1016/S1359-6446(03)02933-7 |
| 24. | Whiting, M.; Muldoon, J.; Lin, Y.-C.; Silverman, S. M.; Lindstrom, W.; Olson, A. J.; Kolb, H. C.; Finn, M. G.; Sharpless, K. B.; Elder, J. H.; Fokin, V. V. Angew. Chem., Int. Ed. 2006, 45, 1435. doi:10.1002/anie.200502161 |
| 25. | Oh, K.; Guan, Z. Chem. Commun. 2006, 3069. doi:10.1039/b606185k |
| 26. | Bock, V. D.; Speijer, D.; Hiemstra, H.; van Maarseveen, J. H. Org. Biomol. Chem. 2007, 5, 971. doi:10.1039/b616751a |
| 27. | Angell, Y. L.; Burgess, K. Chem. Soc. Rev. 2007, 36, 1674. doi:10.1039/b701444a |
| 28. | Nagarajan, S.; Das, T. M. Carbohydr. Res. 2009, 344, 1028. doi:10.1016/j.carres.2009.03.009 |
| 29. | Nagarajan, S.; Arjun, P.; Raaman, N.; Das, T. M. Carbohydr. Res. 2010, 345, 1988. doi:10.1016/j.carres.2010.07.016 |
| 30. | Prasad, V.; Kale, R. R.; Kumar, V.; Tiwari, V. K. Curr. Org. Synth. 2010, 7, 506. doi:10.2174/157017910792246063 |
| 31. | Pandey, V. P.; Bisht, S. S.; Mishra, M.; Kumar, A.; Siddiqi, M. I.; Verma, A.; Mittal, M.; Sane, S. A.; Gupta, S.; Tripathi, R. P. Eur. J. Med. Chem. 2010, 45, 2381. doi:10.1016/j.ejmech.2010.02.018 |
| 49. | Ding, F.; William, R.; Wang, F.; Ma, J.; Ji, L.; Liu, X.-W. Org. Lett. 2011, 13, 652. doi:10.1021/ol102891t |
| 50. | Ding, F. Q.; William, R.; Wang, S.; Gorityala, B. K.; Liu, X.-W. Org. Biomol. Chem. 2011, 9, 3929. doi:10.1039/c1ob05068k |
| 51. | Ding, F.; William, R.; Cai, S.; Ma, J.; Liu, X.-W. J. Org. Chem. 2012, 77, 5245. doi:10.1021/jo300791v |
| 52. | Ding, F.; William, R.; Liu, X.-W. J. Org. Chem. 2013, 78, 1293. doi:10.1021/jo302619b |
| 53. | Ding, F.; Cai, S.; William, R.; Liu, X.-W. RSC Adv. 2013, 3, 13594. doi:10.1039/C3RA40595H |
© 2014 Ding et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)