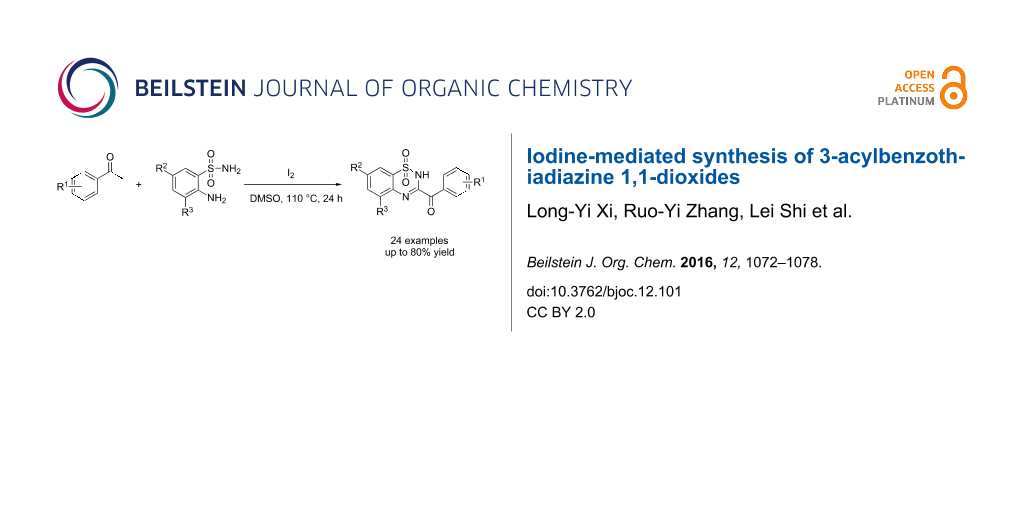
Abstract
An iodine-mediated synthesis of 3-acylbenzothiadizine 1,1-dioxides is described. A range of electronically diverse acetophenones reacted well with several 2-aminobenzenesulfonamides, affording 3-acylbenzothiadiazine 1,1-dioxides in good yields.
Graphical Abstract
Introduction
Benzothiadiazine 1,1-dioxide moieties have attracted remarkable attention in the pharmacological area because of their broad spectrum of activities [1-4], such as antihypertensive [5,6] or antiviral [7,8] and they are also used as cardiovascular agents [9-11] (Scheme 1). In this context, several synthetic methods have been developed to synthesize benzothiadiazine 1,1-dioxides and their analogues. The condensations of 2-aminobenzenesulfonamides with urea, isocyanates, carboxylic acid derivatives or other carbonyl reagents are the most used methods [12-15]. These reactions were usually carried out under harsh reaction conditions, causing the formation of byproducts. An alternative route to the benzothiadiazine 1,1-dioxide ring are transition metal-catalyzed reactions. Various benzothiadiazine 1,1-dioxides were successfully prepared using this approach [16-19]. However, the separation of transitional metal catalysts from pharmaceutical chemicals was cumbersome. Therefore, the development of efficient metal-free routes to benzothiadiazine 1,1-dioxides is necessary. On the other hand, despite that so many benzothiadiazine 1,1-dioxide derivatives have been studied extensively, 3-acylbenzothiadiazine 1,1-dioxides were ignored, because of the lack of efficient methods to prepare them. Readily available acetophenones have been shown to be good starting materials for the synthesis of various heterocyclic compounds [20-24]. The Wu group has reported an efficient protocol for the synthesis of luotonin F and derivatives from aromatic ketones and 2-aminobenzamides via iodination/Kornblum oxidation/annulation [25]. We envisioned that 2-aminobenzenesulfonamides would undergo a similar reaction to afford 3-acylbenzothiadiazine 1,1-dioxides. Herein, we report the first synthesis of 3-acylbenzothiadiazine 1,1-dioxides from 2-aminobenzenesulfonamides and acetophenones via iodine-mediated sp3 C–H functionalization.
Scheme 1: Selected benzothiadaiazine 1,1-dioxides with potent biological activities.
Scheme 1: Selected benzothiadaiazine 1,1-dioxides with potent biological activities.
Results and Discussion
We commenced our studies by heating acetophenone (1a), 2-aminobenzenesulfonamide (2a) and I2 in DMSO for 12 h. To our delight, the desired product was isolated in 60% yield. To improve the yield of this product, a series of additives including acids, metal salt and bases were tested. However, to our disappointment, all of them could not promote this transformation (Table 1, entries 2–7). After optimizing the ratios of 1a and 2a, we found that increasing the amount of 1a or 2a resulted in lower yields (Table 1, entries 8 and 9). Iodinated compound 4a was isolated as a major byproduct, therefore we set out to optimize the amount of I2 (Table 1, entry 1 and entries 10–14). 0.75 equiv of iodine gave the best result, and the yield increased to 73% (Table 1, entry 11). This result was different from the synthesis of quinazolin-4-ones reported by Wu group, in which reducing the amount of iodine leaded to low yield [25]. Raising or reducing temperature both resulted in decreased yields (Table 1, entries 15 and 16). Under argon atmosphere, the yield decreased slightly (Table 1, entry 17 vs entry 11), while it decreased obviously when the amount of I2 was reduced to 0.4 equiv (Table 1, entry 18 vs entry 14) [26]. We further found that the yield increased slightly to 80% by doubling the reaction time (Table 1, entry 19). Consequently, we decided to set the conditions described in entry 19 as the standard conditions.
Table 1: Optimization of reaction conditions.a
|
|
||||
| entry |
I2
(equiv) |
additives
(equiv) |
time
(h) |
yield
(%)b |
|---|---|---|---|---|
| 1 | 1.1 | none | 12 | 60 |
| 2 | 1.1 | HI (0.1) | 12 | 58 |
| 3 | 1.1 | AcOH (0.1) | 12 | 46 |
| 4 | 1.1 | TsOH·H2O (0.1) | 12 | 39 |
| 5 | 1.1 | CuI (0.1) | 12 | 51 |
| 6 | 1.1 | KOH (0.1) | 12 | 46 |
| 7 | 1.1 | NaOt-Bu (0.1) | 12 | 53 |
| 8c | 1.1 | none | 12 | 55 |
|
9d
10 |
1.1
0.9 |
none
none |
12
12 |
41
59 |
|
11
12 |
0.75
0.6 |
none
none |
12
12 |
73
62 |
| 13 | 0.5 | none | 12 | 56 |
|
14
15e 16f 17g |
0.4
0.75 0.75 0.75 |
none
none none none |
12
12 12 12 |
42
21 65 66 |
| 18g | 0.4 | none | 12 | 25 |
| 19 | 0.75 | none | 24 | 80 |
aReaction conditions: 1a (0.33 mmol), 2a (0.3 mmol), DMSO (2 mL). bIsolated yields. c1.5 equiv of acetophenone. d1.5 equiv of 2-aminobenzenesulfonamide. e80 °C. f130 °C. gAr atmosphere.
With optimized conditions in hand, we next explored the substrate scope of acetophenones. As shown in Scheme 2, a variety of acetophenones were compatible with this transformation, both electron-rich and electron-poor functional groups at the para-position of the benzene ring of acetophenones were well tolerated in this reaction. Acetophenones bearing halides (1j–l) such as fluoro, chloro and bromo substituents proceeded smoothly to give the corresponding products in good yields, which provided opportunities for further syntheses of more complex benzothiadiazine 1,1-dioxides. Besides acetophenones, heteroaryl methyl ketones also underwent this transformation, affording the corresponding products in moderate yields (3n and 3o). When 1-(naphthalen-2-yl)ethanone was subjected to this reaction, a good yield of the desired product 3p was isolated.
Scheme 2: Scope of acetophenones (reaction conditions: 1 (0.33 mmol), 2a (0.3 mmol), DMSO (2 mL), I2 (0.75 equiv), 110 °C, 24 h, isolated yields).
Scheme 2: Scope of acetophenones (reaction conditions: 1 (0.33 mmol), 2a (0.3 mmol), DMSO (2 mL), I2 (0.75 eq...
To widen the scope of substrates, we further explored several 2-aminobenzenesulfonamides bearing substituents on the benzene ring. 2-Aminobenzenesulfonamides bearing halides proceeded well, providing the corresponding products in good yields (Scheme 3). Interestingly, when 2-aminobenzenesulfonamides bearing an alknyl group were subjected to this reaction, besides the formation of 3-acylbenzothiadiazine 1,1,-dioxide skeletons, the triple bond was further transformed into an ortho-diketone functionality (Scheme 4) [27-29]. 1,2-Dicarbonyl functionalities are one of the most important skeletons found in biologically active molecules and versatile building blocks for chemical transformations [30-32].
Scheme 3: Scope of 2-aminobenzenesulfonamides (reaction conditions: 1 (0.33 mmol), 2a (0.3 mmol), DMSO (2 mL), I2 (0.75 equiv), 110 °C, 24 h, isolated yields).
Scheme 3: Scope of 2-aminobenzenesulfonamides (reaction conditions: 1 (0.33 mmol), 2a (0.3 mmol), DMSO (2 mL)...
Scheme 4: Reactions of 2-aminobenzenesulfonamides bearing an alknyl group (reaction conditions: 1 (0.33 mmol), 2 (0.3 mmol), DMSO (2 mL), I2 (0.75 equiv), 110 °C, 24 h, isolated yields.)
Scheme 4: Reactions of 2-aminobenzenesulfonamides bearing an alknyl group (reaction conditions: 1 (0.33 mmol)...
In support of the application of this method, we conducted the reaction on a gram scale, and it also showed good performance (Scheme 5). The structure of 4b was characterized by X-ray diffraction (Figure 1).
Scheme 5: Gram scale reaction between 1a and 2a.
Scheme 5: Gram scale reaction between 1a and 2a.
![[1860-5397-12-101-1]](/bjoc/content/figures/1860-5397-12-101-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: X-ray crystal structure of 4b (CCDC 1444753).
Figure 1: X-ray crystal structure of 4b (CCDC 1444753).
To shed light on the mechanism of this reaction, a control experiment was conducted. It is known that acetophenones can undergo halogenation and further Kornblum oxidation to give phenylglyoxal in a I2/DMSO system [33,34]. We isolated the product in 85% yield by heating phenylglyoxal and 2a in DMSO at 110 °C for 24 h without I2 (Scheme 6). To further probe the reaction process, we monitored the reaction system at 80 °C by 1H NMR spectroscopic studies. A signal appeared at 9.63 ppm, which was assigned to phenylglyoxal [35] (see Supporting Information File 1). Moreover, intermediate D was isolated after stirring 12 h at 80 °C.
On the basis of this experiment described above and literature [13,35,36], a possible mechanism was proposed in Scheme 7. The halogenation of 1a with iodine results in the formation of compound A, which, via Kornblum oxidation, provides phenylglyoxal B. The condensation of phenylglyoxal with 2-aminobenzenesulfonamide affords intermediate C, followed by intramolecular addition giving intermediate D. Intermediate D undergoes autoxidation leading to the desired product 3a.
Conclusion
In conclusion, we have developed a novel one-pot protocol for the synthesis of 3-acylbenzothiadiazine 1,1-dioxides from readily available aromatic ketones and 2-aminobenzenesulfonamides. Various aryl and heteroaryl ketones reacted well with 2-aminobenzenesulfonamides, affording the corresponding products in moderate to good yields. This metal-free method would provide opportunities to develop new biologically active benzothiadiazine 1,1-dioxides.
Experimental
Typical procedure for the synthesis of 3-acylbenzothiadiazine 1,1-dioxides: A mixture of acetophenone (0.040 mL, 0.33 mmol), I2 (0.057 g, 0.225 mmol) and 2-aminobenzenesulfonamide (0.051 g, 0.3 mmol) in DMSO (2 mL) was stirred at 110 °C under air atmosphere in a sealed 50 mL Schlenk tube for 24 h. After the reaction was finished, the reaction mixture was cooled to room temperature. The resulting mixture was taken up by dichloromethane (60 mL) and washed with saturated Na2S2O3 solution until the brown color disappeared. The organic phase was dried over Na2SO4 (anhydrous), concentrated in vacuum, and the resulting residue was purified by column chromatography on silica gel with EtOAc/petroleum (1:4) to afford the product.
Supporting Information
| Supporting Information File 1: Experimental part and copies of NMR spectra. | ||
| Format: PDF | Size: 4.5 MB | Download |
References
-
Majumdar, K. C.; Ganai, S. Beilstein J. Org. Chem. 2013, 9, 503–509. doi:10.3762/bjoc.9.54
Return to citation in text: [1] -
Khelili, S.; Kihal, N.; Yekhlef, M.; de Tullio, P.; Lebrun, P.; Pirotte, B. Eur. J. Med. Chem. 2012, 54, 873–878. doi:10.1016/j.ejmech.2012.05.011
Return to citation in text: [1] -
De Tullio, P.; Servais, A.-C.; Fillet, M.; Gillotin, F.; Somers, F.; Chiap, P.; Lebrun, P.; Pirotte, B. J. Med. Chem. 2011, 54, 8353–8361. doi:10.1021/jm200786z
Return to citation in text: [1] -
Francotte, P.; Goffin, E.; Fraikin, P.; Lestage, P.; Van Heugen, J.-C.; Gillotin, F.; Danober, L.; Thomas, J.-Y.; Chiap, P.; Caignard, D.-H.; Pirotte, B.; de Tullio, P. J. Med. Chem. 2010, 53, 1700–1711. doi:10.1021/jm901495t
Return to citation in text: [1] -
Topliss, J. G.; Yudis, M. D. J. Med. Chem. 1972, 15, 394–400. doi:10.1021/jm00274a017
Return to citation in text: [1] -
Tait, A.; Luppi, A.; Franchini, S.; Preziosi, E.; Parenti, C.; Buccioni, M.; Marucci, G.; Leonardi, A.; Poggessi, E.; Brasili, L. Bioorg. Med. Chem. Lett. 2005, 15, 1185–1188. doi:10.1016/j.bmcl.2004.12.004
Return to citation in text: [1] -
Zhan, P.; Liu, X.; De Clercq, E. Curr. Med. Chem. 2008, 15, 1529–1540. doi:10.2174/092986708784638898
Return to citation in text: [1] -
Tripathi, R. L.; Krishnan, P.; He, Y.; Middleton, T.; Pilot-Matias, T.; Chen, C.-M.; Lau, D. T. Y.; Lemon, S. M.; Mo, H.; Kati, W.; Molla, A. Antiviral Res. 2007, 73, 40–49. doi:10.1016/j.antiviral.2006.07.005
Return to citation in text: [1] -
Costantino, L.; Parenti, C.; Di Bella, M.; Zanoli, P.; Baraldi, M. Pharmacol. Res. 1993, 27, 349–358. doi:10.1006/phrs.1993.1034
Return to citation in text: [1] -
Khelili, S.; Leclerc, G.; Faury, G.; Verdetti, J. Bioorg. Med. Chem. 1995, 3, 495–503. doi:10.1016/0968-0896(95)00040-N
Return to citation in text: [1] -
Kim, D.; Mantlo, N. B.; Chang, R. S. L.; Kivlighn, S. D.; Greenlee, W. J. Bioorg. Med. Chem. Lett. 1994, 4, 41–44. doi:10.1016/S0960-894X(01)81119-7
Return to citation in text: [1] -
Watson, A. J. A.; Maxwell, A. C.; Williams, J. M. J. Org. Biomol. Chem. 2012, 10, 240–243. doi:10.1039/C1OB06516E
Return to citation in text: [1] -
Sharif, M.; Opalach, J.; Langer, P.; Beller, M.; Wu, X.-F. RSC Adv. 2014, 4, 8–17. doi:10.1039/C3RA45765F
Return to citation in text: [1] [2] -
Lachenicht, S.; Fischer, A.; Schmidt, C.; Winkler, M.; Rood, A.; Lemoine, H.; Braun, M. ChemMedChem 2009, 4, 1850–1858. doi:10.1002/cmdc.200900261
Return to citation in text: [1] -
Wang, G.; He, Y.; Sun, J.; Das, D.; Hu, M.; Huang, J.; Ruhrmund, D.; Hooi, L.; Misialek, S.; Rajagopalan, P. T. R.; Stoycheva, A.; Buckman, B. O.; Kossen, K.; Seiwert, S. D.; Beigelman, L. Bioorg. Med. Chem. Lett. 2009, 19, 4476–4479. doi:10.1016/j.bmcl.2009.05.063
Return to citation in text: [1] -
Yang, D.; Liu, H.; Yang, H.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. Adv. Synth. Catal. 2009, 351, 1999–2004. doi:10.1002/adsc.200900101
Return to citation in text: [1] -
Yang, D.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. J. Comb. Chem. 2009, 11, 653–657. doi:10.1021/cc9000339
Return to citation in text: [1] -
Zhang, S.; Chen, X.; Parveen, S.; Hussain, S.; Yang, Y.; Jing, C.; Zhu, C. ChemMedChem 2013, 8, 603–613. doi:10.1002/cmdc.201200386
Return to citation in text: [1] -
Yang, D.; An, B.; Wei, W.; Tian, L.; Huang, B.; Wang, H. ACS Comb. Sci. 2015, 17, 113–119. doi:10.1021/co500125n
Return to citation in text: [1] -
Shu, W.-M.; Zheng, K.-L.; Ma, J.-R.; Wu, A.-X. Org. Lett. 2015, 17, 5216–5219. doi:10.1021/acs.orglett.5b02548
Return to citation in text: [1] -
Xi, L.-Y.; Zhang, R.-Y.; Liang, S.; Chen, S.-Y.; Yu, X.-Q. Org. Lett. 2014, 16, 5269–5271. doi:10.1021/ol5023596
Return to citation in text: [1] -
Wu, K.; Huang, Z.; Liu, C.; Zhang, H.; Lei, A. Chem. Commun. 2015, 51, 2286–2289. doi:10.1039/C4CC08074B
Return to citation in text: [1] -
Mohan, D. C.; Donthiri, R. R.; Rao, S. N.; Adimurthy, S. Adv. Synth. Catal. 2013, 355, 2217–2221. doi:10.1002/adsc.201300456
Return to citation in text: [1] -
Pilgrim, B. S.; Gatland, A. E.; McTernan, C. T.; Procopiou, P. A.; Donohoe, T. J. Org. Lett. 2013, 15, 6190–6193. doi:10.1021/ol4030309
Return to citation in text: [1] -
Zhu, Y.-P.; Fei, Z.; Liu, M.-C.; Jia, F.-C.; Wu, A.-X. Org. Lett. 2013, 15, 378–381. doi:10.1021/ol303331g
Return to citation in text: [1] [2] -
Several experiments about the effect of oxygen on the reaction were carried out (see Supporting Information File 1, control experiments).
Return to citation in text: [1] -
Xu, C.-F.; Xu, M.; Jia, Y.-X.; Li, C.-Y. Org. Lett. 2011, 13, 1556–1559. doi:10.1021/ol200270t
Return to citation in text: [1] -
Min, H.; Palani, T.; Park, K.; Hwang, J.; Lee, S. J. Org. Chem. 2014, 79, 6279–6285. doi:10.1021/jo501089k
Return to citation in text: [1] -
Byun, S.; Chung, J.; Lim, T.; Kwon, J.; Kim, B. M. RSC Adv. 2014, 4, 34084–34088. doi:10.1039/C4RA04833D
Return to citation in text: [1] -
Nicolaou, K. C.; Gray, D. L. F.; Tae, J. J. Am. Chem. Soc. 2004, 126, 613–627. doi:10.1021/ja030498f
Return to citation in text: [1] -
Wolkenberg, S. E.; Wisnoski, D. D.; Leister, W. H.; Wang, Y.; Zhao, Z.; Lindsley, C. W. Org. Lett. 2004, 6, 1453–1456. doi:10.1021/ol049682b
Return to citation in text: [1] -
Boyce, G. R.; Johnson, J. S. Angew. Chem., Int. Ed. 2010, 49, 8930–8933. doi:10.1002/anie.201003470
Return to citation in text: [1] -
Kornblum, N.; Powers, J. W.; Anderson, G. J.; Jones, W. J.; Larson, H. O.; Levand, O.; Weaver, W. M. J. Am. Chem. Soc. 1957, 79, 6562. doi:10.1021/ja01581a057
Return to citation in text: [1] -
Yin, G.; Gao, M.; She, N.; Hu, S.; Wu, A.; Pan, Y. Synthesis 2007, 3113–3116. doi:10.1055/s-2007-983880
Return to citation in text: [1] -
Zhu, Y.-P.; Lian, M.; Jia, F.-C.; Liu, M.-C.; Yuan, J.-J.; Gao, Q.-H.; Wu, A.-X. Chem. Commun. 2012, 48, 9086–9088. doi:10.1039/C2CC34561G
Return to citation in text: [1] [2] -
Jiang, H.; Huang, H.; Cao, H.; Qi, C. Org. Lett. 2010, 12, 5561–5563. doi:10.1021/ol1023085
Return to citation in text: [1]
| 1. | Majumdar, K. C.; Ganai, S. Beilstein J. Org. Chem. 2013, 9, 503–509. doi:10.3762/bjoc.9.54 |
| 2. | Khelili, S.; Kihal, N.; Yekhlef, M.; de Tullio, P.; Lebrun, P.; Pirotte, B. Eur. J. Med. Chem. 2012, 54, 873–878. doi:10.1016/j.ejmech.2012.05.011 |
| 3. | De Tullio, P.; Servais, A.-C.; Fillet, M.; Gillotin, F.; Somers, F.; Chiap, P.; Lebrun, P.; Pirotte, B. J. Med. Chem. 2011, 54, 8353–8361. doi:10.1021/jm200786z |
| 4. | Francotte, P.; Goffin, E.; Fraikin, P.; Lestage, P.; Van Heugen, J.-C.; Gillotin, F.; Danober, L.; Thomas, J.-Y.; Chiap, P.; Caignard, D.-H.; Pirotte, B.; de Tullio, P. J. Med. Chem. 2010, 53, 1700–1711. doi:10.1021/jm901495t |
| 12. | Watson, A. J. A.; Maxwell, A. C.; Williams, J. M. J. Org. Biomol. Chem. 2012, 10, 240–243. doi:10.1039/C1OB06516E |
| 13. | Sharif, M.; Opalach, J.; Langer, P.; Beller, M.; Wu, X.-F. RSC Adv. 2014, 4, 8–17. doi:10.1039/C3RA45765F |
| 14. | Lachenicht, S.; Fischer, A.; Schmidt, C.; Winkler, M.; Rood, A.; Lemoine, H.; Braun, M. ChemMedChem 2009, 4, 1850–1858. doi:10.1002/cmdc.200900261 |
| 15. | Wang, G.; He, Y.; Sun, J.; Das, D.; Hu, M.; Huang, J.; Ruhrmund, D.; Hooi, L.; Misialek, S.; Rajagopalan, P. T. R.; Stoycheva, A.; Buckman, B. O.; Kossen, K.; Seiwert, S. D.; Beigelman, L. Bioorg. Med. Chem. Lett. 2009, 19, 4476–4479. doi:10.1016/j.bmcl.2009.05.063 |
| 13. | Sharif, M.; Opalach, J.; Langer, P.; Beller, M.; Wu, X.-F. RSC Adv. 2014, 4, 8–17. doi:10.1039/C3RA45765F |
| 35. | Zhu, Y.-P.; Lian, M.; Jia, F.-C.; Liu, M.-C.; Yuan, J.-J.; Gao, Q.-H.; Wu, A.-X. Chem. Commun. 2012, 48, 9086–9088. doi:10.1039/C2CC34561G |
| 36. | Jiang, H.; Huang, H.; Cao, H.; Qi, C. Org. Lett. 2010, 12, 5561–5563. doi:10.1021/ol1023085 |
| 9. | Costantino, L.; Parenti, C.; Di Bella, M.; Zanoli, P.; Baraldi, M. Pharmacol. Res. 1993, 27, 349–358. doi:10.1006/phrs.1993.1034 |
| 10. | Khelili, S.; Leclerc, G.; Faury, G.; Verdetti, J. Bioorg. Med. Chem. 1995, 3, 495–503. doi:10.1016/0968-0896(95)00040-N |
| 11. | Kim, D.; Mantlo, N. B.; Chang, R. S. L.; Kivlighn, S. D.; Greenlee, W. J. Bioorg. Med. Chem. Lett. 1994, 4, 41–44. doi:10.1016/S0960-894X(01)81119-7 |
| 7. | Zhan, P.; Liu, X.; De Clercq, E. Curr. Med. Chem. 2008, 15, 1529–1540. doi:10.2174/092986708784638898 |
| 8. | Tripathi, R. L.; Krishnan, P.; He, Y.; Middleton, T.; Pilot-Matias, T.; Chen, C.-M.; Lau, D. T. Y.; Lemon, S. M.; Mo, H.; Kati, W.; Molla, A. Antiviral Res. 2007, 73, 40–49. doi:10.1016/j.antiviral.2006.07.005 |
| 33. | Kornblum, N.; Powers, J. W.; Anderson, G. J.; Jones, W. J.; Larson, H. O.; Levand, O.; Weaver, W. M. J. Am. Chem. Soc. 1957, 79, 6562. doi:10.1021/ja01581a057 |
| 34. | Yin, G.; Gao, M.; She, N.; Hu, S.; Wu, A.; Pan, Y. Synthesis 2007, 3113–3116. doi:10.1055/s-2007-983880 |
| 5. | Topliss, J. G.; Yudis, M. D. J. Med. Chem. 1972, 15, 394–400. doi:10.1021/jm00274a017 |
| 6. | Tait, A.; Luppi, A.; Franchini, S.; Preziosi, E.; Parenti, C.; Buccioni, M.; Marucci, G.; Leonardi, A.; Poggessi, E.; Brasili, L. Bioorg. Med. Chem. Lett. 2005, 15, 1185–1188. doi:10.1016/j.bmcl.2004.12.004 |
| 35. | Zhu, Y.-P.; Lian, M.; Jia, F.-C.; Liu, M.-C.; Yuan, J.-J.; Gao, Q.-H.; Wu, A.-X. Chem. Commun. 2012, 48, 9086–9088. doi:10.1039/C2CC34561G |
| 25. | Zhu, Y.-P.; Fei, Z.; Liu, M.-C.; Jia, F.-C.; Wu, A.-X. Org. Lett. 2013, 15, 378–381. doi:10.1021/ol303331g |
| 27. | Xu, C.-F.; Xu, M.; Jia, Y.-X.; Li, C.-Y. Org. Lett. 2011, 13, 1556–1559. doi:10.1021/ol200270t |
| 28. | Min, H.; Palani, T.; Park, K.; Hwang, J.; Lee, S. J. Org. Chem. 2014, 79, 6279–6285. doi:10.1021/jo501089k |
| 29. | Byun, S.; Chung, J.; Lim, T.; Kwon, J.; Kim, B. M. RSC Adv. 2014, 4, 34084–34088. doi:10.1039/C4RA04833D |
| 25. | Zhu, Y.-P.; Fei, Z.; Liu, M.-C.; Jia, F.-C.; Wu, A.-X. Org. Lett. 2013, 15, 378–381. doi:10.1021/ol303331g |
| 30. | Nicolaou, K. C.; Gray, D. L. F.; Tae, J. J. Am. Chem. Soc. 2004, 126, 613–627. doi:10.1021/ja030498f |
| 31. | Wolkenberg, S. E.; Wisnoski, D. D.; Leister, W. H.; Wang, Y.; Zhao, Z.; Lindsley, C. W. Org. Lett. 2004, 6, 1453–1456. doi:10.1021/ol049682b |
| 32. | Boyce, G. R.; Johnson, J. S. Angew. Chem., Int. Ed. 2010, 49, 8930–8933. doi:10.1002/anie.201003470 |
| 20. | Shu, W.-M.; Zheng, K.-L.; Ma, J.-R.; Wu, A.-X. Org. Lett. 2015, 17, 5216–5219. doi:10.1021/acs.orglett.5b02548 |
| 21. | Xi, L.-Y.; Zhang, R.-Y.; Liang, S.; Chen, S.-Y.; Yu, X.-Q. Org. Lett. 2014, 16, 5269–5271. doi:10.1021/ol5023596 |
| 22. | Wu, K.; Huang, Z.; Liu, C.; Zhang, H.; Lei, A. Chem. Commun. 2015, 51, 2286–2289. doi:10.1039/C4CC08074B |
| 23. | Mohan, D. C.; Donthiri, R. R.; Rao, S. N.; Adimurthy, S. Adv. Synth. Catal. 2013, 355, 2217–2221. doi:10.1002/adsc.201300456 |
| 24. | Pilgrim, B. S.; Gatland, A. E.; McTernan, C. T.; Procopiou, P. A.; Donohoe, T. J. Org. Lett. 2013, 15, 6190–6193. doi:10.1021/ol4030309 |
| 16. | Yang, D.; Liu, H.; Yang, H.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. Adv. Synth. Catal. 2009, 351, 1999–2004. doi:10.1002/adsc.200900101 |
| 17. | Yang, D.; Fu, H.; Hu, L.; Jiang, Y.; Zhao, Y. J. Comb. Chem. 2009, 11, 653–657. doi:10.1021/cc9000339 |
| 18. | Zhang, S.; Chen, X.; Parveen, S.; Hussain, S.; Yang, Y.; Jing, C.; Zhu, C. ChemMedChem 2013, 8, 603–613. doi:10.1002/cmdc.201200386 |
| 19. | Yang, D.; An, B.; Wei, W.; Tian, L.; Huang, B.; Wang, H. ACS Comb. Sci. 2015, 17, 113–119. doi:10.1021/co500125n |
| 26. | Several experiments about the effect of oxygen on the reaction were carried out (see Supporting Information File 1, control experiments). |
© 2016 Xi et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)