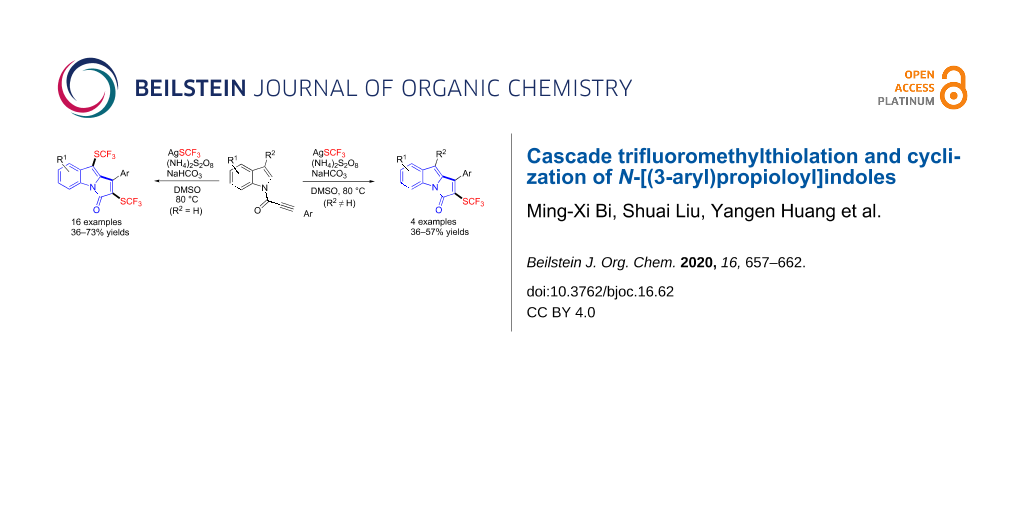
Abstract
A cascade oxidative trifluoromethylthiolation and cyclization of N-[(3-aryl)propioloyl]indoles with AgSCF3 is described. This protocol allows for the synthesis of novel bis(trifluoromethylthiolated) or trifluoromethylthiolated pyrrolo[1,2-a]indol-3-ones in moderate to good yields. Mechanistic investigations indicated that radical processes were probably involved in these transformations.
Graphical Abstract
Introduction
The trifluoromethylthio (SCF3) group could significantly improve the lipophilicity of organic molecules as shown by its high Hansch constant (π = 1.44 for SCF3, 0.88 for CF3, and 0.61 for SMe) [1] that helps permeation across biological membranes. Furthermore, the strong electron-withdrawing properties of the SCF3 group (Hammett constants: σp = 0.50, σm = 0.40) [2] with respect to metabolic stability have attracted considerable interest in pharmaceutical and agrochemical industries [3-5]. Traditional methods to access these compounds mainly include halogen–fluorine exchange of halomethyl sulfides and trifluoromethylation of sulfur-containing compounds [6-8]. Over the last decade, tremendous efforts have been triggered to develop methods for the direct incorporation of the SCF3 group into organic compounds [9-16], such as alkynes, alkenes, arenes, and alkanes. Despite these impressive advances, there is a continued strong demand for new methods that enable the efficient synthesis of SCF3-containing compounds, especially those featuring medicinally promising scaffolds.
Pyrrolo[1,2-a]indol-3-ones are prevalent scaffolds that widely exist in many bioactive compounds and natural products [17-20]. Representative examples of biologically active pyrrolo[1,2-a]indol-3-one derivatives are shown in Figure 1. Recently, the development of efficient methods for the synthesis of pyrrolo[1,2-a]indol-3-one derivatives has attracted considerable attention. For instance, Song [21] and Liang [22] reported the one-pot synthesis of novel phosphorylated and sulfonylated pyrrolo[1,2-a]indol-3-ones from N-[(3-phenyl)propioloyl]indole and N-propargylindoles, respectively. Inspired by these elegant results, we became interested in the preparation of SCF3-substituted pyrrolo[1,2-a]indol-3-ones, which might be potentially useful in medicinal chemistry.
Figure 1: Representative examples of biologically active pyrrolo[1,2-a]indol-3-one derivatives.
Figure 1: Representative examples of biologically active pyrrolo[1,2-a]indol-3-one derivatives.
Radical cascade reactions constitute highly efficient strategies for the construction of compounds with structural diversity and complexity. In 2014, Wang reported the first radical cascade trifluoromethylthiolation and cyclization of activated alkenes (Scheme 1a) [23]. Afterward, Nevado [24], Hopkinson and Glorius [25], Dagousset and Magnier [26], as well as Fu [27] applied this strategy in the synthesis of a series of CH2SCF3-substituted heterocycles. For the construction of SCF3-substituted cyclic compounds, normally proper alkynes are chosen as the substrates for cascade reactions [28-32]. In 2015, Wang developed an oxidative radical cyclization of aryl alkynoate esters with AgSCF3 for the synthesis of trifluoromethylthiolated coumarins (Scheme 1b) [28]. In 2016, Liu exploited the tandem trifluoromethylthiolation/cyclization of N-arylpropiolamides to construct the SCF3-substituted spiro[4,5]trienones (Scheme 1c) [29]. In the same year, Zhang and Chen disclosed the transformation of arylpropynones to SCF3-substituted indenones through the tandem trifluoromethylthiolation/cyclization processes (Scheme 1d) [30]. As part of our continuing research interest in radical trifluoromethylthiolation reactions [33-38], herein we disclose a cascade trifluoromethylthiolation and cyclization of N-[(3-aryl)propioloyl]indoles to access SCF3-substituted pyrrolo[1,2-a]indol-3-ones (Scheme 1e).
Scheme 1: Radical cascade trifluoromethylthiolation and cyclization reactions.
Scheme 1: Radical cascade trifluoromethylthiolation and cyclization reactions.
Results and Discussion
On the outset, 1-(1H-indol-1-yl)-3-phenylprop-2-yn-1-one (1a) was chosen as the model substrate for optimization of the reaction conditions (Table 1). To our surprise, the reaction of 1a and AgSCF3 in the presence of K2S2O8 and KHCO3 in DMSO at 80 °C gave bis(trifluoromethylthiolated) product 2a in 28% yield (Table 1, entry 1). Only trace of mono(trifluoromethylthiolated) product was detected, and most of the substrate 1a was not converted. To the best of our knowledge, the combination of bis(trifluoromethylthiolation) [36,39-41] and cascade cyclization reactions has not been reported before. Thus, the amounts of AgSCF3 and K2S2O8 were increased to deliver 2a in 52% yield (Table 1, entry 2). Other oxidants including Na2S2O8 and (NH4)2S2O8 afforded 2a in slightly higher yields, respectively (Table 1, entries 3 and 4). Switching KHCO3 to NaHCO3 could enhance the yield (Table 1, entry 6), whereas K2CO3 and DBU reduced the reaction efficiency (Table 1, entries 5 and 7). Subsequent evaluation of solvents revealed that MeCN and DMF were inferior to DMSO (Table 1, entries 8 and 9). Gratifyingly, the yield was improved to 80% by reducing the amount of base to 1.0 equivalent (Table 1, entry 10).
Table 1: Optimization of the reaction conditionsa.
|
|
||||
| entry | oxidant | base | solvent | yield (%)b |
| 1 | K2S2O8 | KHCO3 | DMSO | 28 |
| 2c | K2S2O8 | KHCO3 | DMSO | 52 |
| 3c | Na2S2O8 | KHCO3 | DMSO | 55 |
| 4c | (NH4)2S2O8 | KHCO3 | DMSO | 58 |
| 5c | (NH4)2S2O8 | K2CO3 | DMSO | 40 |
| 6c | (NH4)2S2O8 | NaHCO3 | DMSO | 72 |
| 7c | (NH4)2S2O8 | DBU | DMSO | 34 |
| 8c | (NH4)2S2O8 | NaHCO3 | MeCN | 8 |
| 9c | (NH4)2S2O8 | NaHCO3 | DMF | trace |
| 10d | (NH4)2S2O8 | NaHCO3 | DMSO | 80 |
aReaction conditions: 1a (0.1 mmol), AgSCF3 (0.15 mmol), oxidant (0.2 mmol), base (0.15 mmol), solvent (2.0 mL), 80 °C, 12 h. bYield was determined by 19F NMR using trifluorotoluene as an internal standard. cAgSCF3 (0.3 mmol), oxidant (0.3 mmol). dAgSCF3 (0.3 mmol), oxidant (0.3 mmol), base (0.1 mmol).
With the optimized reaction conditions in hand, we then set out to explore the substrate scope of N-[(3-aryl)propioloyl]indoles (Scheme 2). First, we explored the effect of the substitution on the indole ring. Both electron-donating and withdrawing groups at different positions of the indole ring produced the bis(trifluoromethylthiolated) products 2a–o in moderate to good yields. A wide range of functionalities such as alkyl, alkoxy, nitro, nitrile, ester, aldehyde, fluoro, chloro, and bromo were well-tolerated and compatible under the mild reaction conditions. Substrate 1p containing a methyl substituent on the phenyl ring could also participate in the reaction and furnish the desired product in moderate yield. However, attempts to prepare the substrates bearing an alkyl or electron-deficient aryl substituent on the alkynone were not successful. The structure of product 2a was unambiguously identified by single-crystal X-ray analysis.
Scheme 2: Cascade bis(trifluoromethylthiolation) and cyclization of N-[(3-aryl)propioloyl]indoles 1. Reaction conditions: 1 (0.25 mmol), AgSCF3 (0.75 mmol), (NH4)2S2O8 (0.75 mmol), NaHCO3 (0.25 mmol), DMSO (5.0 mL), 80 °C, 12 h, isolated yields.
Scheme 2: Cascade bis(trifluoromethylthiolation) and cyclization of N-[(3-aryl)propioloyl]indoles 1. Reaction...
When the N-[(3-aryl)propioloyl]indole substrates (3a–d) with different substituents at the 3-position of the indole ring were subjected to the standard conditions, the cascade trifluoromethylthiolation and cyclization occurred to yield trifluoromethylthiolated pyrrolo[1,2-a]indol-3-ones (4a–d) in moderate yields (Scheme 3). The functionalities including alkyl, aryl, nitrile, and acyl were also well tolerated in this reaction.
Scheme 3: Cascade trifluoromethylthiolation and cyclization of N-[(3-aryl)propioloyl]indoles 3. Reaction conditions: 3 (0.25 mmol), AgSCF3 (0.75 mmol), (NH4)2S2O8 (0.75 mmol), NaHCO3 (0.25 mmol), DMSO (5.0 mL), 80 °C, 12 h, isolated yields.
Scheme 3: Cascade trifluoromethylthiolation and cyclization of N-[(3-aryl)propioloyl]indoles 3. Reaction cond...
In order to gain insight into the reaction mechanism, the radical scavenger 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) was added to the standard reactions of 1a and 3b, respectively. The desired product 2a was not formed and only trace of 4b was detected (see the Supporting Information File 1), which suggested that the radical process was probably involved in these transformations. Notably, no TEMPO-trapped product was detected by 19F NMR spectra of the crude reaction mixtures. On the basis of these results and literature studies [21,23,42-47], a plausible reaction mechanism was proposed in Scheme 4. First, oxidation of AgSCF3 by (NH4)2S2O8 generates AgIISCF3, which could be further transformed to the CF3S radical or CF3SSCF3 [23,36]. Then, the addition of a CF3S radical to the alkyne function of substrates 1 or 3 afforded intermediate A. Subsequently, cyclization of intermediate A, followed by oxidation with (NH4)2S2O8, gave intermediate C [21,42-47]. Finally, deprotonation of intermediate C (R2 ≠ H) with NaHCO3 delivered the aromatized product 4. In the case of intermediate C (R2 = H), intermediate D was probably formed, and further underwent electrophilic trifluoromethylthiolation with CF3SSCF3 [48,49] to furnish the bis(trifluoromethylthiolated) product 2.
Conclusion
We have reported the cascade trifluoromethylthiolation and cyclization reactions for the preparation of novel and potentially useful SCF3-containing pyrrolo[1,2-a]indol-3-ones. Oxidative trifluoromethylthiolation of N-[(3-aryl)propioloyl]indoles without substituent at the 3-position of the indole ring with AgSCF3 afforded the bis(trifluoromethylthiolated) products in moderate to good yields, whereas the substrates with a substituent at the 3-position of the indole ring were converted to the mono(trifluoromethylthiolated) products in moderate yields. Further studies on applying radical cascade reactions to the construction of fluorine-containing heterocyclic scaffolds are in progress in our laboratory.
Supporting Information
| Supporting Information File 1: Experimental procedures, spectroscopic and X-ray data (CCDC 1968129 for compound 2a) and copies of NMR spectra. | ||
| Format: PDF | Size: 3.7 MB | Download |
References
-
Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207–1216. doi:10.1021/jm00269a003
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
Return to citation in text: [1] -
Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c
Return to citation in text: [1] -
Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879
Return to citation in text: [1] -
Meanwell, N. A. J. Med. Chem. 2018, 61, 5822–5880. doi:10.1021/acs.jmedchem.7b01788
Return to citation in text: [1] -
Leroux, F.; Jeschke, P.; Schlosser, M. Chem. Rev. 2005, 105, 827–856. doi:10.1021/cr040075b
Return to citation in text: [1] -
Boiko, V. N. Beilstein J. Org. Chem. 2010, 6, 880–921. doi:10.3762/bjoc.6.88
Return to citation in text: [1] -
Manteau, B.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. J. Fluorine Chem. 2010, 131, 140–158. doi:10.1016/j.jfluchem.2009.09.009
Return to citation in text: [1] -
Chu, L.; Qing, F.-L. Acc. Chem. Res. 2014, 47, 1513–1522. doi:10.1021/ar4003202
Return to citation in text: [1] -
Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2415–2428. doi:10.1002/ejoc.201301857
Return to citation in text: [1] -
Shao, X.; Xu, C.; Lu, L.; Shen, Q. Acc. Chem. Res. 2015, 48, 1227–1236. doi:10.1021/acs.accounts.5b00047
Return to citation in text: [1] -
Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b
Return to citation in text: [1] -
Chachignon, H.; Cahard, D. Chin. J. Chem. 2016, 34, 445–454. doi:10.1002/cjoc.201500890
Return to citation in text: [1] -
Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 7150–7182. doi:10.1039/c6ob00763e
Return to citation in text: [1] -
Zheng, H.; Huang, Y.; Weng, Z. Tetrahedron Lett. 2016, 57, 1397–1409. doi:10.1016/j.tetlet.2016.02.073
Return to citation in text: [1] -
Hardy, M. A.; Chachignon, H.; Cahard, D. Asian J. Org. Chem. 2019, 8, 591–609. doi:10.1002/ajoc.201900004
Return to citation in text: [1] -
Toyota, M.; Ihara, M. Nat. Prod. Rep. 1998, 15, 327–340. doi:10.1039/a815327y
Return to citation in text: [1] -
Elmegeed, G. A.; Baiuomy, A. R.; Abdel-Salam, O. M. E. Eur. J. Med. Chem. 2007, 42, 1285–1292. doi:10.1016/j.ejmech.2007.01.027
Return to citation in text: [1] -
Liu, J.-F.; Jiang, Z.-Y.; Wang, R.-R.; Zheng, Y.-T.; Chen, J.-J.; Zhang, X.-M.; Ma, Y.-B. Org. Lett. 2007, 9, 4127–4129. doi:10.1021/ol701540y
Return to citation in text: [1] -
Bass, P. D.; Gubler, D. A.; Judd, T. C.; Williams, R. M. Chem. Rev. 2013, 113, 6816–6863. doi:10.1021/cr3001059
Return to citation in text: [1] -
Xu, J.; Yu, X.; Song, Q. Org. Lett. 2017, 19, 980–983. doi:10.1021/acs.orglett.6b03713
Return to citation in text: [1] [2] [3] -
Zhu, X.-Y.; Han, Y.-P.; Li, M.; Li, X.-S.; Liang, Y.-M. Adv. Synth. Catal. 2018, 360, 3460–3465. doi:10.1002/adsc.201800414
Return to citation in text: [1] -
Yin, F.; Wang, X.-S. Org. Lett. 2014, 16, 1128–1131. doi:10.1021/ol403739w
Return to citation in text: [1] [2] [3] -
Fuentes, N.; Kong, W.; Fernández-Sánchez, L.; Merino, E.; Nevado, C. J. Am. Chem. Soc. 2015, 137, 964–973. doi:10.1021/ja5115858
Return to citation in text: [1] -
Honeker, R.; Garza-Sanchez, R. A.; Hopkinson, M. N.; Glorius, F. Chem. – Eur. J. 2016, 22, 4395–4399. doi:10.1002/chem.201600190
Return to citation in text: [1] -
Dagousset, G.; Simon, C.; Anselmi, E.; Tuccio, B.; Billard, T.; Magnier, E. Chem. – Eur. J. 2017, 23, 4282–4286. doi:10.1002/chem.201700734
Return to citation in text: [1] -
Zhu, M.; Fu, W.; Guo, W.; Tian, Y.; Wang, Z.; Ji, B. Org. Biomol. Chem. 2019, 17, 3374–3380. doi:10.1039/c9ob00342h
Return to citation in text: [1] -
Zeng, Y.-F.; Tan, D.-H.; Chen, Y.; Lv, W.-X.; Liu, X.-G.; Li, Q.; Wang, H. Org. Chem. Front. 2015, 2, 1511–1515. doi:10.1039/c5qo00271k
Return to citation in text: [1] [2] -
Jin, D.-P.; Gao, P.; Chen, D.-Q.; Chen, S.; Wang, J.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2016, 18, 3486–3489. doi:10.1021/acs.orglett.6b01702
Return to citation in text: [1] [2] -
Song, Y.-K.; Qian, P.-C.; Chen, F.; Deng, C.-L.; Zhang, X.-G. Tetrahedron 2016, 72, 7589–7593. doi:10.1016/j.tet.2016.10.013
Return to citation in text: [1] [2] -
Qiu, Y.-F.; Zhu, X.-Y.; Li, Y.-X.; He, Y.-T.; Yang, F.; Wang, J.; Hua, H.-L.; Zheng, L.; Wang, L.-C.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2015, 17, 3694–3697. doi:10.1021/acs.orglett.5b01657
Return to citation in text: [1] -
Qiu, Y.-F.; Niu, Y.-J.; Wei, X.; Cao, B.-Q.; Wang, X.-C.; Quan, Z.-J. J. Org. Chem. 2019, 84, 4165–4178. doi:10.1021/acs.joc.9b00181
Return to citation in text: [1] -
Zhang, K.; Liu, J.-B.; Qing, F.-L. Chem. Commun. 2014, 50, 14157–14160. doi:10.1039/c4cc07062c
Return to citation in text: [1] -
Pan, S.; Huang, Y.; Qing, F.-L. Chem. – Asian J. 2016, 11, 2854–2858. doi:10.1002/asia.201601098
Return to citation in text: [1] -
Li, H.; Liu, S.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Chem. Commun. 2017, 53, 10136–10139. doi:10.1039/c7cc06232j
Return to citation in text: [1] -
Pan, S.; Li, H.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2017, 19, 3247–3250. doi:10.1021/acs.orglett.7b01366
Return to citation in text: [1] [2] [3] -
Pan, S.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2017, 19, 4624–4627. doi:10.1021/acs.orglett.7b02249
Return to citation in text: [1] -
Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2019, 58, 18508–18512. doi:10.1002/anie.201911323
Return to citation in text: [1] -
Tlili, A.; Alazet, S.; Glenadel, Q.; Billard, T. Chem. – Eur. J. 2016, 22, 10230–10234. doi:10.1002/chem.201601338
Return to citation in text: [1] -
Mesgar, M.; Daugulis, O. Org. Lett. 2017, 19, 4247–4250. doi:10.1021/acs.orglett.7b01901
Return to citation in text: [1] -
Liu, Y.-L.; Qing, F.-L.; Xu, X.-H. Eur. J. Org. Chem. 2020, 1015–1018. doi:10.1002/ejoc.201901836
Return to citation in text: [1] -
Zhang, H.; Li, W.; Zhu, C. J. Org. Chem. 2017, 82, 2199–2204. doi:10.1021/acs.joc.6b02673
Return to citation in text: [1] [2] -
Zhu, X.-Y.; Li, M.; Han, Y.-P.; Chen, S.; Li, X.-S.; Liang, Y.-M. J. Org. Chem. 2017, 82, 8761–8768. doi:10.1021/acs.joc.7b01497
Return to citation in text: [1] [2] -
Zhang, P.; Gao, Y.; Chen, S.; Tang, G.; Zhao, Y. Org. Chem. Front. 2017, 4, 1350–1353. doi:10.1039/c7qo00167c
Return to citation in text: [1] [2] -
Chen, H.; Liu, M.; Qiu, G.; Wu, J. Adv. Synth. Catal. 2019, 361, 146–150. doi:10.1002/adsc.201801038
Return to citation in text: [1] [2] -
Sun, K.; Chen, X.-L.; Zhang, Y.-L.; Li, K.; Huang, X.-Q.; Peng, Y.-Y.; Qu, L.-B.; Yu, B. Chem. Commun. 2019, 55, 12615–12618. doi:10.1039/c9cc06924k
Return to citation in text: [1] [2] -
Gharpure, S. J.; Shelke, Y. G. Org. Lett. 2017, 19, 5022–5025. doi:10.1021/acs.orglett.7b02005
Return to citation in text: [1] [2] -
Ma, L.; Cheng, X.-F.; Li, Y.; Wang, X.-S. Tetrahedron Lett. 2016, 57, 2972–2975. doi:10.1016/j.tetlet.2016.05.086
Return to citation in text: [1] -
Chachignon, H.; Maeno, M.; Kondo, H.; Shibata, N.; Cahard, D. Org. Lett. 2016, 18, 2467–2470. doi:10.1021/acs.orglett.6b01026
Return to citation in text: [1]
| 36. | Pan, S.; Li, H.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2017, 19, 3247–3250. doi:10.1021/acs.orglett.7b01366 |
| 39. | Tlili, A.; Alazet, S.; Glenadel, Q.; Billard, T. Chem. – Eur. J. 2016, 22, 10230–10234. doi:10.1002/chem.201601338 |
| 40. | Mesgar, M.; Daugulis, O. Org. Lett. 2017, 19, 4247–4250. doi:10.1021/acs.orglett.7b01901 |
| 41. | Liu, Y.-L.; Qing, F.-L.; Xu, X.-H. Eur. J. Org. Chem. 2020, 1015–1018. doi:10.1002/ejoc.201901836 |
| 30. | Song, Y.-K.; Qian, P.-C.; Chen, F.; Deng, C.-L.; Zhang, X.-G. Tetrahedron 2016, 72, 7589–7593. doi:10.1016/j.tet.2016.10.013 |
| 33. | Zhang, K.; Liu, J.-B.; Qing, F.-L. Chem. Commun. 2014, 50, 14157–14160. doi:10.1039/c4cc07062c |
| 34. | Pan, S.; Huang, Y.; Qing, F.-L. Chem. – Asian J. 2016, 11, 2854–2858. doi:10.1002/asia.201601098 |
| 35. | Li, H.; Liu, S.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Chem. Commun. 2017, 53, 10136–10139. doi:10.1039/c7cc06232j |
| 36. | Pan, S.; Li, H.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2017, 19, 3247–3250. doi:10.1021/acs.orglett.7b01366 |
| 37. | Pan, S.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2017, 19, 4624–4627. doi:10.1021/acs.orglett.7b02249 |
| 38. | Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2019, 58, 18508–18512. doi:10.1002/anie.201911323 |
| 1. | Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207–1216. doi:10.1021/jm00269a003 |
| 9. | Chu, L.; Qing, F.-L. Acc. Chem. Res. 2014, 47, 1513–1522. doi:10.1021/ar4003202 |
| 10. | Toulgoat, F.; Alazet, S.; Billard, T. Eur. J. Org. Chem. 2014, 2415–2428. doi:10.1002/ejoc.201301857 |
| 11. | Shao, X.; Xu, C.; Lu, L.; Shen, Q. Acc. Chem. Res. 2015, 48, 1227–1236. doi:10.1021/acs.accounts.5b00047 |
| 12. | Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b |
| 13. | Chachignon, H.; Cahard, D. Chin. J. Chem. 2016, 34, 445–454. doi:10.1002/cjoc.201500890 |
| 14. | Barata-Vallejo, S.; Bonesi, S.; Postigo, A. Org. Biomol. Chem. 2016, 14, 7150–7182. doi:10.1039/c6ob00763e |
| 15. | Zheng, H.; Huang, Y.; Weng, Z. Tetrahedron Lett. 2016, 57, 1397–1409. doi:10.1016/j.tetlet.2016.02.073 |
| 16. | Hardy, M. A.; Chachignon, H.; Cahard, D. Asian J. Org. Chem. 2019, 8, 591–609. doi:10.1002/ajoc.201900004 |
| 28. | Zeng, Y.-F.; Tan, D.-H.; Chen, Y.; Lv, W.-X.; Liu, X.-G.; Li, Q.; Wang, H. Org. Chem. Front. 2015, 2, 1511–1515. doi:10.1039/c5qo00271k |
| 6. | Leroux, F.; Jeschke, P.; Schlosser, M. Chem. Rev. 2005, 105, 827–856. doi:10.1021/cr040075b |
| 7. | Boiko, V. N. Beilstein J. Org. Chem. 2010, 6, 880–921. doi:10.3762/bjoc.6.88 |
| 8. | Manteau, B.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. J. Fluorine Chem. 2010, 131, 140–158. doi:10.1016/j.jfluchem.2009.09.009 |
| 29. | Jin, D.-P.; Gao, P.; Chen, D.-Q.; Chen, S.; Wang, J.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2016, 18, 3486–3489. doi:10.1021/acs.orglett.6b01702 |
| 3. | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c |
| 4. | Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879 |
| 5. | Meanwell, N. A. J. Med. Chem. 2018, 61, 5822–5880. doi:10.1021/acs.jmedchem.7b01788 |
| 27. | Zhu, M.; Fu, W.; Guo, W.; Tian, Y.; Wang, Z.; Ji, B. Org. Biomol. Chem. 2019, 17, 3374–3380. doi:10.1039/c9ob00342h |
| 2. | Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004 |
| 28. | Zeng, Y.-F.; Tan, D.-H.; Chen, Y.; Lv, W.-X.; Liu, X.-G.; Li, Q.; Wang, H. Org. Chem. Front. 2015, 2, 1511–1515. doi:10.1039/c5qo00271k |
| 29. | Jin, D.-P.; Gao, P.; Chen, D.-Q.; Chen, S.; Wang, J.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2016, 18, 3486–3489. doi:10.1021/acs.orglett.6b01702 |
| 30. | Song, Y.-K.; Qian, P.-C.; Chen, F.; Deng, C.-L.; Zhang, X.-G. Tetrahedron 2016, 72, 7589–7593. doi:10.1016/j.tet.2016.10.013 |
| 31. | Qiu, Y.-F.; Zhu, X.-Y.; Li, Y.-X.; He, Y.-T.; Yang, F.; Wang, J.; Hua, H.-L.; Zheng, L.; Wang, L.-C.; Liu, X.-Y.; Liang, Y.-M. Org. Lett. 2015, 17, 3694–3697. doi:10.1021/acs.orglett.5b01657 |
| 32. | Qiu, Y.-F.; Niu, Y.-J.; Wei, X.; Cao, B.-Q.; Wang, X.-C.; Quan, Z.-J. J. Org. Chem. 2019, 84, 4165–4178. doi:10.1021/acs.joc.9b00181 |
| 25. | Honeker, R.; Garza-Sanchez, R. A.; Hopkinson, M. N.; Glorius, F. Chem. – Eur. J. 2016, 22, 4395–4399. doi:10.1002/chem.201600190 |
| 21. | Xu, J.; Yu, X.; Song, Q. Org. Lett. 2017, 19, 980–983. doi:10.1021/acs.orglett.6b03713 |
| 42. | Zhang, H.; Li, W.; Zhu, C. J. Org. Chem. 2017, 82, 2199–2204. doi:10.1021/acs.joc.6b02673 |
| 43. | Zhu, X.-Y.; Li, M.; Han, Y.-P.; Chen, S.; Li, X.-S.; Liang, Y.-M. J. Org. Chem. 2017, 82, 8761–8768. doi:10.1021/acs.joc.7b01497 |
| 44. | Zhang, P.; Gao, Y.; Chen, S.; Tang, G.; Zhao, Y. Org. Chem. Front. 2017, 4, 1350–1353. doi:10.1039/c7qo00167c |
| 45. | Chen, H.; Liu, M.; Qiu, G.; Wu, J. Adv. Synth. Catal. 2019, 361, 146–150. doi:10.1002/adsc.201801038 |
| 46. | Sun, K.; Chen, X.-L.; Zhang, Y.-L.; Li, K.; Huang, X.-Q.; Peng, Y.-Y.; Qu, L.-B.; Yu, B. Chem. Commun. 2019, 55, 12615–12618. doi:10.1039/c9cc06924k |
| 47. | Gharpure, S. J.; Shelke, Y. G. Org. Lett. 2017, 19, 5022–5025. doi:10.1021/acs.orglett.7b02005 |
| 22. | Zhu, X.-Y.; Han, Y.-P.; Li, M.; Li, X.-S.; Liang, Y.-M. Adv. Synth. Catal. 2018, 360, 3460–3465. doi:10.1002/adsc.201800414 |
| 26. | Dagousset, G.; Simon, C.; Anselmi, E.; Tuccio, B.; Billard, T.; Magnier, E. Chem. – Eur. J. 2017, 23, 4282–4286. doi:10.1002/chem.201700734 |
| 48. | Ma, L.; Cheng, X.-F.; Li, Y.; Wang, X.-S. Tetrahedron Lett. 2016, 57, 2972–2975. doi:10.1016/j.tetlet.2016.05.086 |
| 49. | Chachignon, H.; Maeno, M.; Kondo, H.; Shibata, N.; Cahard, D. Org. Lett. 2016, 18, 2467–2470. doi:10.1021/acs.orglett.6b01026 |
| 21. | Xu, J.; Yu, X.; Song, Q. Org. Lett. 2017, 19, 980–983. doi:10.1021/acs.orglett.6b03713 |
| 21. | Xu, J.; Yu, X.; Song, Q. Org. Lett. 2017, 19, 980–983. doi:10.1021/acs.orglett.6b03713 |
| 23. | Yin, F.; Wang, X.-S. Org. Lett. 2014, 16, 1128–1131. doi:10.1021/ol403739w |
| 42. | Zhang, H.; Li, W.; Zhu, C. J. Org. Chem. 2017, 82, 2199–2204. doi:10.1021/acs.joc.6b02673 |
| 43. | Zhu, X.-Y.; Li, M.; Han, Y.-P.; Chen, S.; Li, X.-S.; Liang, Y.-M. J. Org. Chem. 2017, 82, 8761–8768. doi:10.1021/acs.joc.7b01497 |
| 44. | Zhang, P.; Gao, Y.; Chen, S.; Tang, G.; Zhao, Y. Org. Chem. Front. 2017, 4, 1350–1353. doi:10.1039/c7qo00167c |
| 45. | Chen, H.; Liu, M.; Qiu, G.; Wu, J. Adv. Synth. Catal. 2019, 361, 146–150. doi:10.1002/adsc.201801038 |
| 46. | Sun, K.; Chen, X.-L.; Zhang, Y.-L.; Li, K.; Huang, X.-Q.; Peng, Y.-Y.; Qu, L.-B.; Yu, B. Chem. Commun. 2019, 55, 12615–12618. doi:10.1039/c9cc06924k |
| 47. | Gharpure, S. J.; Shelke, Y. G. Org. Lett. 2017, 19, 5022–5025. doi:10.1021/acs.orglett.7b02005 |
| 17. | Toyota, M.; Ihara, M. Nat. Prod. Rep. 1998, 15, 327–340. doi:10.1039/a815327y |
| 18. | Elmegeed, G. A.; Baiuomy, A. R.; Abdel-Salam, O. M. E. Eur. J. Med. Chem. 2007, 42, 1285–1292. doi:10.1016/j.ejmech.2007.01.027 |
| 19. | Liu, J.-F.; Jiang, Z.-Y.; Wang, R.-R.; Zheng, Y.-T.; Chen, J.-J.; Zhang, X.-M.; Ma, Y.-B. Org. Lett. 2007, 9, 4127–4129. doi:10.1021/ol701540y |
| 20. | Bass, P. D.; Gubler, D. A.; Judd, T. C.; Williams, R. M. Chem. Rev. 2013, 113, 6816–6863. doi:10.1021/cr3001059 |
| 24. | Fuentes, N.; Kong, W.; Fernández-Sánchez, L.; Merino, E.; Nevado, C. J. Am. Chem. Soc. 2015, 137, 964–973. doi:10.1021/ja5115858 |
| 23. | Yin, F.; Wang, X.-S. Org. Lett. 2014, 16, 1128–1131. doi:10.1021/ol403739w |
| 36. | Pan, S.; Li, H.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Org. Lett. 2017, 19, 3247–3250. doi:10.1021/acs.orglett.7b01366 |
© 2020 Bi et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)