Abstract
The well-known aminoazoles, 3-amino-5-methylisoxazole and 5-amino-N-aryl-1H-pyrazole-4-carboxamides, were studied as an amine component in Ugi and Groebke–Blackburn–Bienaymé multicomponent reactions. The first example of an application of aminoazoles in an Ugi four-component reaction was discovered and novel features of a Groebke–Blackburn–Bienaymé cyclocondensation are established and discussed. The heterocycles obtained were evaluated for their antibacterial activity and several of them demonstrated a weak antimicrobial effect, but for most of the compounds a 30–50% increase in biomass of Gram-positive strains (mainly B. subtilis) compared to control was observed.
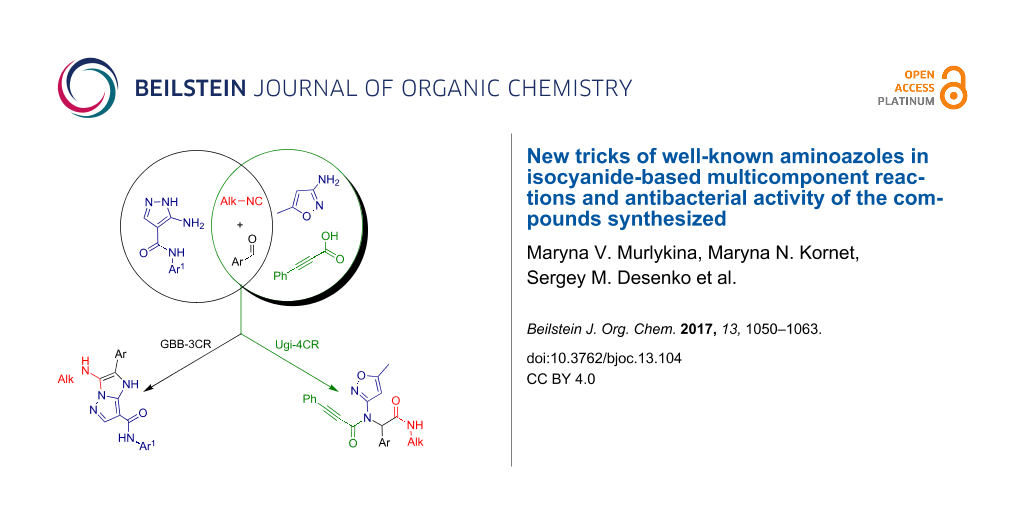
Graphical Abstract
Introduction
An intensive progress in pharmaceutical and medicinal chemistry, as well as in the generation and improvement of medicinal technologies has led to defeating a wide scope of diseases. However, we are still facing the problem of untreated ones, together with the appearance of unknown disorders and the dramatical growth of antimicrobial resistance caused by the continuous evolution of microorganisms [1-5]. Therefore, there is urgency in careful screening the chemical space with the aim of finding new biologically active structures. Modern chemistry offers several approaches, for instance, diversity oriented synthesis (DOS) for the generation of diverse compound libraries [6-8]. From this point of view, multicomponent reactions (MCRs), including isocyanide-based MCRs as the Ugi four-component reaction (Ugi-4CR) and the Groebke–Blackburn–Bienaymé reaction (GBB-3CR) in combination with post-cyclizations are powerful tools to access diversity as well as complexity in a one-pot procedure; in this way they largely cover the available chemical space [9-26]. The imidazoheterocyclic scaffold represents a promising area for the discovery of novel synthetic drug molecules [27-52]. Particularly, there are several drugs containing the imidazo[1,2-a]pyridine moiety such as zolpidem (treatment of insomnia) and olprinone (cardiotonic drug) and a lot of compounds in biological testing and preclinical evaluation such as soraprazan (clinical antiulcer compound), necopidem (sedative effect), and saripidem (anxiolytic) [27]. The activity of different imidazoheterocycles was also studied against migraine [30], gastric [31,32], heart [34-36], viral diseases [37-41] and an array of neurological syndromes [52]. The imidazo[1,2-b]pyrazole core shows also a pharmacological potential. Among others, anti-inflammatory [28,43], antiviral [39,44], antidiabetic [45] effects and cancer cell growth-inhibitory features should be mentioned [29,46-48].
Ugi-4CR has been applied in the synthesis of natural products, as bicyclomycin, furanomycin, penicillin etc. [53]. The high combinatorial potential of Ugi-4CR together with the ability to incorporate a variety of functionalities and modifications extend its application for the generation of organic compound libraries, following hit-to-lead optimization, choosing the hit structure and final marketed drug production [54-58]. Moreover, it has been acknowledged that the combination of two privileged scaffolds in a single molecule (e.g., the combination of a peptidomimetic structure with an azole fragment [59]) potentially creates more active, new entities with unusual bioproperties [20,60,61]. In addition, the application of polyfunctional reagents in Ugi-4CR opens ways to different post-cyclization reactions, thereby broadening the scope. Thus the Ugi-4CR involving substituted propiolic acids, can be followed by electrophilic ipso-iodocyclization [62] or transition-metal-initiated [63-68] and metal-free cyclizations [69,70].
There are examples of using aminoazoles as an amine component in GBB-3CR (Scheme 1). They mostly involve different substituted 3-amino-1,2,4-triazoles [71-75] and 2-amino(benzo)thiazoles [71,72,76-88]. Several publications deal with 2-amino-1,3,4-thiadiazoles [71,83,84,89,90], 2-aminoimidazoles [71,72,91,92], 2-aminoxazoles [71] and 1,2,5-oxadiazole-3,4-diamine [93] with the formation of imidazoazoles. Among the pyrazoles only 5-amino-3-methylpyrazole, 5-aminopyrazole-4-carbonitrile and ethyl 5-aminopyrazole-4-carboxylate are described in GBB-3CR [29,71,83,84,94-96]. To the best of our knowledge, there is no information about the reactivity of aminoazoles as an amine component in Ugi-4CR.
Scheme 1: Aminoazoles in GBB-3CR and Ugi-4CR.
Scheme 1: Aminoazoles in GBB-3CR and Ugi-4CR.
Taking into account the above-mentioned facts, several aminoazoles, whose reactivity in isocyanide-based reactions had not been studied yet, were examined as an amine component in Ugi-4CR and GBB-3CR. The generated compounds were screened for their biological activity towards Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa.
Results and Discussion
Since aminoazoles contain an exocyclic NH2 group and an endocyclic nucleophilic center, they can act both as primary amines and as 1,3-binucleophiles, therefore, their treatments with isocyanides, aldehydes and carboxylic acids may proceed either as Ugi-4CR (aminoazole – primary amine, acid – reagent) or as GBB-3CR (aminoazole – 1,3-binucleophile, acid – catalyst). Literature data indicate [14,25,59,97-101] that 5-aminopyrazoles bearing in the fourth position electron-withdrawing substituents like carboxamide, carboxylate or a carbonitrile group, posses chemical properties being different from other 5-aminopyrazoles but sometimes similar to 3-amino-1,2,4-triazole that was described as a component of GBB-3CR earlier [71-75]. Therefore, the first type of aminoazoles studied in our work was 5-amino-N-aryl-1H-pyrazole-4-carboxamide that showed 1,3-binucleophile properties in the condensation with aromatic aldehydes and alkylisocyanides giving the product resulting from GBB-3CR. On the other hand, 3-amino-5-methylisoxazole in MCRs often acted as primary amine [102-106], that was confirmed in our case by treatment with aromatic aldehydes, alkylisocyanides and phenylpropiolic acid resulting in the corresponding products of the Ugi-4CR (Scheme 2).
Scheme 2: Reactivity of 5-amino-N-aryl-1H-pyrazole-4-carboxamide and 3-amino-5-methylisoxazole in GBB-3CR and Ugi-4CR.
Scheme 2: Reactivity of 5-amino-N-aryl-1H-pyrazole-4-carboxamide and 3-amino-5-methylisoxazole in GBB-3CR and...
The scope and limitations of isocyanide-based reactions involving 5-amino-N-aryl-1H-pyrazole-4-carboxamides and 3-amino-5-methylisoxazole were studied in detail. It was established that the optimal reaction conditions of a GBB-3CR involving 5-amino-N-aryl-1H-pyrazole-4-carboxamides 2a–d were different depending on the other substrates. Particularly, the condensation involving tert-butylisocyanide (3a) and aldehydes 1a–e bearing electron-donating substituents was effectively carried out in EtOH/H2O mixture with TFA (10 mol %) at room temperature for 24 h (method A) resulting in the formation of imidazopyrazoles 4a–m (Table 1, entries 1–13).
Table 1: CBB-3CR involving tert-butylisocyanide.
|
|
|||||||
| Entry | Starting materials | Method | Product | Yield, % | |||
|---|---|---|---|---|---|---|---|
| Aldehydes | R1 | Aminopyrazoles | R2 | ||||
| 1 | 1a | H | 2a | 4-F | A | 4a | 54 |
| 2 | 1b | 2-CH3O | 2a | 4-F | A | 4b | 75 |
| 3 | 1c | 3-CH3O | 2a | 4-F | A | 4c | 77 |
| 4 | 1d | 4-CH3O | 2a | 4-F | A | 4d | 75 |
| 5 | 1e | 4-Cl | 2a | 4-F | A | 4e | 72 |
| 6 | 1b | 2-CH3O | 2b | 3-F | A | 4f | 83 |
| 7 | 1d | 4-CH3O | 2b | 3-F | A | 4g | 64 |
| 8 | 1e | 4-Cl | 2b | 3-F | A | 4h | 89 |
| 9 | 1b | 2-CH3O | 2c | 2-CH2CH3 | A | 4i | 82 |
| 10 | 1d | 4-CH3O | 2c | 2-CH2CH3 | A | 4j | 64 |
| 11 | 1e | 4-Cl | 2c | 2-CH2CH3 | A | 4k | 66 |
| 12 | 1b | 2-CH3O | 2d | 4-CH2CH3 | A | 4l | 85 |
| 13 | 1e | 4-Cl | 2d | 4-CH2CH3 | A | 4m | 53 |
| 14 | 1f | 4-CO2CH3 | 2a | 4-F | B | 4n | 85 |
| 15 | 1g | 4-NO2 | 2a | 4-F | B | 4o | 87 |
| 16 | 1h | 4-CN | 2a | 4-F | B | 4p | 90 |
| 17 | 1f | 4-CO2CH3 | 2b | 3-F | B | 4q | 88 |
| 18 | 1g | 4-NO2 | 2b | 3-F | B | 4r | 87 |
| 19 | 1h | 4-CN | 2b | 3-F | B | 4s | 82 |
| 20 | 1f | 4-CO2CH3 | 2c | 2-CH2CH3 | B | 4t | 61 |
| 21 | 1g | 4-NO2 | 2c | 2-CH2CH3 | B | 4u | 79 |
| 22 | 1h | 4-CN | 2c | 2-CH2CH3 | B | 4v | 83 |
This condensation was also carried out in TFE or MeOH with addition of HClO4 (10 mol %), but a significant amount of Schiff base 5 (Table 1) was observed in this case. The reaction involving aldehydes 1f–h bearing strong electron-withdrawing groups under all the abovementioned conditions allowed isolating a mixture of imidazopyrazoles 4n–v with a large quantity of Schiff bases 5.
Therefore, the conditions for the synthesis of compounds 4n–v were optimized employing 5-amino-N-(3-fluorophenyl)-1H-pyrazole-4-carboxamide (2b), methyl benzaldehyde-4-carboxylate (1f) and tert-butylisocyanide (3a, Table 2).
Table 2: Optimization of the reaction conditions for the synthesis of compound 4q.
|
|
|||||
| Entry | Solvent, acid | Time, hours | T, °C | Products (ratio) | Yield, %a |
|---|---|---|---|---|---|
| 1 | TFE, HClO4, (10 mol %) | 72 | 25 | 4 + 5 (2:1) | – |
| 2 | EtOH/H2O (1:1), TFA, (10 mol %) | 24 | 25 | 4 + 5 (1:1) | – |
| 3 | EtOH/H2O (1:1), TFA, (20 mol %) | 24 | 25 | 4 + 5 (1:1) | – |
| 4 | EtOH/H2O (1:1), TFA, (10 mol %) | 12 | 80 | 4 + 5 (2:1) | – |
| 5 | EtOH/H2O (1:1), TFA, (10 mol %) | 0.3b | 140 | 4 + 5 (4:5) | – |
| 6 | EtOH/H2O (1:1), TFA, (10 mol %) | 2c | 25 | 4 + 5d | – |
| 7 | EtOH/H2O (1:1), TFA, (10 mol %) | 48 | 85 | 4 | 56 |
| 8 | EtOH/H2O (1:1), TFA, (10 mol %) | 5b | 140 | 4 + 5 (2:3) | – |
| 9 | DMF, HClO4, (10 mol %) | 48 | 25 | 4 | 88 |
aThe yields are indicated for compound 4 and are not calculated for the mixtures; bupon MW irradiation; cupon US irradiation; din a mixture with starting materials and undetected impurities.
Obviously, this reaction requires a longer reaction time (min. 48 h) and a moderate temperature (not more than 85 °C) to avoid tarring. Thus, after 48 h of heating (oil bath) at 85 °C the starting materials 1f, 2b and 3a in EtOH/H2O with TFA (10 mol %), imidazopyrazole 4q was isolated in 56% yield. However, the mother liquor still contained unreacted Shiff base 5q (entry 7, Table 2 ). On the other hand, using DMF/HClO4 (10 mol %, method B) allowed obtaining the target compound 4q in 88 % yield with no impurities (entry 9, Table 2).
Similarly the reaction of 5-amino-N-(4-fluorophenyl)-1H-pyrazole-4-carboxamide (2a) with 4-nitrobenzaldehyde (1g) and tert-butylisocyanide (3a) in EtOH/H2O with TFA (10 mol %) also led to Shiff base 5o while stirring the starting materials in DMF-HClO4 (10 mol %) for 48 h gave compound 4o (Table 3).
Table 3: Optimization of the reaction conditions for the synthesis of compound 4o.
|
|
|||||
| Entry | Solvent | Time, hours | T, °C | Main product | Yield, (%)a |
|---|---|---|---|---|---|
| 1 | EtOH/H2O (1:1), TFA, (10 mol %) | 72 | 25 | 5b | – |
| 2 | EtOH/H2O (1:1), TFA, (10 mol %) | 2 | 140c | 5b | – |
| 3 | EtOH/H2O (1:1), TFA, (10 mol %) | 72 | 90 | 5b | – |
| 4 | DMF, HClO4, (10 mol %) | 48 | 25 | 4 | 87 |
aThe yields are indicated for compound 4o; bin a mixture with starting materials and undetected impurities; cupon MW irradiation.
It should be noted that the conditions of method B are also suitable for obtaining imidazopyrazoles 4a–m in comparatively high yields; however, the synthesis in EtOH/H2O medium is preferred from the point of view of green chemistry. Thereby, the optimal methodology for obtaining compounds 4a–m is the synthesis according to the method A (H2O/EtOH (1:1), TFA (10 mol %), rt, 24 h) while for compounds 4n–v method B (DMF, HClO4 (10 mol %), rt, 48 h) proved to be superior (Table 1, entries 14–22).
We presume that such difference in the outcome of GBB-3CR depending on the substitution pattern in the carbonyl component is related with the ability of the intermediate Schiff bases to be protonated as well as with their solubility. In case of the presence of electron-withdrawing substituents in the aldehyde the corresponding Schiff bases 5 are less soluble and less basic. DMF increases solubility of imines 5, while the application of strong acid (HClO4) promotes Shiff bases protonation.
Phenylpyruvic acid (1') was also applied as carbonyl component in GBB-3CR to obtain imidazopyrazoles having a carboxylic group. However, the process of decarboxylation took place in the reaction and 1-H-imidazopyrazole carboxamides 4w,x were isolated as the sole reaction products (Table 4).
When replacing tert-butylisocyanide (3a) with ethyl 2-isocyanoacetate (3b), imidazo[1,2-b]pyrazole-7-carboxamides 6a–h were isolated. The condensation proceeded well in TFE with the addition of HClO4 (10 mol %) upon stirring for 24 h (Table 5). On the other hand, condensation of the starting reagents 1d–g with 2a,b and 3b in EtOH/H2O (1:1) with TFA (10 mol %) resulted in the formation of the target products 6 in a mixture with a substantial amount of Schiff bases 5. Moreover, only imines 5 with impurities of the starting compounds were isolated when applying DMF with HClO4.
Table 5: GBB-3CR involving ethyl 2-isocyanoacetate.
|
|
||||||
| Entry | Starting materials | Product | Yield, % | |||
|---|---|---|---|---|---|---|
| Aldehydes | R1 | Aminopyrazoles | R2 | |||
| 1 | 1d | 4-CH3O | 2a | 4-F | 6a | 77 |
| 2 | 1e | 4-Cl | 2a | 4-F | 6b | 95 |
| 3 | 1f | 4-CO2CH3 | 2a | 4-F | 6c | 67 |
| 4 | 1g | 4-NO2 | 2a | 4-F | 6d | 85 |
| 5 | 1d | 4-CH3O | 2b | 3-F | 6e | 78 |
| 6 | 1e | 4-Cl | 2b | 3-F | 6f | 84 |
| 7 | 1f | 4-CO2CH3 | 2b | 3-F | 6g | 82 |
| 8 | 1g | 4-NO2 | 2b | 3-F | 6h | 80 |
Interestingly, in case of ethyl 2-isocyanoacetate (3b) the reaction proceeded equally well regardless of the substituent in the aldehyde. This can be connected with an increased reactivity of ethyl 2-isocyanoacetate (3b) compared to tert-butylisocyanide (3a) due to sterical reasons thus leveling the influence of the solubility factor of imines 5.
As it has been already mentioned above, in contrast to 5-amino-N-aryl-1H-pyrazole-4-carboxamides 2, 3-amino-5-methylisoxazole (7) exhibited only properties of primary amines that allowed using it as an amine component in Ugi-4CR. Particularly, its reaction with aromatic aldehydes 1a–h, phenylpropiolic acid (8) and tert-butylisocyanide (3a) gave peptidomimetics 9 under stirring the starting reagents in MeOH at room temperature for 24 h. In the presence of strong electron-withdrawing substituents as nitro or cyano groups in para-position of the aldehyde, despite the variation of the reaction conditions, only imines 10 were isolated as the major products. In case of 4-cyanobenzaldehyde (1h) we managed to isolate Ugi product 9g in a low yield of 18% (Table 6).
Table 6: Ugi-4CR involving 3-amino-5-methylisoxazole.
|
|
||||
| Entry | Starting materials | Product | Yieldb, % | |
|---|---|---|---|---|
| Aldehydes | R1 | |||
| 1 | 1a | H | 9a | 49 |
| 2 | 1b | 2-CH3O | 9b | 67 |
| 3 | 1c | 3-CH3O | 9c | 51 |
| 4 | 1d | 4-CH3O | 9d | 73 |
| 5 | 1e | 4-Cl | 9e | 43 |
| 6 | 1f | 4-CO2CH3 | 9f | 43 |
| 7 | 1g | 4-NO2 | 10ga | –b |
| 8 | 1h | 4-CN | 9h | 18 |
a9g was not isolated; bthe yields are indicated for compounds 9.
It should be noted that 5-amino-N-aryl-1H-pyrazole-4-carboxamides 2 were also introduced into Ugi-4CR with aromatic aldehydes 1, alkylisocyanides 3 and phenylpropiolic acid 8; however, the acid 8 acted as a catalyst favouring the formation of GBB-3CR-products 4. The attempts to carry out GBB-3CR involving 3-amino-5-methylisoxazole (7) according to the elaborated procedures (Table 1, methods A or B) as well as under other conditions were not successful.
Structure elucidation
The purity and structures of the heterocycles obtained were established by means of mass spectrometry (including HRMS), NMR spectroscopy and X-ray diffraction study.
The 1H NMR spectra of imidazo[1,2-b]pyrazole-7-carboxamides 4 exhibit a broad signal for the NH group in the position 1 at ca. 11.8 ppm, a broad signal for the carboxamide NH at ≈9.5 ppm, a singlet for pyrazole CH in the position 6 at ≈8.2 ppm, a broad signal for the NH group near the position 3 at ≈5.1 ppm, a singlet for tert-butyl CH3 groups at ≈1.0 ppm, resonances for the aromatic protons around 6.9–8.2 ppm as well as signals for other substituents. In case of imidazo[1,2-b]pyrazole-7-carboxamides 4w,x a broad signal for the tert-butyl NH group is shifted upfield to 4.2 ppm and an additional singlet for the benzyl CH2 group is present at 3.9 ppm.
The 1H NMR spectra of imidazo[1,2-b]pyrazole-7-carboxamides 6 exhibit a broad signal for the NH group in the position 1 at ≈11.8 ppm, a broad signal for the carboxamide NH group at ≈9.6 ppm, a singlet for the pyrazole CH in the position 6 at ≈8.2 ppm, a broad signal for the NH group near the position 3 at ≈5.7 ppm, a singlet for the CH2 group in the acetate moiety at ≈4.2 ppm, peaks for the ethoxy group: a quartet for CH2 group at ≈4.0 ppm and a triplet for the CH3 group at ≈1.0 ppm, peaks for the aromatic protons around 6.7–7.7 ppm as well as signals for other substituents.
The 1H NMR spectra of N-(1-arylethyl-2-(tert-butylamino)-2-oxo)-N-(5-methylisoxazol-3-yl)-3-phenylpropiolamides 9 exhibit a broad signal for the amide NH group at ≈7.9 ppm, singlet for the isoxazole CH at ≈6.3 ppm, a singlet for the CH group in the position 1 at ≈6.0 ppm, a singlet for the isoxazole CH3 group at ≈2.3 ppm, a singlet for the tert-butyl CH3 groups at ≈1.2 ppm, peaks for the aromatic protons around 6.8–7.5 ppm as well as signals for other substituents.
As it was found earlier for 2-aminopyrimidines [107-109] GBB-3CR may lead to the formation of two positional isomers A and B (Figure 1).
Figure 1: Alternative structures A and B for compounds 4 and 6.
Figure 1: Alternative structures A and B for compounds 4 and 6.
Experiment with D2O allowed to identify the signals of NH protons while the HSQC spectrum showed the correlations between the signals of protons and corresponding carbon atoms (in the position 6 and in tert-butyl group). The correlations between the signals of NH protons and corresponding carbon atoms (through two and three bonds, Figure 2) allowed final distinguishing the shifts of three NH groups signals in 1H NMR spectra.
Figure 2: Selected data of HSQC and HMBC experiments for compound 4a.
Figure 2: Selected data of HSQC and HMBC experiments for compound 4a.
However, the final assignment of the structure A for heterocycles 4 was made with the help of X-ray analysis (Figure 3).
![[1860-5397-13-104-3]](/bjoc/content/figures/1860-5397-13-104-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Molecular structure of 3-(tert-butylamino)-2-(4-chlorophenyl)-N-(4-fluorophenyl)-1H-imidazo[1,2-b]pyrazole-7-carboxamide (4e) (X-ray diffraction data). Non-hydrogen atoms are presented as thermal ellipsoids with 50% probability.
Figure 3: Molecular structure of 3-(tert-butylamino)-2-(4-chlorophenyl)-N-(4-fluorophenyl)-1H-imidazo[1,2-b]p...
In the case of compounds 9 the presence of NOE between the signals of the methyl group and the CH group in the isoxazole moiety allowed to distinguish closely located signals of two CH groups (Figure 4).
Figure 4: Selected data of NOE and HSQC experiments for compound 9d.
Figure 4: Selected data of NOE and HSQC experiments for compound 9d.
Ultimately, the structure of compounds 9 was proven by an X-ray analysis of compound 9e (Figure 5).
![[1860-5397-13-104-5]](/bjoc/content/figures/1860-5397-13-104-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Molecular structure of N-(2-(tert-butylamino)-1-(4-chlorophenyl)-2-oxoethyl)-N-(5-methylisoxazol-3-yl)-3-phenylpropiolamide (9e) (X-ray diffraction data). Non-hydrogen atoms are presented as thermal ellipsoids with 50% probability.
Figure 5: Molecular structure of N-(2-(tert-butylamino)-1-(4-chlorophenyl)-2-oxoethyl)-N-(5-methylisoxazol-3-...
Antibacterial activity
The antibacterial activity of compounds 4, 6 and 9 (Table 7) was studied (see Experimental part in Supporting Information File 1 for details) against reference bacterial cultures: Bacillus subtilis (strain 1211), Staphylococcus aureus (strain 2231) (Gram-positive) and Escherichia coli (strain 1257), Pseudomonas aeruginosa (strain 1111) (Gram-negative).
Table 7: Antibacterial activity results.
| Entry | Substance |
MICa/MBCb,
mg/L |
Strains of test cultures | |||
|---|---|---|---|---|---|---|
|
Escherichia
coli |
Pseudomonas aeruginosa |
Staphylococcus
aureus |
Bacillus
subtilis |
|||
| 1 | 4a | MIC | 125 | –c | – | – |
| MBC | – | – | – | – | ||
| 2 | 4b | MIC | 500 | – | – | – |
| MBC | – | – | – | – | ||
| 3 | 4c | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 4 | 4d | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 5 | 4e | MIC | 500 | – | – | – |
| MBC | – | – | – | – | ||
| 6 | 4f | MIC | – | – | – | –*d |
| MBC | – | – | – | – | ||
| 7 | 4g | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 8 | 4i | MIC | 500 | – | – | 500 |
| MBC | – | – | – | – | ||
| 9 | 4j | MIC | 250 | – | – | –* |
| MBC | – | – | – | – | ||
| 10 | 4k | MIC | – | – | –* | – |
| MBC | – | – | – | – | ||
| 11 | 4n | MIC | – | – | – | –* |
| MBC | – | – | – | – | ||
| 12 | 4o | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 13 | 4p | MIC | 500 | – | – | – |
| MBC | – | – | – | – | ||
| 14 | 4q | MIC | – | – | – | –* |
| MBC | – | – | – | – | ||
| 15 | 4r | MIC | 250 | – | – | – |
| MBC | – | – | – | – | ||
| 16 | 4s | MIC | 500 | – | – | –* |
| MBC | – | – | – | – | ||
| 17 | 4t | MIC | – | – | – | –* |
| MBC | – | – | – | – | ||
| 18 | 4u | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 19 | 4v | MIC | 500 | 500 | – | – |
| MBC | – | – | – | – | ||
| 20 | 4w | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 21 | 4x | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 22 | 6a | MIC | 500 | – | – | –* |
| MBC | – | – | – | – | ||
| 23 | 6b | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 24 | 6c | MIC | 500 | – | – | – |
| MBC | – | – | – | – | ||
| 25 | 6d | MIC | – | – | – | 500 |
| MBC | – | – | – | – | ||
| 26 | 6e | MIC | – | 500 | – | – |
| MBC | – | – | – | – | ||
| 27 | 6f | MIC | 500 | – | – | – |
| MBC | – | – | – | – | ||
| 28 | 6g | MIC | 125 | – | – | – |
| MBC | – | – | – | – | ||
| 29 | 6h | MIC | 500 | – | 500 | – |
| MBC | – | – | – | – | ||
| 30 | 9a | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 31 | 9b | MIC | – | – | – | –* |
| MBC | – | – | – | – | ||
| 32 | 9c | MIC | – | – | – | –* |
| MBC | – | – | – | – | ||
| 33 | 9d | MIC | – | – | – | –* |
| MBC | – | – | – | – | ||
| 34 | 9e | MIC | – | – | – | – |
| MBC | – | – | – | – | ||
| 35 | 9f | MIC | – | – | – | –* |
| MBC | – | – | – | – | ||
| 36 | Nitroxoline | MIC | 15.6 | 62.5 | 31.25 | 1.9 |
| MBC | 15.6 | 62.5 | 31.25 | 1.9 | ||
aMIC – minimum inhibitory concentration; bMBC – minimum bactericidal concentration; cthe substance in concentration ≤500 mg/L does not inhibit the culture growth; dincrease in biomass compared to control.
As it follows from the results obtained several of the substances studied inhibit the growth of test-microorganisms demonstrating a weak antimicrobial effect (Table 7). Generally, the compounds were found to be less active than nitroxoline (being the reference substance).
The antimicrobial effect of the heterocycles studied is different depending on each bacterical strain; however, some rules can be seen. Only a few substances inhibited the growth of Gram-negative bacteria (strains of E. coli and P. aeruginosa) in an effective way. Particularly, compounds 4a and 6g inhibited the growth of E. coli in concentration 125 mg/L. The bacteriostatic activity against P. aeruginosa of compounds 4v and 6e was fixed only in the highest concentration 500 mg/L. The Gram-positive bacterium S. aureus showed the resistance to almost all the compounds tested in the given concentration range. The strain of B. subtilis was found to be sensitive to compounds 4i and 6d, but the bacteriostatic activity was fixed only in the highest concentration 500 mg/L. The information about the influence of the compounds on bacteria is important from the point of view of choosing the further strategy for the investigations of their biological action. An absence or a low level of antibacterial activity of the heterocycles synthesized is a good prerequisite for carrying out the research on the other promising types of activity, e.g., anticancer, antidiabetic, etc., because in this case a negative influence on a microflora of the organism is decreased [110].
The other interesting feature of most of the compounds was the 30–50% increase in biomass of Gram-positive strains (mainly B. subtilis) compared to control. As it follows from a brief literature overview there are a lot of applications of metabolites (recombinant insulin [111], polyhydroxyalkanoates [112,113] etc. [114-119]) produced by the strains studied. Therefore, the found probiotic effect of the heterocycles has a very promising area for the further application while scaling up the production of biomass with the aim of shortening the time and saving resources [120,121]. Although this is a subject for a future detailed study the results of antibacterial activity allowed outlining the positive tendency.
Conclusion
In summary, the behavior of 5-amino-N-aryl-1H-pyrazole-4-carboxamide and 3-amino-5-methylisoxazole as an amine component in isocyanide-based multicomponent reactions was studied. Particularly, 5-amino-N-aryl-1H-pyrazole-4-carboxamide reacted as 1,3-binucleophile with aromatic aldehydes and alkylisocyanides with the formation of 3-(alkylamino)-N,2-diaryl-1H-imidazo[1,2-b]pyrazole-7-carboxamides (Groebke–Blackburn–Bienaymé reaction). In contrast, 3-amino-5-methylisoxazole acted as a primary amine in Ugi four-component reaction with aromatic aldehydes, phenylpropiolic acid and tert-butylisocyanide giving N-(1-arylethyl-2-(tert-butylamino)-2-oxo)-N-(5-methylisoxazol-3-yl)-3-phenylpropiolamides.
The optimal reaction conditions were different depending on the substituent in the carbonyl component and the structure of the isocyanide. Thus, GBB-3CR involving tert-butylisocyanide in the case of aldehydes with electron-donating substituents was carried out in an EtOH/H2O mixture with TFA (10 mol %) at rt for 24 h and in DMF/HClO4 (10 mol %) at rt for 48 h in case of electron-withdrawing groups. When replacing tert-butylisocyanide with ethyl 2-isocyanoacetate the similar imidazo[1,2-b]pyrazole-7-carboxamides were isolated from the treatment in TFE/HClO4 (10 mol %) at rt for 24 h. Ugi-4CR involving tert-butylisocyanide proceeded under standard conditions in MeOH.
The broad antibacterial activity of the obtained compounds was studied as well. Several of the substances inhibited the growth of test microorganisms demonstrating a weak antimicrobial effect. For most of the stuctures a 30–50% increase in biomass of Gram-positive strains (mainly B. subtilis) compared to control was observed. After a detailed study this effect may be used to stimulate the growth of producers of biologically active compounds.
Acknowledgements
V. Chebanov was supported by a scholarship of KU Leuven (SF/14/006). M. Murlykina was supported by an Erasmus Mundus scholarship. We thank K. Duerinckx and D. Sysoiev for recording some 1H NMR and 13C NMR spectra, B. Demarsin and D. Sysoiev for measuring HRMS and LC–MC spectra, N. Gorobets and D. Sysoiev for the help with discussing the results.
References
-
Navigating the threat of antimicrobial resistance. In Pharmacol. Matters; Gavins, F., Ed.; 2014; Vol. 7 (3), pp 1–26.
Return to citation in text: [1] -
Nordberg, P.; Monnet, L. D.; Cars, O. “A Public Health Approach to Innovation.”. WHO project: Priority Medicines for Europe and the World; 2005; pp 1–40.
Return to citation in text: [1] -
Coates, A.; Hu, Y.; Bax, R.; Page, C. Nat. Rev. Drug Discovery 2002, 1, 895–910. doi:10.1038/nrd940
Return to citation in text: [1] -
Carlet, J.; Jarlier, V.; Harbarth, S.; Voss, A.; Goossens, H.; Pittet, D. Antimicrob. Resist. Infect. Control 2012, 1, No. 11. doi:10.1186/2047-2994-1-11
Return to citation in text: [1] -
Scott, M. G.; Davidson, D. J.; Gold, M. R.; Bowdish, D.; Hancock, R. E. W. J. J. Immunol. 2002, 169, 3883–3891. doi:10.4049/jimmunol.169.7.3883
Return to citation in text: [1] -
Ruijter, E.; Scheffelaar, R.; Orru, R. V. A. Angew. Chem., Int. Ed. 2011, 50, 6234–6246. doi:10.1002/anie.201006515
Return to citation in text: [1] -
Marcaurelle, L. A.; Foley, M. A. Curr. Opin. Chem. Biol. 2010, 14, 285–288. doi:10.1016/j.cbpa.2010.05.001
Return to citation in text: [1] -
Biggs-Houck, J. E.; Younai, A.; Shaw, J. T. Curr. Opin. Chem. Biol. 2010, 14, 371–382. doi:10.1016/j.cbpa.2010.03.003
Return to citation in text: [1] -
Ganem, B. Acc. Chem. Res. 2009, 42, 463–472. doi:10.1021/ar800214s
Return to citation in text: [1] -
Sunderhaus, J. D.; Martin, S. F. Chem. – Eur. J. 2009, 15, 1300–1308. doi:10.1002/chem.200802140
Return to citation in text: [1] -
Touré, B. B.; Hall, D. G. Chem. Rev. 2009, 109, 4439–4486. doi:10.1021/cr800296p
Return to citation in text: [1] -
Isambert, N.; Lavilla, R. Chem. – Eur. J. 2008, 14, 8444–8454. doi:10.1002/chem.200800473
Return to citation in text: [1] -
Mironov, M. A. QSAR Comb. Sci. 2006, 25, 423–431. doi:10.1002/qsar.200540190
Return to citation in text: [1] -
Chebanov, V. A.; Desenko, S. M. Diversity-Oriented Synth.; 2014; Vol. 1, pp 43–63. doi:10.2478/dos-2014-0003
Return to citation in text: [1] [2] -
Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U
Return to citation in text: [1] -
Banfi, L.; Basso, A.; Riva, R. In Synthesis of Heterocycles via Multicomponent Reactions I; Orru, R. V. A.; Ruijter, E., Eds.; Springer-Verlag : Berlin Heidelberg, 2010; Vol. 23, pp 1–39. doi:10.1007/7081_2009_23
Return to citation in text: [1] -
Zhu, J.; Wang, Q.; Wang, M.-X., Eds. Multicomponent reactions in organic chemistry; Wiley-VCH, 2015.
Return to citation in text: [1] -
Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem. – Eur. J. 2000, 6, 3321–3329. doi:10.1002/1521-3765(20000915)6:18<3321::AID-CHEM3321>3.0.CO;2-A
Return to citation in text: [1] -
Dolle, R. E.; Le Bourdonnec, B.; Goodman, A. J.; Morales, G. A.; Thomas, C. J.; Zhang, W. J. Comb. Chem. 2008, 10, 753–802. doi:10.1021/cc800119z
Return to citation in text: [1] -
Zarganes-Tzitzikas, T.; Chandgude, A. L.; Dömling, A. Chem. Rec. 2015, 15, 981–996. doi:10.1002/tcr.201500201
Return to citation in text: [1] [2] -
Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958–2975. doi:10.1039/c4gc00013g
Return to citation in text: [1] -
Shaaban, S.; Abdel-Wahab, B. F. Mol. Diversity 2016, 20, 233–254. doi:10.1007/s11030-015-9602-6
Return to citation in text: [1] -
Váradi, A.; Palmer, T. C.; Dardashti, R. N.; Majumdar, S. Molecules 2016, 21, 19. doi:10.3390/molecules21010019
Return to citation in text: [1] -
Sadjadi, S.; Nahavandi, F.; Heravi, M. J. Iran. Chem. Soc. 2015, 12, 1049–1052. doi:10.1007/s13738-014-0564-x
Return to citation in text: [1] -
Chebanov, V. A.; Desenko, S. M.; Gurley, T. W. Azaheterocycles Based on a,b-Unsaturated Carbonyls; Springer-Verlag: Berlin Heidelberg, 2008.
Return to citation in text: [1] [2] -
Kaur, T.; Wadhwa, P.; Bagchi, S.; Sharma, A. Chem. Commun. 2016, 52, 6958–6976. doi:10.1039/C6CC01562J
Return to citation in text: [1] -
Devi, N.; Rawal, R. K.; Singh, V. Tetrahedron 2015, 71, 183–232. doi:10.1016/j.tet.2014.10.032
Return to citation in text: [1] [2] -
Bruno, O.; Brullo, C.; Bondavalli, F.; Ranise, A.; Schenone, S.; Falzarano, M. S.; Varani, K.; Spisani, S. Bioorg. Med. Chem. Lett. 2007, 17, 3696–3701. doi:10.1016/j.bmcl.2007.04.036
Return to citation in text: [1] [2] -
Baviskar, A. T.; Madaan, C.; Preet, R.; Mohapatra, P.; Jain, V.; Agarwal, A.; Guchhait, S. K.; Kundu, C. N.; Banerjee, U. C.; Bharatam, P. V. J. Med. Chem. 2011, 54, 5013–5030. doi:10.1021/jm200235u
Return to citation in text: [1] [2] [3] -
Maul, C.; Sundermann, B.; Hennies, H.; Schneider, J.; Gerlach, M. Tert-butyl-(7-methylimidazo[1,2-a]pyridin-3-yl)-amine-derivative. WO PCT Patent Application WO/2001/027109, April 19, 2001.
Return to citation in text: [1] [2] -
Starrett, J. E., Jr.; Montzka, T. A.; Crosswell, A. R.; Cavanagh, R. L. J. Med. Chem. 1989, 32, 2204–2210. doi:10.1021/jm00129a028
Return to citation in text: [1] [2] -
Palmer, A. M.; Chrismann, S.; Münch, G.; Brehm, C.; Zimmermann, P. J.; Buhr, W.; Senn-Bilfinger, J.; Feth, M. P.; Simon, W. A. Bioorg. Med. Chem. 2009, 17, 368–384. doi:10.1016/j.bmc.2008.10.055
Return to citation in text: [1] [2] -
Okubo, T.; Yoshikawa, R.; Chaki, S.; Okuyama, S.; Nakazato, A. Bioorg. Med. Chem. 2004, 12, 423–438. doi:10.1016/j.bmc.2003.10.050
Return to citation in text: [1] -
Mizushige, K.; Ueda, T.; Yukiiri, K.; Suzuki, H. Cardiovasc. Drug Rev. 2002, 20, 163–174. doi:10.1111/j.1527-3466.2002.tb00085.x
Return to citation in text: [1] [2] -
Mori, H.; Tanaka, M.; Kayasuga, R.; Masuda, T.; Ochi, Y.; Yamada, H.; Kishikawa, K.; Ito, M.; Nakamura, T. Bone 2008, 43, 840–848. doi:10.1016/j.bone.2008.07.242
Return to citation in text: [1] [2] -
Tominaga, M.; Yang, Y.-H.; Nakagawa, K.; Ogawa, H. Carbostyrill compounds, compositions containing same and processes for preparing same. Eur. Pat. Appl. EP52016 (A1), May 19, 1981.
Return to citation in text: [1] [2] -
Gueiffier, A.; Mavel, S.; Lhassani, M.; Elhakmaoui, A.; Snoeck, R.; Andrei, G.; Chavignon, O.; Teulade, J.-C.; Witvrouw, M.; Balzarini, J.; De Clercq, E.; Chapat, J. P. J. Med. Chem. 1998, 41, 5108–5112. doi:10.1021/jm981051y
Return to citation in text: [1] [2] -
Defosse, G.; Le Ber, P.; Saarmets, A.; Wick, A. New bicyclic imidazole ketone derivs. FR Patent FR2699919 (A1), July 1, 1994.
Return to citation in text: [1] [2] -
Elleder, D.; Young, J. A. T.; Baiga, T. J.; Noel, J. P. Non-nucleoside reverse transcriptase inhibitors. WO PCT Pat. Appl. WO2009/061856, May 14, 2009.
Return to citation in text: [1] [2] [3] -
Véron, J.-B.; Allouchi, H.; Enguehard-Gueiffier, C.; Snoeck, R.; Andrei, G.; De Clercq, E.; Gueiffier, A. Bioorg. Med. Chem. 2008, 16, 9536–9545. doi:10.1016/j.bmc.2008.09.027
Return to citation in text: [1] [2] -
Gudmundsson, K. S.; Johns, B. A. Bioorg. Med. Chem. Lett. 2007, 17, 2735–2739. doi:10.1016/j.bmcl.2007.02.079
Return to citation in text: [1] [2] -
Al-Tel, T. H.; Al-Qawasmeh, R. A.; Zaarour, R. Eur. J. Med. Chem. 2011, 46, 1874–1881. doi:10.1016/j.ejmech.2011.02.051
Return to citation in text: [1] -
Terada, A.; Wachi, K.; Miyazawa, H.; Iizuka, Y.; Hasegawa, K.; Tabata, K. Use of imidazopyrazole derivatives as analgesics and anti-inflammatory agents. U.S. Patent 5,232,939, Aug 3, 1993.
Return to citation in text: [1] [2] -
Frey, B.; Hufton, R.; Harding, M.; Draffan, A. G. Compounds for the treatment of HCV. WO PCT Pat. Appl. WO 2013/036994 A1, March 21, 2013.
Return to citation in text: [1] [2] -
Mascitti, V.; McClure, K. F.; Munchhof, M. J.; Robinson,, R. P. Imidazo-pyrazoles as GPR119 inhibitors. WO PCT Pat. Appl. WO2011/061679, May 26, 2011.
Return to citation in text: [1] [2] -
Zhang, J.; Singh, R.; Goff, D.; Kinoshita, T. Small molecule inhibitors of spleen tyrosine kinase (SYK). U.S. Pat. Appl. 20100316649 A1, Dec 16, 2010.
Return to citation in text: [1] [2] -
Ennis, H. L.; Möller, L.; Wang, J. J.; Selawry, O. S. Biochem. Pharmacol. 1971, 20, 2639–2646. doi:10.1016/0006-2952(71)90173-0
Return to citation in text: [1] [2] -
Oku, T.; Kawai, Y.; Marusawa, H.; Yamazaki, H.; Abe, Y.; Tanaka, H. E. 3-(Heteroaryl)-pyrazololi[1,5-a]pyrimidines. U.S. Patent 5,356,897, Oct 18, 1994.
Return to citation in text: [1] [2] -
Kuehnert, S.; Oberboersch, S.; Sundermann, C.; Haurand, M.; Jostock,, R.; Schiene, K.; Tzschentke, T.; Christoph, T.; Kaulartz, D. Substituted bicyclic imidazo-3-ylamin compounds. WO PCT Pat. Appl. WO2006/029980 A1, March 23, 2006.
Return to citation in text: [1] -
Berset, C.; Audetat, S.; Tietz, J.; Gunde, T.; Barberis, A.; Schumacher, A.; Traxler, P. Protein kinase inhibitors. WO PCT Pat. Appl. WO2005/120513 A1, Dec 22, 2005.
Return to citation in text: [1] -
Goldfarb, D. S. Method for altering the lifespan of eukaryotic organisms. U.S. Pat. Appl. 2009/0163545 A1, 2009.
Return to citation in text: [1] -
Van Niel, M. B.; Miah, A. Substituted imidazo[1,2-a]pyridines and their use as agonists at GABA-A receptors for treating or preventing neurological or psychlatric disorders. UK Pat. Appl. GB2448808 (A), Oct 29, 2008.
Return to citation in text: [1] [2] -
Ugi, I. Angew. Chem., Int. Ed. Engl. 1982, 21, 810–819. doi:10.1002/anie.198208101
Return to citation in text: [1] -
Basso, A.; Banfi, L.; Riva, R.; Guanti, G. J. Org. Chem. 2005, 70, 575–579. doi:10.1021/jo048389m
Return to citation in text: [1] -
Domling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728
Return to citation in text: [1] -
Hulme, C.; Gore, V. Curr. Med. Chem. 2003, 10, 51–80. doi:10.2174/0929867033368600
Return to citation in text: [1] -
Akritopoulou-Zanze, I. Curr. Opin. Chem. Biol. 2008, 12, 324–331. doi:10.1016/j.cbpa.2008.02.004
Return to citation in text: [1] -
Koopmanschap, G.; Ruijter, E.; Orru, R. V. A. Beilstein J. Org. Chem. 2014, 10, 544–598. doi:10.3762/bjoc.10.50
Return to citation in text: [1] -
Chebanov, V. A.; Gura, K. A.; Desenko, S. M. In Synthesis of Heterocycles via Multicomponent Reactions I; Orru, R. V. A.; Ruijter, E., Eds.; Springer: Berlin Heidelberg, 2010; Vol. 23, pp 41–84. doi:10.1007/7081_2009_21
Return to citation in text: [1] [2] -
Soural, M.; Bouillon, I.; Krchňák, V. J. Comb. Chem. 2008, 10, 923–933. doi:10.1021/cc8001074
Return to citation in text: [1] -
Maiti, B.; Chanda, K.; Selvaraju, M.; Tseng, C.-C.; Sun, C.-M. ACS Comb. Sci. 2013, 15, 291–297. doi:10.1021/co400010y
Return to citation in text: [1] -
Yugandhar, D.; Srivastava, A. K. ACS Comb. Sci. 2015, 17, 474–481. doi:10.1021/acscombsci.5b00065
Return to citation in text: [1] -
Vachhani, D. D.; Galli, M.; Jacobs, J.; Van Meervelt, L.; Van der Eycken, E. V. Chem. Commun. 2013, 49, 7171–7173. doi:10.1039/c3cc43418d
Return to citation in text: [1] -
Moni, L.; Deniβen, M.; Valentini, G.; Müller, T. J. J.; Riva, R. Chem. – Eur. J. 2015, 21 , 753–762. doi:10.1002/chem.201404209
Return to citation in text: [1] -
Vachhani, D. D.; Kumar, A.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van Der Eycken, E. V. Eur. J. Org. Chem. 2013, 1223–1227. doi:10.1002/ejoc.201201587
Return to citation in text: [1] -
Kumar, A.; Vachhani, D. D.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van Der Eycken, E. V. Synthesis 2013, 45, 2571–2582. doi:10.1055/s-0033-1339474
Return to citation in text: [1] -
Balalaie, S.; Motaghedi, H.; Bararjanian, M.; Tahmassebi, D.; Bijanzadeh, H. R. Tetrahedron 2011, 67, 9134–9141. doi:10.1016/j.tet.2011.09.089
Return to citation in text: [1] -
Welsch, S. J.; Umkehrer, M.; Ross, G.; Kolb, J.; Burdack, C.; Wessjohann, L. A. Tetrahedron Lett. 2011, 52, 6295–6297. doi:10.1016/j.tetlet.2011.09.094
Return to citation in text: [1] -
Ambasana, P. A.; Vachhani, D. D.; Galli, M.; Jacobs, J.; Van Meervelt, L.; Shah, A. K.; Van Der Eycken, E. V. Org. Biomol. Chem. 2014, 12, 8861–8865. doi:10.1039/C4OB01644K
Return to citation in text: [1] -
Ghabraie, E.; Balalaie, S.; Mehrparvar, S.; Rominger, F. J. Org. Chem. 2014, 79, 7926–7934. doi:10.1021/jo5010422
Return to citation in text: [1] -
Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. Engl. 1998, 37, 2234–2237. doi:10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Che, C.; Xiang, J.; Wang, G.-X.; Fathi, R.; Quan, J.-M.; Yang, Z. J. Comb. Chem. 2007, 9, 982–989. doi:10.1021/cc070058a
Return to citation in text: [1] [2] [3] [4] -
Aouali, M.; Mhalla, D.; Allouche, F.; El Kaim, L.; Tounsi, S.; Trigui, M.; Chabchoub, F. Med. Chem. Res. 2015, 24, 2732–2741. doi:10.1007/s00044-015-1322-z
Return to citation in text: [1] [2] -
Huang, Y.; Hu, X.-Q.; Shen, D.-P.; Chen, Y.-F.; Xu, P.-F. Mol. Diversity 2007, 11, 73–80. doi:10.1007/s11030-007-9059-3
Return to citation in text: [1] [2] -
Parchinsky, V. Z.; Koleda, V. V.; Shuvalova, O.; Kravchenko, D. V.; Krasavin, M. Tetrahedron Lett. 2006, 47, 6891–6894. doi:10.1016/j.tetlet.2006.07.037
Return to citation in text: [1] [2] -
Akritopoulou-Zanze, I.; Wakefield, B. D.; Gasiecki, A.; Kalvin, D.; Johnson, E. F.; Kovar, P.; Djuric, S. W. Bioorg. Med. Chem. Lett. 2011, 21, 1480–1483. doi:10.1016/j.bmcl.2011.01.001
Return to citation in text: [1] -
Hieke, M.; Rödl, C. B.; Wisniewska, J. M.; La Buscató, E.; Stark, H.; Schubert-Zsilavecz, M.; Steinhilber, D.; Hofmann, B.; Proschak, E. Bioorg. Med. Chem. Lett. 2012, 22, 1969–1975. doi:10.1016/j.bmcl.2012.01.038
Return to citation in text: [1] -
Al-Tel, T. H.; Al-Qawasmeh, R. A.; Voelter, W. Eur. J. Org. Chem. 2010, 5586–5593. doi:10.1002/ejoc.201000808
Return to citation in text: [1] -
Vidyacharan, S.; Shinde, A. H.; Satpathi, B.; Sharada, D. S. Green Chem. 2014, 16, 1168–1175. doi:10.1039/c3gc42130a
Return to citation in text: [1] -
Burchak, O. N.; Mugherli, L.; Ostuni, M.; Lacapre, J. J.; Balakirev, M. Y. J. Am. Chem. Soc. 2011, 133, 10058–10061. doi:10.1021/ja204016e
Return to citation in text: [1] -
Guchhait, S. K.; Madaan, C. Org. Biomol. Chem. 2010, 8, 3631–3634. doi:10.1039/c0ob00022a
Return to citation in text: [1] -
Hatamjafari, F.; Javad, M.; Mohtasham, M.; Chakoli, F. A. Orient. J. Chem. 2012, 28, 1271–1273. doi:10.13005/ojc/280323
Return to citation in text: [1] -
Guchhait, S. K.; Madaan, C. Synlett 2009, 628–632. doi:10.1055/s-0028-1087915
Return to citation in text: [1] [2] [3] -
Guchhait, S. K.; Madaan, C.; Thakkar, B. S. Synthesis 2009, 3293–3300. doi:10.1055/s-0029-1216916
Return to citation in text: [1] [2] [3] -
Rostamnia, S.; Lamei, K.; Mohammadquli, M.; Sheykhan, M.; Heydari, A. Tetrahedron Lett. 2012, 53, 5257–5260. doi:10.1016/j.tetlet.2012.07.075
Return to citation in text: [1] -
Adib, M.; Mahdavi, M.; Noghani, M. A.; Mirzaei, P. Tetrahedron Lett. 2007, 48, 7263–7265. doi:10.1016/j.tetlet.2007.08.049
Return to citation in text: [1] -
Tsirulnikov, S.; Kysil, V.; Ivachtchenko, A.; Krasavin, M. Synth. Commun. 2009, 40, 111–119. doi:10.1080/00397910902953331
Return to citation in text: [1] -
Mouradzadegun, A.; Ma’mani, L.; Mahdavi, M.; Rashid, Z.; Shafiee, A.; Foroumadi, A.; Dianat, S. RSC Adv. 2015, 5, 83530–83537. doi:10.1039/C5RA12307K
Return to citation in text: [1] -
Krasavin, M.; Tsirulnikov, S.; Nikulnikov, M.; Kysil, V.; Ivachtchenko, A. Tetrahedron Lett. 2008, 49, 5241–5243. doi:10.1016/j.tetlet.2008.06.113
Return to citation in text: [1] -
Wadhwa, P.; Kaur, T.; Sharma, A. RSC Adv. 2015, 5, 44353–44360. doi:10.1039/C5RA06747B
Return to citation in text: [1] -
Lee, C.-H.; Hsu, W.-S.; Chen, C.-H.; Sun, C.-M. Eur. J. Org. Chem. 2013, 2201–2208. doi:10.1002/ejoc.201201645
Return to citation in text: [1] -
Pereshivko, O. P.; Peshkov, V. A.; Ermolat’ev, D. S.; Van Der Eycken, E. V. Synlett 2013, 24, 351–354. doi:10.1055/s-0032-1317986
Return to citation in text: [1] -
Kysil, V.; Khvat, A.; Tsirulnikov, S.; Tkachenko, S.; Williams, C.; Churakova, M.; Ivachtchenko, A. Eur. J. Org. Chem. 2010, 1525–1543. doi:10.1002/ejoc.200901360
Return to citation in text: [1] -
Rahmati, A.; Kouzehrash, M. A. Synthesis 2011, 2913–2920. doi:10.1055/s-0030-1260154
Return to citation in text: [1] -
Rahmati, A.; Eskandari-Vashareh, M.; Alizadeh-Kouzehrash, M. Tetrahedron 2013, 69, 4199–4204. doi:10.1016/j.tet.2013.03.103
Return to citation in text: [1] -
Demjén, A.; Gyuris, M.; Wölfling, J.; Puskás, L. G.; Kanizsai, I. Beilstein J. Org. Chem. 2014, 10, 2338–2344. doi:10.3762/bjoc.10.243
Return to citation in text: [1] -
Sakhno, Y. I.; Shishkina, S. V.; Shishkin, O. V.; Musatov, V. I.; Vashchenko, E. V.; Desenko, S. M.; Chebanov, V. A. Mol. Diversity 2010, 14, 523–531. doi:10.1007/s11030-010-9226-9
Return to citation in text: [1] -
Chebanov, V. A.; Saraev, V. E.; Shishkina, S. V.; Shishkin, O. V.; Musatov, V. I.; Desenko, S. M. Eur. J. Org. Chem. 2012, 5515–5524. doi:10.1002/ejoc.201200669
Return to citation in text: [1] -
Chebanov, V. A.; Sakhno, Y. I.; Desenko, S. M.; Chernenko, V. N.; Musatov, V. I.; Shishkina, S. V.; Shishkin, O. V.; Kappe, C. O. Tetrahedron 2007, 63, 1229–1242. doi:10.1016/j.tet.2006.11.048
Return to citation in text: [1] -
Chebanov, V. A.; Desenko, S. M. Chem. Heterocycl. Compd. 2012, 48, 566–583. doi:10.1007/s10593-012-1030-2
Return to citation in text: [1] -
Muravyova, E. A.; Desenko, S. M.; Rudenko, R. V.; Shishkina, S. V.; Shishkin, O. V.; Sen’ko, Y. V.; Vashchenko, E. V.; Chebanov, V. A. Tetrahedron 2011, 67, 9389–9400. doi:10.1016/j.tet.2011.09.138
Return to citation in text: [1] -
Morozova, A. D.; Muravyova, E. A.; Shishkina, S. V.; Vashchenko, E. V.; Sen’ko, Y. V.; Chebanov, V. A. J. Heterocycl. Chem. 2017, 54, 932–943. doi:10.1002/jhet.2656
Return to citation in text: [1] -
Ryabukhin, S. V.; Panov, D. M.; Plaskon, A. S.; Grygorenko, O. O. ACS Comb. Sci. 2012, 14, 631–635. doi:10.1021/co300082t
Return to citation in text: [1] -
Rajanarendar, E.; Reddy, M. N.; Raju, S. Indian J. Chem. 2011, 50B, 751–755.
Return to citation in text: [1] -
Shafiee, M.; Khosropour, A. R.; Mohammadpoor-Baltork, I.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Tetrahedron Lett. 2012, 53, 3086–3090. doi:10.1016/j.tetlet.2012.04.037
Return to citation in text: [1] -
Rajanarendar, E.; Murthy, K. R.; Reddy, M. N. Indian J. Chem. 2011, 50B, 926–930.
Return to citation in text: [1] -
Mandair, G. S.; Light, M.; Russell, A.; Hursthouse, M.; Bradley, M. Tetrahedron Lett. 2002, 43, 4267–4269. doi:10.1016/S0040-4039(02)00709-8
Return to citation in text: [1] -
Parchinsky, V. Z.; Shuvalova, O.; Ushakova, O.; Kravchenko, D. V.; Krasavin, M. Tetrahedron Lett. 2006, 47, 947–951. doi:10.1016/j.tetlet.2005.11.152
Return to citation in text: [1] -
Thompson, M. J.; Hurst, J. M.; Chen, B. Synlett 2008, 3183–3187. doi:10.1055/s-0028-1087274
Return to citation in text: [1] -
Albert, A. Selective Toxicity: the physico-chemical basis of therapy; Springer: Netherlands, 1985. doi:10.1007/978-94-009-4846-4
Return to citation in text: [1] -
Baeshen, N. A.; Baeshen, M. N.; Sheikh, A.; Bora, R. S.; Ahmed, M. M. M.; Ramadan, H. A. I.; Saini, K. S.; Redwan, E. M. Microb. Cell Fact. 2014, 13, No. 141. doi:10.1186/s12934-014-0141-0
Return to citation in text: [1] -
Gomaa, E. Z. Braz. Arch. Biol. Technol. 2014, 57, 145–154. doi:10.1590/S1516-89132014000100020
Return to citation in text: [1] -
Wang, Y.; Ruan, L.; Lo, W.-H.; Chua, H.; Yu, H.-F. Appl. Biochem. Biotechnol. 2006, 132, 1015–1022. doi:10.1385/ABAB:132:1:1015
Return to citation in text: [1] -
Kamionka, M. Curr. Pharm. Biotechnol. 2011, 12, 268–274. doi:10.2174/138920111794295693
Return to citation in text: [1] -
Sánchez Blancoa, A.; Palacios Durivea, O.; Batista Péreza, S.; Díaz Montesa, Z.; Pérez Guerra, N. Braz. J. Microbiol. 2016, 47, 665–674. doi:10.1016/j.bjm.2016.04.019
Return to citation in text: [1] -
Oyeleke, S. B.; Oyewole, O. A.; Egwim, E. C. Adv. Life Sci. 2011, 1, 49–53. doi:10.5923/j.als.20110102.09
Return to citation in text: [1] -
Markkanen,, P. H.; Bailey, M. J. J. Appl. Chem. Biotechnol. 1974, 24, 93–103. doi:10.1002/jctb.5020240111
Return to citation in text: [1] -
López-Valdez, F.; Fernández-Luqueño, F.; Ceballos-Ramírez, J. M.; Marsch, R.; Olalde-Portugal, V.; Dendooven, L. Sci. Hortic. (Amsterdam, Neth.) 2011, 128, 499–505. doi:10.1016/j.scienta.2011.02.006
Return to citation in text: [1] -
Qiao, J.-Q.; Wu, H.-J.; Huo, R.; Gao, X.-W.; Borriss, R. Chem. Biol. Technol. Agric. 2014, 1, No. 12. doi:10.1186/s40538-014-0012-2
Return to citation in text: [1] -
Lee, S. Y.; Kim, H. U. Nat. Biotechnol. 2015, 33, 1061–1072. doi:10.1038/nbt.3365
Return to citation in text: [1] -
Stewart, E. J. J. Bacteriol. 2012, 194, 4151–4160. doi:10.1128/JB.00345-12
Return to citation in text: [1]
| 29. | Baviskar, A. T.; Madaan, C.; Preet, R.; Mohapatra, P.; Jain, V.; Agarwal, A.; Guchhait, S. K.; Kundu, C. N.; Banerjee, U. C.; Bharatam, P. V. J. Med. Chem. 2011, 54, 5013–5030. doi:10.1021/jm200235u |
| 71. | Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. Engl. 1998, 37, 2234–2237. doi:10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R |
| 83. | Guchhait, S. K.; Madaan, C. Synlett 2009, 628–632. doi:10.1055/s-0028-1087915 |
| 84. | Guchhait, S. K.; Madaan, C.; Thakkar, B. S. Synthesis 2009, 3293–3300. doi:10.1055/s-0029-1216916 |
| 94. | Rahmati, A.; Kouzehrash, M. A. Synthesis 2011, 2913–2920. doi:10.1055/s-0030-1260154 |
| 95. | Rahmati, A.; Eskandari-Vashareh, M.; Alizadeh-Kouzehrash, M. Tetrahedron 2013, 69, 4199–4204. doi:10.1016/j.tet.2013.03.103 |
| 96. | Demjén, A.; Gyuris, M.; Wölfling, J.; Puskás, L. G.; Kanizsai, I. Beilstein J. Org. Chem. 2014, 10, 2338–2344. doi:10.3762/bjoc.10.243 |
| 14. | Chebanov, V. A.; Desenko, S. M. Diversity-Oriented Synth.; 2014; Vol. 1, pp 43–63. doi:10.2478/dos-2014-0003 |
| 25. | Chebanov, V. A.; Desenko, S. M.; Gurley, T. W. Azaheterocycles Based on a,b-Unsaturated Carbonyls; Springer-Verlag: Berlin Heidelberg, 2008. |
| 59. | Chebanov, V. A.; Gura, K. A.; Desenko, S. M. In Synthesis of Heterocycles via Multicomponent Reactions I; Orru, R. V. A.; Ruijter, E., Eds.; Springer: Berlin Heidelberg, 2010; Vol. 23, pp 41–84. doi:10.1007/7081_2009_21 |
| 97. | Sakhno, Y. I.; Shishkina, S. V.; Shishkin, O. V.; Musatov, V. I.; Vashchenko, E. V.; Desenko, S. M.; Chebanov, V. A. Mol. Diversity 2010, 14, 523–531. doi:10.1007/s11030-010-9226-9 |
| 98. | Chebanov, V. A.; Saraev, V. E.; Shishkina, S. V.; Shishkin, O. V.; Musatov, V. I.; Desenko, S. M. Eur. J. Org. Chem. 2012, 5515–5524. doi:10.1002/ejoc.201200669 |
| 99. | Chebanov, V. A.; Sakhno, Y. I.; Desenko, S. M.; Chernenko, V. N.; Musatov, V. I.; Shishkina, S. V.; Shishkin, O. V.; Kappe, C. O. Tetrahedron 2007, 63, 1229–1242. doi:10.1016/j.tet.2006.11.048 |
| 100. | Chebanov, V. A.; Desenko, S. M. Chem. Heterocycl. Compd. 2012, 48, 566–583. doi:10.1007/s10593-012-1030-2 |
| 101. | Muravyova, E. A.; Desenko, S. M.; Rudenko, R. V.; Shishkina, S. V.; Shishkin, O. V.; Sen’ko, Y. V.; Vashchenko, E. V.; Chebanov, V. A. Tetrahedron 2011, 67, 9389–9400. doi:10.1016/j.tet.2011.09.138 |
| 71. | Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. Engl. 1998, 37, 2234–2237. doi:10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R |
| 72. | Che, C.; Xiang, J.; Wang, G.-X.; Fathi, R.; Quan, J.-M.; Yang, Z. J. Comb. Chem. 2007, 9, 982–989. doi:10.1021/cc070058a |
| 73. | Aouali, M.; Mhalla, D.; Allouche, F.; El Kaim, L.; Tounsi, S.; Trigui, M.; Chabchoub, F. Med. Chem. Res. 2015, 24, 2732–2741. doi:10.1007/s00044-015-1322-z |
| 74. | Huang, Y.; Hu, X.-Q.; Shen, D.-P.; Chen, Y.-F.; Xu, P.-F. Mol. Diversity 2007, 11, 73–80. doi:10.1007/s11030-007-9059-3 |
| 75. | Parchinsky, V. Z.; Koleda, V. V.; Shuvalova, O.; Kravchenko, D. V.; Krasavin, M. Tetrahedron Lett. 2006, 47, 6891–6894. doi:10.1016/j.tetlet.2006.07.037 |
| 1. | Navigating the threat of antimicrobial resistance. In Pharmacol. Matters; Gavins, F., Ed.; 2014; Vol. 7 (3), pp 1–26. |
| 2. | Nordberg, P.; Monnet, L. D.; Cars, O. “A Public Health Approach to Innovation.”. WHO project: Priority Medicines for Europe and the World; 2005; pp 1–40. |
| 3. | Coates, A.; Hu, Y.; Bax, R.; Page, C. Nat. Rev. Drug Discovery 2002, 1, 895–910. doi:10.1038/nrd940 |
| 4. | Carlet, J.; Jarlier, V.; Harbarth, S.; Voss, A.; Goossens, H.; Pittet, D. Antimicrob. Resist. Infect. Control 2012, 1, No. 11. doi:10.1186/2047-2994-1-11 |
| 5. | Scott, M. G.; Davidson, D. J.; Gold, M. R.; Bowdish, D.; Hancock, R. E. W. J. J. Immunol. 2002, 169, 3883–3891. doi:10.4049/jimmunol.169.7.3883 |
| 27. | Devi, N.; Rawal, R. K.; Singh, V. Tetrahedron 2015, 71, 183–232. doi:10.1016/j.tet.2014.10.032 |
| 53. | Ugi, I. Angew. Chem., Int. Ed. Engl. 1982, 21, 810–819. doi:10.1002/anie.198208101 |
| 120. | Lee, S. Y.; Kim, H. U. Nat. Biotechnol. 2015, 33, 1061–1072. doi:10.1038/nbt.3365 |
| 121. | Stewart, E. J. J. Bacteriol. 2012, 194, 4151–4160. doi:10.1128/JB.00345-12 |
| 27. | Devi, N.; Rawal, R. K.; Singh, V. Tetrahedron 2015, 71, 183–232. doi:10.1016/j.tet.2014.10.032 |
| 28. | Bruno, O.; Brullo, C.; Bondavalli, F.; Ranise, A.; Schenone, S.; Falzarano, M. S.; Varani, K.; Spisani, S. Bioorg. Med. Chem. Lett. 2007, 17, 3696–3701. doi:10.1016/j.bmcl.2007.04.036 |
| 29. | Baviskar, A. T.; Madaan, C.; Preet, R.; Mohapatra, P.; Jain, V.; Agarwal, A.; Guchhait, S. K.; Kundu, C. N.; Banerjee, U. C.; Bharatam, P. V. J. Med. Chem. 2011, 54, 5013–5030. doi:10.1021/jm200235u |
| 30. | Maul, C.; Sundermann, B.; Hennies, H.; Schneider, J.; Gerlach, M. Tert-butyl-(7-methylimidazo[1,2-a]pyridin-3-yl)-amine-derivative. WO PCT Patent Application WO/2001/027109, April 19, 2001. |
| 31. | Starrett, J. E., Jr.; Montzka, T. A.; Crosswell, A. R.; Cavanagh, R. L. J. Med. Chem. 1989, 32, 2204–2210. doi:10.1021/jm00129a028 |
| 32. | Palmer, A. M.; Chrismann, S.; Münch, G.; Brehm, C.; Zimmermann, P. J.; Buhr, W.; Senn-Bilfinger, J.; Feth, M. P.; Simon, W. A. Bioorg. Med. Chem. 2009, 17, 368–384. doi:10.1016/j.bmc.2008.10.055 |
| 33. | Okubo, T.; Yoshikawa, R.; Chaki, S.; Okuyama, S.; Nakazato, A. Bioorg. Med. Chem. 2004, 12, 423–438. doi:10.1016/j.bmc.2003.10.050 |
| 34. | Mizushige, K.; Ueda, T.; Yukiiri, K.; Suzuki, H. Cardiovasc. Drug Rev. 2002, 20, 163–174. doi:10.1111/j.1527-3466.2002.tb00085.x |
| 35. | Mori, H.; Tanaka, M.; Kayasuga, R.; Masuda, T.; Ochi, Y.; Yamada, H.; Kishikawa, K.; Ito, M.; Nakamura, T. Bone 2008, 43, 840–848. doi:10.1016/j.bone.2008.07.242 |
| 36. | Tominaga, M.; Yang, Y.-H.; Nakagawa, K.; Ogawa, H. Carbostyrill compounds, compositions containing same and processes for preparing same. Eur. Pat. Appl. EP52016 (A1), May 19, 1981. |
| 37. | Gueiffier, A.; Mavel, S.; Lhassani, M.; Elhakmaoui, A.; Snoeck, R.; Andrei, G.; Chavignon, O.; Teulade, J.-C.; Witvrouw, M.; Balzarini, J.; De Clercq, E.; Chapat, J. P. J. Med. Chem. 1998, 41, 5108–5112. doi:10.1021/jm981051y |
| 38. | Defosse, G.; Le Ber, P.; Saarmets, A.; Wick, A. New bicyclic imidazole ketone derivs. FR Patent FR2699919 (A1), July 1, 1994. |
| 39. | Elleder, D.; Young, J. A. T.; Baiga, T. J.; Noel, J. P. Non-nucleoside reverse transcriptase inhibitors. WO PCT Pat. Appl. WO2009/061856, May 14, 2009. |
| 40. | Véron, J.-B.; Allouchi, H.; Enguehard-Gueiffier, C.; Snoeck, R.; Andrei, G.; De Clercq, E.; Gueiffier, A. Bioorg. Med. Chem. 2008, 16, 9536–9545. doi:10.1016/j.bmc.2008.09.027 |
| 41. | Gudmundsson, K. S.; Johns, B. A. Bioorg. Med. Chem. Lett. 2007, 17, 2735–2739. doi:10.1016/j.bmcl.2007.02.079 |
| 42. | Al-Tel, T. H.; Al-Qawasmeh, R. A.; Zaarour, R. Eur. J. Med. Chem. 2011, 46, 1874–1881. doi:10.1016/j.ejmech.2011.02.051 |
| 43. | Terada, A.; Wachi, K.; Miyazawa, H.; Iizuka, Y.; Hasegawa, K.; Tabata, K. Use of imidazopyrazole derivatives as analgesics and anti-inflammatory agents. U.S. Patent 5,232,939, Aug 3, 1993. |
| 44. | Frey, B.; Hufton, R.; Harding, M.; Draffan, A. G. Compounds for the treatment of HCV. WO PCT Pat. Appl. WO 2013/036994 A1, March 21, 2013. |
| 45. | Mascitti, V.; McClure, K. F.; Munchhof, M. J.; Robinson,, R. P. Imidazo-pyrazoles as GPR119 inhibitors. WO PCT Pat. Appl. WO2011/061679, May 26, 2011. |
| 46. | Zhang, J.; Singh, R.; Goff, D.; Kinoshita, T. Small molecule inhibitors of spleen tyrosine kinase (SYK). U.S. Pat. Appl. 20100316649 A1, Dec 16, 2010. |
| 47. | Ennis, H. L.; Möller, L.; Wang, J. J.; Selawry, O. S. Biochem. Pharmacol. 1971, 20, 2639–2646. doi:10.1016/0006-2952(71)90173-0 |
| 48. | Oku, T.; Kawai, Y.; Marusawa, H.; Yamazaki, H.; Abe, Y.; Tanaka, H. E. 3-(Heteroaryl)-pyrazololi[1,5-a]pyrimidines. U.S. Patent 5,356,897, Oct 18, 1994. |
| 49. | Kuehnert, S.; Oberboersch, S.; Sundermann, C.; Haurand, M.; Jostock,, R.; Schiene, K.; Tzschentke, T.; Christoph, T.; Kaulartz, D. Substituted bicyclic imidazo-3-ylamin compounds. WO PCT Pat. Appl. WO2006/029980 A1, March 23, 2006. |
| 50. | Berset, C.; Audetat, S.; Tietz, J.; Gunde, T.; Barberis, A.; Schumacher, A.; Traxler, P. Protein kinase inhibitors. WO PCT Pat. Appl. WO2005/120513 A1, Dec 22, 2005. |
| 51. | Goldfarb, D. S. Method for altering the lifespan of eukaryotic organisms. U.S. Pat. Appl. 2009/0163545 A1, 2009. |
| 52. | Van Niel, M. B.; Miah, A. Substituted imidazo[1,2-a]pyridines and their use as agonists at GABA-A receptors for treating or preventing neurological or psychlatric disorders. UK Pat. Appl. GB2448808 (A), Oct 29, 2008. |
| 54. | Basso, A.; Banfi, L.; Riva, R.; Guanti, G. J. Org. Chem. 2005, 70, 575–579. doi:10.1021/jo048389m |
| 55. | Domling, A. Chem. Rev. 2006, 106, 17–89. doi:10.1021/cr0505728 |
| 56. | Hulme, C.; Gore, V. Curr. Med. Chem. 2003, 10, 51–80. doi:10.2174/0929867033368600 |
| 57. | Akritopoulou-Zanze, I. Curr. Opin. Chem. Biol. 2008, 12, 324–331. doi:10.1016/j.cbpa.2008.02.004 |
| 58. | Koopmanschap, G.; Ruijter, E.; Orru, R. V. A. Beilstein J. Org. Chem. 2014, 10, 544–598. doi:10.3762/bjoc.10.50 |
| 9. | Ganem, B. Acc. Chem. Res. 2009, 42, 463–472. doi:10.1021/ar800214s |
| 10. | Sunderhaus, J. D.; Martin, S. F. Chem. – Eur. J. 2009, 15, 1300–1308. doi:10.1002/chem.200802140 |
| 11. | Touré, B. B.; Hall, D. G. Chem. Rev. 2009, 109, 4439–4486. doi:10.1021/cr800296p |
| 12. | Isambert, N.; Lavilla, R. Chem. – Eur. J. 2008, 14, 8444–8454. doi:10.1002/chem.200800473 |
| 13. | Mironov, M. A. QSAR Comb. Sci. 2006, 25, 423–431. doi:10.1002/qsar.200540190 |
| 14. | Chebanov, V. A.; Desenko, S. M. Diversity-Oriented Synth.; 2014; Vol. 1, pp 43–63. doi:10.2478/dos-2014-0003 |
| 15. | Dömling, A.; Ugi, I. Angew. Chem., Int. Ed. 2000, 39, 3168–3210. doi:10.1002/1521-3773(20000915)39:18<3168::AID-ANIE3168>3.0.CO;2-U |
| 16. | Banfi, L.; Basso, A.; Riva, R. In Synthesis of Heterocycles via Multicomponent Reactions I; Orru, R. V. A.; Ruijter, E., Eds.; Springer-Verlag : Berlin Heidelberg, 2010; Vol. 23, pp 1–39. doi:10.1007/7081_2009_23 |
| 17. | Zhu, J.; Wang, Q.; Wang, M.-X., Eds. Multicomponent reactions in organic chemistry; Wiley-VCH, 2015. |
| 18. | Bienaymé, H.; Hulme, C.; Oddon, G.; Schmitt, P. Chem. – Eur. J. 2000, 6, 3321–3329. doi:10.1002/1521-3765(20000915)6:18<3321::AID-CHEM3321>3.0.CO;2-A |
| 19. | Dolle, R. E.; Le Bourdonnec, B.; Goodman, A. J.; Morales, G. A.; Thomas, C. J.; Zhang, W. J. Comb. Chem. 2008, 10, 753–802. doi:10.1021/cc800119z |
| 20. | Zarganes-Tzitzikas, T.; Chandgude, A. L.; Dömling, A. Chem. Rec. 2015, 15, 981–996. doi:10.1002/tcr.201500201 |
| 21. | Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958–2975. doi:10.1039/c4gc00013g |
| 22. | Shaaban, S.; Abdel-Wahab, B. F. Mol. Diversity 2016, 20, 233–254. doi:10.1007/s11030-015-9602-6 |
| 23. | Váradi, A.; Palmer, T. C.; Dardashti, R. N.; Majumdar, S. Molecules 2016, 21, 19. doi:10.3390/molecules21010019 |
| 24. | Sadjadi, S.; Nahavandi, F.; Heravi, M. J. Iran. Chem. Soc. 2015, 12, 1049–1052. doi:10.1007/s13738-014-0564-x |
| 25. | Chebanov, V. A.; Desenko, S. M.; Gurley, T. W. Azaheterocycles Based on a,b-Unsaturated Carbonyls; Springer-Verlag: Berlin Heidelberg, 2008. |
| 26. | Kaur, T.; Wadhwa, P.; Bagchi, S.; Sharma, A. Chem. Commun. 2016, 52, 6958–6976. doi:10.1039/C6CC01562J |
| 45. | Mascitti, V.; McClure, K. F.; Munchhof, M. J.; Robinson,, R. P. Imidazo-pyrazoles as GPR119 inhibitors. WO PCT Pat. Appl. WO2011/061679, May 26, 2011. |
| 112. | Gomaa, E. Z. Braz. Arch. Biol. Technol. 2014, 57, 145–154. doi:10.1590/S1516-89132014000100020 |
| 113. | Wang, Y.; Ruan, L.; Lo, W.-H.; Chua, H.; Yu, H.-F. Appl. Biochem. Biotechnol. 2006, 132, 1015–1022. doi:10.1385/ABAB:132:1:1015 |
| 6. | Ruijter, E.; Scheffelaar, R.; Orru, R. V. A. Angew. Chem., Int. Ed. 2011, 50, 6234–6246. doi:10.1002/anie.201006515 |
| 7. | Marcaurelle, L. A.; Foley, M. A. Curr. Opin. Chem. Biol. 2010, 14, 285–288. doi:10.1016/j.cbpa.2010.05.001 |
| 8. | Biggs-Houck, J. E.; Younai, A.; Shaw, J. T. Curr. Opin. Chem. Biol. 2010, 14, 371–382. doi:10.1016/j.cbpa.2010.03.003 |
| 29. | Baviskar, A. T.; Madaan, C.; Preet, R.; Mohapatra, P.; Jain, V.; Agarwal, A.; Guchhait, S. K.; Kundu, C. N.; Banerjee, U. C.; Bharatam, P. V. J. Med. Chem. 2011, 54, 5013–5030. doi:10.1021/jm200235u |
| 46. | Zhang, J.; Singh, R.; Goff, D.; Kinoshita, T. Small molecule inhibitors of spleen tyrosine kinase (SYK). U.S. Pat. Appl. 20100316649 A1, Dec 16, 2010. |
| 47. | Ennis, H. L.; Möller, L.; Wang, J. J.; Selawry, O. S. Biochem. Pharmacol. 1971, 20, 2639–2646. doi:10.1016/0006-2952(71)90173-0 |
| 48. | Oku, T.; Kawai, Y.; Marusawa, H.; Yamazaki, H.; Abe, Y.; Tanaka, H. E. 3-(Heteroaryl)-pyrazololi[1,5-a]pyrimidines. U.S. Patent 5,356,897, Oct 18, 1994. |
| 114. | Kamionka, M. Curr. Pharm. Biotechnol. 2011, 12, 268–274. doi:10.2174/138920111794295693 |
| 115. | Sánchez Blancoa, A.; Palacios Durivea, O.; Batista Péreza, S.; Díaz Montesa, Z.; Pérez Guerra, N. Braz. J. Microbiol. 2016, 47, 665–674. doi:10.1016/j.bjm.2016.04.019 |
| 116. | Oyeleke, S. B.; Oyewole, O. A.; Egwim, E. C. Adv. Life Sci. 2011, 1, 49–53. doi:10.5923/j.als.20110102.09 |
| 117. | Markkanen,, P. H.; Bailey, M. J. J. Appl. Chem. Biotechnol. 1974, 24, 93–103. doi:10.1002/jctb.5020240111 |
| 118. | López-Valdez, F.; Fernández-Luqueño, F.; Ceballos-Ramírez, J. M.; Marsch, R.; Olalde-Portugal, V.; Dendooven, L. Sci. Hortic. (Amsterdam, Neth.) 2011, 128, 499–505. doi:10.1016/j.scienta.2011.02.006 |
| 119. | Qiao, J.-Q.; Wu, H.-J.; Huo, R.; Gao, X.-W.; Borriss, R. Chem. Biol. Technol. Agric. 2014, 1, No. 12. doi:10.1186/s40538-014-0012-2 |
| 37. | Gueiffier, A.; Mavel, S.; Lhassani, M.; Elhakmaoui, A.; Snoeck, R.; Andrei, G.; Chavignon, O.; Teulade, J.-C.; Witvrouw, M.; Balzarini, J.; De Clercq, E.; Chapat, J. P. J. Med. Chem. 1998, 41, 5108–5112. doi:10.1021/jm981051y |
| 38. | Defosse, G.; Le Ber, P.; Saarmets, A.; Wick, A. New bicyclic imidazole ketone derivs. FR Patent FR2699919 (A1), July 1, 1994. |
| 39. | Elleder, D.; Young, J. A. T.; Baiga, T. J.; Noel, J. P. Non-nucleoside reverse transcriptase inhibitors. WO PCT Pat. Appl. WO2009/061856, May 14, 2009. |
| 40. | Véron, J.-B.; Allouchi, H.; Enguehard-Gueiffier, C.; Snoeck, R.; Andrei, G.; De Clercq, E.; Gueiffier, A. Bioorg. Med. Chem. 2008, 16, 9536–9545. doi:10.1016/j.bmc.2008.09.027 |
| 41. | Gudmundsson, K. S.; Johns, B. A. Bioorg. Med. Chem. Lett. 2007, 17, 2735–2739. doi:10.1016/j.bmcl.2007.02.079 |
| 28. | Bruno, O.; Brullo, C.; Bondavalli, F.; Ranise, A.; Schenone, S.; Falzarano, M. S.; Varani, K.; Spisani, S. Bioorg. Med. Chem. Lett. 2007, 17, 3696–3701. doi:10.1016/j.bmcl.2007.04.036 |
| 43. | Terada, A.; Wachi, K.; Miyazawa, H.; Iizuka, Y.; Hasegawa, K.; Tabata, K. Use of imidazopyrazole derivatives as analgesics and anti-inflammatory agents. U.S. Patent 5,232,939, Aug 3, 1993. |
| 110. | Albert, A. Selective Toxicity: the physico-chemical basis of therapy; Springer: Netherlands, 1985. doi:10.1007/978-94-009-4846-4 |
| 34. | Mizushige, K.; Ueda, T.; Yukiiri, K.; Suzuki, H. Cardiovasc. Drug Rev. 2002, 20, 163–174. doi:10.1111/j.1527-3466.2002.tb00085.x |
| 35. | Mori, H.; Tanaka, M.; Kayasuga, R.; Masuda, T.; Ochi, Y.; Yamada, H.; Kishikawa, K.; Ito, M.; Nakamura, T. Bone 2008, 43, 840–848. doi:10.1016/j.bone.2008.07.242 |
| 36. | Tominaga, M.; Yang, Y.-H.; Nakagawa, K.; Ogawa, H. Carbostyrill compounds, compositions containing same and processes for preparing same. Eur. Pat. Appl. EP52016 (A1), May 19, 1981. |
| 39. | Elleder, D.; Young, J. A. T.; Baiga, T. J.; Noel, J. P. Non-nucleoside reverse transcriptase inhibitors. WO PCT Pat. Appl. WO2009/061856, May 14, 2009. |
| 44. | Frey, B.; Hufton, R.; Harding, M.; Draffan, A. G. Compounds for the treatment of HCV. WO PCT Pat. Appl. WO 2013/036994 A1, March 21, 2013. |
| 111. | Baeshen, N. A.; Baeshen, M. N.; Sheikh, A.; Bora, R. S.; Ahmed, M. M. M.; Ramadan, H. A. I.; Saini, K. S.; Redwan, E. M. Microb. Cell Fact. 2014, 13, No. 141. doi:10.1186/s12934-014-0141-0 |
| 31. | Starrett, J. E., Jr.; Montzka, T. A.; Crosswell, A. R.; Cavanagh, R. L. J. Med. Chem. 1989, 32, 2204–2210. doi:10.1021/jm00129a028 |
| 32. | Palmer, A. M.; Chrismann, S.; Münch, G.; Brehm, C.; Zimmermann, P. J.; Buhr, W.; Senn-Bilfinger, J.; Feth, M. P.; Simon, W. A. Bioorg. Med. Chem. 2009, 17, 368–384. doi:10.1016/j.bmc.2008.10.055 |
| 102. | Morozova, A. D.; Muravyova, E. A.; Shishkina, S. V.; Vashchenko, E. V.; Sen’ko, Y. V.; Chebanov, V. A. J. Heterocycl. Chem. 2017, 54, 932–943. doi:10.1002/jhet.2656 |
| 103. | Ryabukhin, S. V.; Panov, D. M.; Plaskon, A. S.; Grygorenko, O. O. ACS Comb. Sci. 2012, 14, 631–635. doi:10.1021/co300082t |
| 104. | Rajanarendar, E.; Reddy, M. N.; Raju, S. Indian J. Chem. 2011, 50B, 751–755. |
| 105. | Shafiee, M.; Khosropour, A. R.; Mohammadpoor-Baltork, I.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. Tetrahedron Lett. 2012, 53, 3086–3090. doi:10.1016/j.tetlet.2012.04.037 |
| 106. | Rajanarendar, E.; Murthy, K. R.; Reddy, M. N. Indian J. Chem. 2011, 50B, 926–930. |
| 30. | Maul, C.; Sundermann, B.; Hennies, H.; Schneider, J.; Gerlach, M. Tert-butyl-(7-methylimidazo[1,2-a]pyridin-3-yl)-amine-derivative. WO PCT Patent Application WO/2001/027109, April 19, 2001. |
| 52. | Van Niel, M. B.; Miah, A. Substituted imidazo[1,2-a]pyridines and their use as agonists at GABA-A receptors for treating or preventing neurological or psychlatric disorders. UK Pat. Appl. GB2448808 (A), Oct 29, 2008. |
| 107. | Mandair, G. S.; Light, M.; Russell, A.; Hursthouse, M.; Bradley, M. Tetrahedron Lett. 2002, 43, 4267–4269. doi:10.1016/S0040-4039(02)00709-8 |
| 108. | Parchinsky, V. Z.; Shuvalova, O.; Ushakova, O.; Kravchenko, D. V.; Krasavin, M. Tetrahedron Lett. 2006, 47, 947–951. doi:10.1016/j.tetlet.2005.11.152 |
| 109. | Thompson, M. J.; Hurst, J. M.; Chen, B. Synlett 2008, 3183–3187. doi:10.1055/s-0028-1087274 |
| 62. | Yugandhar, D.; Srivastava, A. K. ACS Comb. Sci. 2015, 17, 474–481. doi:10.1021/acscombsci.5b00065 |
| 59. | Chebanov, V. A.; Gura, K. A.; Desenko, S. M. In Synthesis of Heterocycles via Multicomponent Reactions I; Orru, R. V. A.; Ruijter, E., Eds.; Springer: Berlin Heidelberg, 2010; Vol. 23, pp 41–84. doi:10.1007/7081_2009_21 |
| 20. | Zarganes-Tzitzikas, T.; Chandgude, A. L.; Dömling, A. Chem. Rec. 2015, 15, 981–996. doi:10.1002/tcr.201500201 |
| 60. | Soural, M.; Bouillon, I.; Krchňák, V. J. Comb. Chem. 2008, 10, 923–933. doi:10.1021/cc8001074 |
| 61. | Maiti, B.; Chanda, K.; Selvaraju, M.; Tseng, C.-C.; Sun, C.-M. ACS Comb. Sci. 2013, 15, 291–297. doi:10.1021/co400010y |
| 71. | Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. Engl. 1998, 37, 2234–2237. doi:10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R |
| 93. | Kysil, V.; Khvat, A.; Tsirulnikov, S.; Tkachenko, S.; Williams, C.; Churakova, M.; Ivachtchenko, A. Eur. J. Org. Chem. 2010, 1525–1543. doi:10.1002/ejoc.200901360 |
| 71. | Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. Engl. 1998, 37, 2234–2237. doi:10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R |
| 83. | Guchhait, S. K.; Madaan, C. Synlett 2009, 628–632. doi:10.1055/s-0028-1087915 |
| 84. | Guchhait, S. K.; Madaan, C.; Thakkar, B. S. Synthesis 2009, 3293–3300. doi:10.1055/s-0029-1216916 |
| 89. | Krasavin, M.; Tsirulnikov, S.; Nikulnikov, M.; Kysil, V.; Ivachtchenko, A. Tetrahedron Lett. 2008, 49, 5241–5243. doi:10.1016/j.tetlet.2008.06.113 |
| 90. | Wadhwa, P.; Kaur, T.; Sharma, A. RSC Adv. 2015, 5, 44353–44360. doi:10.1039/C5RA06747B |
| 71. | Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. Engl. 1998, 37, 2234–2237. doi:10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R |
| 72. | Che, C.; Xiang, J.; Wang, G.-X.; Fathi, R.; Quan, J.-M.; Yang, Z. J. Comb. Chem. 2007, 9, 982–989. doi:10.1021/cc070058a |
| 91. | Lee, C.-H.; Hsu, W.-S.; Chen, C.-H.; Sun, C.-M. Eur. J. Org. Chem. 2013, 2201–2208. doi:10.1002/ejoc.201201645 |
| 92. | Pereshivko, O. P.; Peshkov, V. A.; Ermolat’ev, D. S.; Van Der Eycken, E. V. Synlett 2013, 24, 351–354. doi:10.1055/s-0032-1317986 |
| 71. | Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. Engl. 1998, 37, 2234–2237. doi:10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R |
| 72. | Che, C.; Xiang, J.; Wang, G.-X.; Fathi, R.; Quan, J.-M.; Yang, Z. J. Comb. Chem. 2007, 9, 982–989. doi:10.1021/cc070058a |
| 73. | Aouali, M.; Mhalla, D.; Allouche, F.; El Kaim, L.; Tounsi, S.; Trigui, M.; Chabchoub, F. Med. Chem. Res. 2015, 24, 2732–2741. doi:10.1007/s00044-015-1322-z |
| 74. | Huang, Y.; Hu, X.-Q.; Shen, D.-P.; Chen, Y.-F.; Xu, P.-F. Mol. Diversity 2007, 11, 73–80. doi:10.1007/s11030-007-9059-3 |
| 75. | Parchinsky, V. Z.; Koleda, V. V.; Shuvalova, O.; Kravchenko, D. V.; Krasavin, M. Tetrahedron Lett. 2006, 47, 6891–6894. doi:10.1016/j.tetlet.2006.07.037 |
| 71. | Bienaymé, H.; Bouzid, K. Angew. Chem., Int. Ed. Engl. 1998, 37, 2234–2237. doi:10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R |
| 72. | Che, C.; Xiang, J.; Wang, G.-X.; Fathi, R.; Quan, J.-M.; Yang, Z. J. Comb. Chem. 2007, 9, 982–989. doi:10.1021/cc070058a |
| 76. | Akritopoulou-Zanze, I.; Wakefield, B. D.; Gasiecki, A.; Kalvin, D.; Johnson, E. F.; Kovar, P.; Djuric, S. W. Bioorg. Med. Chem. Lett. 2011, 21, 1480–1483. doi:10.1016/j.bmcl.2011.01.001 |
| 77. | Hieke, M.; Rödl, C. B.; Wisniewska, J. M.; La Buscató, E.; Stark, H.; Schubert-Zsilavecz, M.; Steinhilber, D.; Hofmann, B.; Proschak, E. Bioorg. Med. Chem. Lett. 2012, 22, 1969–1975. doi:10.1016/j.bmcl.2012.01.038 |
| 78. | Al-Tel, T. H.; Al-Qawasmeh, R. A.; Voelter, W. Eur. J. Org. Chem. 2010, 5586–5593. doi:10.1002/ejoc.201000808 |
| 79. | Vidyacharan, S.; Shinde, A. H.; Satpathi, B.; Sharada, D. S. Green Chem. 2014, 16, 1168–1175. doi:10.1039/c3gc42130a |
| 80. | Burchak, O. N.; Mugherli, L.; Ostuni, M.; Lacapre, J. J.; Balakirev, M. Y. J. Am. Chem. Soc. 2011, 133, 10058–10061. doi:10.1021/ja204016e |
| 81. | Guchhait, S. K.; Madaan, C. Org. Biomol. Chem. 2010, 8, 3631–3634. doi:10.1039/c0ob00022a |
| 82. | Hatamjafari, F.; Javad, M.; Mohtasham, M.; Chakoli, F. A. Orient. J. Chem. 2012, 28, 1271–1273. doi:10.13005/ojc/280323 |
| 83. | Guchhait, S. K.; Madaan, C. Synlett 2009, 628–632. doi:10.1055/s-0028-1087915 |
| 84. | Guchhait, S. K.; Madaan, C.; Thakkar, B. S. Synthesis 2009, 3293–3300. doi:10.1055/s-0029-1216916 |
| 85. | Rostamnia, S.; Lamei, K.; Mohammadquli, M.; Sheykhan, M.; Heydari, A. Tetrahedron Lett. 2012, 53, 5257–5260. doi:10.1016/j.tetlet.2012.07.075 |
| 86. | Adib, M.; Mahdavi, M.; Noghani, M. A.; Mirzaei, P. Tetrahedron Lett. 2007, 48, 7263–7265. doi:10.1016/j.tetlet.2007.08.049 |
| 87. | Tsirulnikov, S.; Kysil, V.; Ivachtchenko, A.; Krasavin, M. Synth. Commun. 2009, 40, 111–119. doi:10.1080/00397910902953331 |
| 88. | Mouradzadegun, A.; Ma’mani, L.; Mahdavi, M.; Rashid, Z.; Shafiee, A.; Foroumadi, A.; Dianat, S. RSC Adv. 2015, 5, 83530–83537. doi:10.1039/C5RA12307K |
| 63. | Vachhani, D. D.; Galli, M.; Jacobs, J.; Van Meervelt, L.; Van der Eycken, E. V. Chem. Commun. 2013, 49, 7171–7173. doi:10.1039/c3cc43418d |
| 64. | Moni, L.; Deniβen, M.; Valentini, G.; Müller, T. J. J.; Riva, R. Chem. – Eur. J. 2015, 21 , 753–762. doi:10.1002/chem.201404209 |
| 65. | Vachhani, D. D.; Kumar, A.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van Der Eycken, E. V. Eur. J. Org. Chem. 2013, 1223–1227. doi:10.1002/ejoc.201201587 |
| 66. | Kumar, A.; Vachhani, D. D.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van Der Eycken, E. V. Synthesis 2013, 45, 2571–2582. doi:10.1055/s-0033-1339474 |
| 67. | Balalaie, S.; Motaghedi, H.; Bararjanian, M.; Tahmassebi, D.; Bijanzadeh, H. R. Tetrahedron 2011, 67, 9134–9141. doi:10.1016/j.tet.2011.09.089 |
| 68. | Welsch, S. J.; Umkehrer, M.; Ross, G.; Kolb, J.; Burdack, C.; Wessjohann, L. A. Tetrahedron Lett. 2011, 52, 6295–6297. doi:10.1016/j.tetlet.2011.09.094 |
| 69. | Ambasana, P. A.; Vachhani, D. D.; Galli, M.; Jacobs, J.; Van Meervelt, L.; Shah, A. K.; Van Der Eycken, E. V. Org. Biomol. Chem. 2014, 12, 8861–8865. doi:10.1039/C4OB01644K |
| 70. | Ghabraie, E.; Balalaie, S.; Mehrparvar, S.; Rominger, F. J. Org. Chem. 2014, 79, 7926–7934. doi:10.1021/jo5010422 |
© 2017 Murlykina et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)