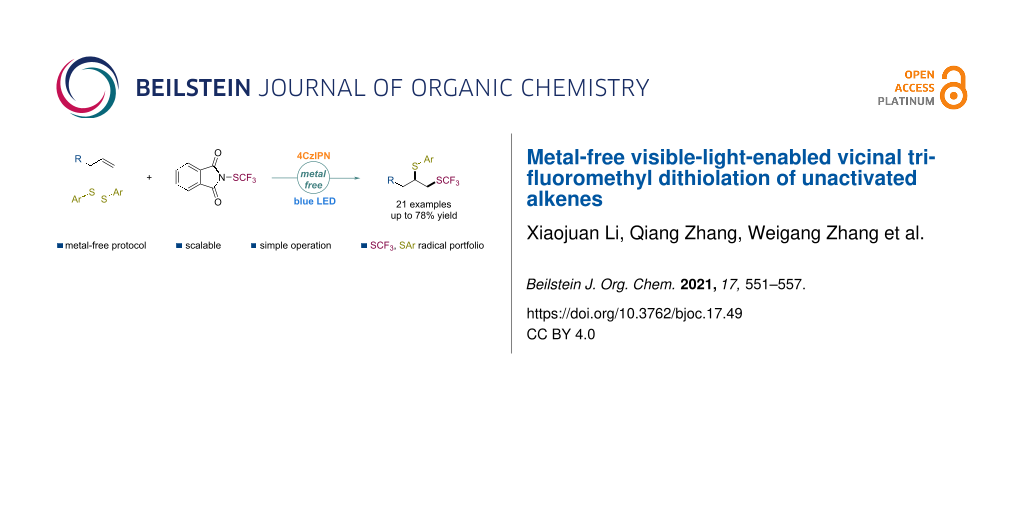
Abstract
The difunctionalization of alkenes involving a trifluoromethylthio group (SCF3) for the conversion of versatile and readily available olefins into structurally more complex molecules has been successfully studied. However, the disproportionate dithiolation of alkenes is unknown. Herein, a transition-metal-free protocol is presented for the vicinal trifluoromethylthio–thiolation of unactivated alkenes via a radical process under mild conditions with a broad substrate scope and excellent tolerance.
Graphical Abstract
Introduction
The incorporation of fluorine atoms into drug molecules will significantly enhance the physical, chemical, and biological properties of the pharmaceuticals [1-6]. Modifying drug candidates by introducing fluorine-containing (such as -CF3, -CF2H, -C2F5, -SCF3, -OCF3) moieties has become a substantial strategy for medicinal research [2,7,8]. Among the fluorinated functionalities, the trifluoromethylthio group (SCF3) has strong electron-withdrawing properties and a higher lipophilicity (πR = 1.44), compared with CF3 (πR = 0.88) and SCH3 (πR = 0.61), which could improve the pharmaceuticals’ ability to cross lipid membranes [9,10]. Along these lines, the introduction of the SCF3 group into small molecules has attracted great attention in organofluorine methodology [11-17].
The vicinal difunctionalization of olefins to introduce two functional groups across a double bond has appeared as a powerful transformation to rapidly increase molecular complexity in synthetic chemistry with improved efficiency [18-22]. Various transition-metal-mediated approaches for the trifluoromethylthio (SCF3) difunctionalization of alkenes, such as cyanation [23], etherification [24-27], amination [28-30], chlorination [31,32], hydrogenation [33], trifluoromethylation [34], phosphonization [35], arylation [36-38], trifluoromethylthiolation [39], fluorination [40], and selenylation [41] have been reported (Scheme 1a). However, the visible-light-induced trifluoromethylthio difunctionalization of alkenes remained underdeveloped. For instance, Magnier and co-workers have documented a practical intramolecular carbotrifluoromethylthiolation of acrylamides under irradiation of visible light [38]. In 2017, the photoredox-catalyzed intermolecular trifluoromethylthio–trifluoromethylation and thiosulfonylation reaction of unactivated alkenes have been respectively developed by Liu [34] and Xu [42]. Recently, Qing [43] and co-workers reported an efficient anti-Markovnikov hydrotrifluoromethylthiolation of alkenes utilizing trifluoromethanesulfonic anhydride (Tf2O) as a radical trifluoromethylthiolating reagent through a deoxygenative reduction and a photoredox radical pathway (Scheme 1b). The C–S bond [44,45] is an important structural motif that is widely present in natural products, drug molecules, biologically active molecules, and functional materials. However, the highly selective incorporation of two different sulfur-bearing moieties across double bonds remains challenging [42]. Herein, we describe a visible-light-enabled cascade radical difunctionalization of unactivated alkenes for the construction of partially trifluoromethylated dithioethers with a broad substrate scope and a good chemical tolerance (Scheme 1c).
Results and Discussion
We evaluated the reaction conditions for this trifluoromethylthio–thiolation and found out that under irradiation of blue LEDs, allyl boronate 1a (0.1 mmol), disulfide 2a (1.0 equiv) and N-(trifluoromethylthio)phthalimide (Phth-SCF3, 3, 1.5 equiv), the desired trifluoromethylthiolated product 4a was obtained in 71% yield with 1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (4CzIPN, 2 mol %) as the photocatalyst and KH2PO4 (10 mol %) as the base (Table 1, entry 1). The yield of 4a was not increased when 2 equiv of K2HPO4 were used (Table 1, entry 2) and no difunctionalized product was observed with DMA as the solvent (Table 1, entry 4). The employment of KH2PO4 as base and [Ir(dF(CF3)ppy)2(dtbby)]PF6 as the photocatalyst furnished the product in very low yields (Table 1, entries 3 and 5). The control experiments indicated that 4CzIPN, K2HPO4 or visible-light were indispensable for the reaction to proceed (Table 1, entries 6–8).
Table 1: Optimization of the reaction conditions.a
|
|
||
| entry | deviation from the reaction conditions | yieldb (%) |
| 1 | none | 71 |
| 2 | 2.0 equiv K2HPO4 | 70 |
| 3 | KH2PO4 instead of K2HPO4 | 34 |
| 4 | DMA instead of DMSO | trace |
| 5 | [Ir(dF(CF3)ppy)2(dtbby)]PF6 instead of 4CzIPN | 43 |
| 6 | no K2HPO4 | 0 |
| 7 | without 4CzIPN | 0 |
| 8 | in darkness | 0 |
aReaction conditions: 1a (0.1 mmol), 2a (1.0 equiv), 3 (1.5 equiv), 4CzIPN (2 mol %), K2HPO4 (10 mol %), rt, Ar, 24 h. bCrude yields were determined by 19F NMR using trifluoromethoxybenzene as an internal standard.
With the optimized reaction conditions determined, we next examined the substrate scope of the disulfides (Scheme 2). Using borate-substituted olefins, the intermolecular trifluoromethylthio–thiolation induced by the sequential radical difunctionalization proceeded smoothly in a chemoselective fashion. Both, with electron-withdrawing and electron-donating groups substituted aryldisulfides were tolerated to access products 4a–g. The homoallylic borate (1b) was also converted into the corresponding product 4h in a moderate yield.
In order to further examine the generality of the reaction, we have extended this protocol to a range of unactivated alkenes. Terminal alkenes containing ester (5a–d) and oxygenated alkyl (5e–g) functionalities were tolerated under this framework (Scheme 3). The tested 1-phenyl-3-butene substrates transformed into the desired products 5h and 5i in good yields. Also olefins containing amido (5j) and sulfonate (5k) functionalities furnished the corresponding SCF3 adducts. In addition, boldenone- and ᴅ-glucose-derived terminal alkenes were compatible with the conditions affording the corresponding products 5l and 5m in moderate to good yields.
Scheme 3: Substrate scope of unactivated alkenes.
Scheme 3: Substrate scope of unactivated alkenes.
To gain insight into the reaction mechanism, control experiments were conducted under the standard conditions (Scheme 4). The radical-trapping agent 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO, 3.0 equiv) completely prevented the reaction. When diphenylethylene (3.0 equiv) was added to the reaction mixture, the desired product 5h was not obtained, and the vinyltrifluoromethylsulfide 7 was afforded in 12% yield [46,47].
Based on the above results and previous literature reports [33,38,48], a plausible mechanism for the trifluoromethylthio–thiolation is proposed (Scheme 5). In solution, two resonance structures can be formulated for Phth-SCF3, 3 (E1/2red = −0.45 V vs SCE) [49] and the intermediate I [50]. The complexation of the intermediate I with K2HPO4 provides the intermediate II. Then, 4CzIPN* (4CzIPN+/4CzIPN*: E1/2red = −1.04 V vs SCE) [51] reduces the intermediate II to generate a phthalimide anion (Phth− [50]) and a trifluoromethylthio radical under irradiation. The SCF3 radical readily reacts with the unactivated alkenes to give the intermediate B. The subsequent addition of B to disulfide 2 affords the difunctionalized products 4 or 5 and the thiophenyl radical C [52]. Finally, the oxidation of radical C by 4CzIPN+ closes the catalytic cycle [53].
Conclusion
In summary, we described a visible-light-induced cascade radical difunctionalization of unactivated alkenes to provide partially trifluoromethylated dithioethers. The approach features practical conditions, good functional group tolerance, and a broad substrate scope allowing the incorporation of two distinctive sulfur-containing motifs into terminal olefins.
Supporting Information
| Supporting Information File 1: Full experimental details, compound characterization, and copies of NMR spectra. | ||
| Format: PDF | Size: 4.1 MB | Download |
Funding
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21772085, 21971107, 22071101) and the Fundamental Research Funds for the Central Universities (Nos. 020514380220, 020514380131, 020514913412, 020514913214). We also thank the Collaborative Innovation Center of Advanced Microstructures and the Jiangsu Provincial Key Laboratory of Photonic and Electronic Materials at Nanjing University for support.
References
-
Yang, X.; Wu, T.; Phipps, R. J.; Toste, F. D. Chem. Rev. 2015, 115, 826–870. doi:10.1021/cr500277b
Return to citation in text: [1] -
Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879
Return to citation in text: [1] [2] -
O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a
Return to citation in text: [1] -
Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881–1886. doi:10.1126/science.1131943
Return to citation in text: [1] -
Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214–8264. doi:10.1002/anie.201206566
Return to citation in text: [1] -
Manteau, B.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. J. Fluorine Chem. 2010, 131, 140–158. doi:10.1016/j.jfluchem.2009.09.009
Return to citation in text: [1] -
Guo, S.; Cong, F.; Guo, R.; Wang, L.; Tang, P. Nat. Chem. 2017, 9, 546–551. doi:10.1038/nchem.2711
Return to citation in text: [1] -
Shao, X.; Hong, X.; Lu, L.; Shen, Q. Tetrahedron 2019, 75, 4156–4166. doi:10.1016/j.tet.2019.05.061
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207–1216. doi:10.1021/jm00269a003
Return to citation in text: [1] -
Xu, C.; Ma, B.; Shen, Q. Angew. Chem., Int. Ed. 2014, 53, 9316–9320. doi:10.1002/anie.201403983
Return to citation in text: [1] -
Glenadel, Q.; Alazet, S.; Billard, T. J. Fluorine Chem. 2015, 179, 89–95. doi:10.1016/j.jfluchem.2015.06.007
Return to citation in text: [1] -
Ferry, A.; Billard, T.; Langlois, B. R.; Bacqué, E. Angew. Chem., Int. Ed. 2009, 48, 8551–8555. doi:10.1002/anie.200903387
Return to citation in text: [1] -
Mukherjee, S.; Maji, B.; Tlahuext-Aca, A.; Glorius, F. J. Am. Chem. Soc. 2016, 138, 16200–16203. doi:10.1021/jacs.6b09970
Return to citation in text: [1] -
Boiko, V. N. Beilstein J. Org. Chem. 2010, 6, 880–921. doi:10.3762/bjoc.6.88
Return to citation in text: [1] -
Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b
Return to citation in text: [1] -
Wu, H.; Xiao, Z.; Wu, J.; Guo, Y.; Xiao, J.-C.; Liu, C.; Chen, Q.-Y. Angew. Chem., Int. Ed. 2015, 54, 4070–4074. doi:10.1002/anie.201411953
Return to citation in text: [1] -
Chen, Y.; Li, L.; Ma, Y.; Li, Z. J. Org. Chem. 2019, 84, 5328–5338. doi:10.1021/acs.joc.9b00339
Return to citation in text: [1] -
Yin, G.; Mu, X.; Liu, G. Acc. Chem. Res. 2016, 49, 2413–2423. doi:10.1021/acs.accounts.6b00328
Return to citation in text: [1] -
Wang, X.-X.; Lu, X.; He, S.-J.; Fu, Y. Chem. Sci. 2020, 11, 7950–7956. doi:10.1039/d0sc02054k
Return to citation in text: [1] -
Tu, H.-Y.; Zhu, S.; Qing, F.-L.; Chu, L. Synthesis 2020, 52, 1346–1356. doi:10.1055/s-0039-1690842
Return to citation in text: [1] -
Dhungana, R. K.; KC, S.; Basnet, P.; Giri, R. Chem. Rec. 2018, 18, 1314–1340. doi:10.1002/tcr.201700098
Return to citation in text: [1] -
Ji, M.; Wu, Z.; Yu, J.; Wan, X.; Zhu, C. Adv. Synth. Catal. 2017, 359, 1959–1962. doi:10.1002/adsc.201700218
Return to citation in text: [1] -
Xiang, H.; Yang, C. Org. Lett. 2014, 16, 5686–5689. doi:10.1021/ol502751k
Return to citation in text: [1] -
Zhu, L.; Wang, G.; Guo, Q.; Xu, Z.; Zhang, D.; Wang, R. Org. Lett. 2014, 16, 5390–5393. doi:10.1021/ol502624z
Return to citation in text: [1] -
Xu, C.; Shen, Q. Org. Lett. 2015, 17, 4561–4563. doi:10.1021/acs.orglett.5b02315
Return to citation in text: [1] -
Zhu, Z.; Luo, J.; Zhao, X. Org. Lett. 2017, 19, 4940–4943. doi:10.1021/acs.orglett.7b02406
Return to citation in text: [1] -
Luo, J.; Liu, Y.; Zhao, X. Org. Lett. 2017, 19, 3434–3437. doi:10.1021/acs.orglett.7b01392
Return to citation in text: [1] -
Xiao, Q.; He, Q.; Li, J.; Wang, J. Org. Lett. 2015, 17, 6090–6093. doi:10.1021/acs.orglett.5b03116
Return to citation in text: [1] -
Luo, J.; Zhu, Z.; Liu, Y.; Zhao, X. Org. Lett. 2015, 17, 3620–3623. doi:10.1021/acs.orglett.5b01727
Return to citation in text: [1] -
Jia, Y.; Qin, H.; Wang, N.; Jiang, Z.-X.; Yang, Z. J. Org. Chem. 2018, 83, 2808–2817. doi:10.1021/acs.joc.7b03261
Return to citation in text: [1] -
Jiang, L.; Ding, T.; Yi, W.-b.; Zeng, X.; Zhang, W. Org. Lett. 2018, 20, 2236–2240. doi:10.1021/acs.orglett.8b00581
Return to citation in text: [1] -
Yang, T.; Lu, L.; Shen, Q. Chem. Commun. 2015, 51, 5479–5481. doi:10.1039/c4cc08655d
Return to citation in text: [1] [2] -
Fang, J.; Wang, Z.-K.; Wu, S.-W.; Shen, W.-G.; Ao, G.-Z.; Liu, F. Chem. Commun. 2017, 53, 7638–7641. doi:10.1039/c7cc01903c
Return to citation in text: [1] [2] -
Xiao, Z.; Liu, Y.; Zheng, L.; Zhou, X.; Xie, Y.; Liu, C.; Guo, Y.; Chen, Q.-Y. Tetrahedron 2018, 74, 6213–6219. doi:10.1016/j.tet.2018.09.019
Return to citation in text: [1] -
Xiao, Z.; Liu, Y.; Zheng, L.; Liu, C.; Guo, Y.; Chen, Q.-Y. J. Org. Chem. 2018, 83, 5836–5843. doi:10.1021/acs.joc.8b00650
Return to citation in text: [1] -
Yin, F.; Wang, X.-S. Org. Lett. 2014, 16, 1128–1131. doi:10.1021/ol403739w
Return to citation in text: [1] -
Dagousset, G.; Simon, C.; Anselmi, E.; Tuccio, B.; Billard, T.; Magnier, E. Chem. – Eur. J. 2017, 23, 4282–4286. doi:10.1002/chem.201700734
Return to citation in text: [1] [2] [3] -
Liu, Y.-L.; Qing, F.-L.; Xu, X.-H. Eur. J. Org. Chem. 2020, 1015–1018. doi:10.1002/ejoc.201901836
Return to citation in text: [1] -
Liu, X.; Liang, Y.; Ji, J.; Luo, J.; Zhao, X. J. Am. Chem. Soc. 2018, 140, 4782–4786. doi:10.1021/jacs.8b01513
Return to citation in text: [1] -
Saravanan, P.; Anbarasan, P. Chem. Commun. 2019, 55, 4639–4642. doi:10.1039/c9cc00815b
Return to citation in text: [1] -
Li, H.; Shan, C.; Tung, C.-H.; Xu, Z. Chem. Sci. 2017, 8, 2610–2615. doi:10.1039/c6sc05093j
Return to citation in text: [1] [2] -
Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2019, 58, 18508–18512. doi:10.1002/anie.201911323
Return to citation in text: [1] -
Beletskaya, I. P.; Ananikov, V. P. Chem. Rev. 2011, 111, 1596–1636. doi:10.1021/cr100347k
Return to citation in text: [1] -
Otsuka, S.; Nogi, K.; Yorimitsu, H. Top. Curr. Chem. 2018, 376, 13. doi:10.1007/s41061-018-0190-7
Return to citation in text: [1] -
Li, H.; Liu, S.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Chem. Commun. 2017, 53, 10136–10139. doi:10.1039/c7cc06232j
Return to citation in text: [1] -
Honeker, R.; Garza-Sanchez, R. A.; Hopkinson, M. N.; Glorius, F. Chem. – Eur. J. 2016, 22, 4395–4399. doi:10.1002/chem.201600190
Return to citation in text: [1] -
Xu, W.; Ma, J.; Yuan, X.-A.; Dai, J.; Xie, J.; Zhu, C. Angew. Chem., Int. Ed. 2018, 57, 10357–10361. doi:10.1002/anie.201805927
Return to citation in text: [1] -
Pluta, R.; Nikolaienko, P.; Rueping, M. Angew. Chem., Int. Ed. 2014, 53, 1650–1653. doi:10.1002/anie.201307484
Return to citation in text: [1] -
Rahman, M.; Buchspies, J.; Szostak, M. Catalysts 2019, 9, 129. doi:10.3390/catal9020129
Return to citation in text: [1] [2] -
Luo, J.; Zhang, J. ACS Catal. 2016, 6, 873–877. doi:10.1021/acscatal.5b02204
Return to citation in text: [1] -
Jin, Y.; Yang, H.; Fu, H. Chem. Commun. 2016, 52, 12909–12912. doi:10.1039/c6cc06994k
Return to citation in text: [1] -
Straathof, N. J. W.; Cramer, S. E.; Hessel, V.; Noël, T. Angew. Chem., Int. Ed. 2016, 55, 15549–15553. doi:10.1002/anie.201608297
Return to citation in text: [1]
| 50. | Rahman, M.; Buchspies, J.; Szostak, M. Catalysts 2019, 9, 129. doi:10.3390/catal9020129 |
| 52. | Jin, Y.; Yang, H.; Fu, H. Chem. Commun. 2016, 52, 12909–12912. doi:10.1039/c6cc06994k |
| 53. | Straathof, N. J. W.; Cramer, S. E.; Hessel, V.; Noël, T. Angew. Chem., Int. Ed. 2016, 55, 15549–15553. doi:10.1002/anie.201608297 |
| 1. | Yang, X.; Wu, T.; Phipps, R. J.; Toste, F. D. Chem. Rev. 2015, 115, 826–870. doi:10.1021/cr500277b |
| 2. | Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879 |
| 3. | O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308–319. doi:10.1039/b711844a |
| 4. | Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881–1886. doi:10.1126/science.1131943 |
| 5. | Liang, T.; Neumann, C. N.; Ritter, T. Angew. Chem., Int. Ed. 2013, 52, 8214–8264. doi:10.1002/anie.201206566 |
| 6. | Manteau, B.; Pazenok, S.; Vors, J.-P.; Leroux, F. R. J. Fluorine Chem. 2010, 131, 140–158. doi:10.1016/j.jfluchem.2009.09.009 |
| 18. | Chen, Y.; Li, L.; Ma, Y.; Li, Z. J. Org. Chem. 2019, 84, 5328–5338. doi:10.1021/acs.joc.9b00339 |
| 19. | Yin, G.; Mu, X.; Liu, G. Acc. Chem. Res. 2016, 49, 2413–2423. doi:10.1021/acs.accounts.6b00328 |
| 20. | Wang, X.-X.; Lu, X.; He, S.-J.; Fu, Y. Chem. Sci. 2020, 11, 7950–7956. doi:10.1039/d0sc02054k |
| 21. | Tu, H.-Y.; Zhu, S.; Qing, F.-L.; Chu, L. Synthesis 2020, 52, 1346–1356. doi:10.1055/s-0039-1690842 |
| 22. | Dhungana, R. K.; KC, S.; Basnet, P.; Giri, R. Chem. Rec. 2018, 18, 1314–1340. doi:10.1002/tcr.201700098 |
| 40. | Liu, X.; Liang, Y.; Ji, J.; Luo, J.; Zhao, X. J. Am. Chem. Soc. 2018, 140, 4782–4786. doi:10.1021/jacs.8b01513 |
| 11. | Xu, C.; Ma, B.; Shen, Q. Angew. Chem., Int. Ed. 2014, 53, 9316–9320. doi:10.1002/anie.201403983 |
| 12. | Glenadel, Q.; Alazet, S.; Billard, T. J. Fluorine Chem. 2015, 179, 89–95. doi:10.1016/j.jfluchem.2015.06.007 |
| 13. | Ferry, A.; Billard, T.; Langlois, B. R.; Bacqué, E. Angew. Chem., Int. Ed. 2009, 48, 8551–8555. doi:10.1002/anie.200903387 |
| 14. | Mukherjee, S.; Maji, B.; Tlahuext-Aca, A.; Glorius, F. J. Am. Chem. Soc. 2016, 138, 16200–16203. doi:10.1021/jacs.6b09970 |
| 15. | Boiko, V. N. Beilstein J. Org. Chem. 2010, 6, 880–921. doi:10.3762/bjoc.6.88 |
| 16. | Xu, X.-H.; Matsuzaki, K.; Shibata, N. Chem. Rev. 2015, 115, 731–764. doi:10.1021/cr500193b |
| 17. | Wu, H.; Xiao, Z.; Wu, J.; Guo, Y.; Xiao, J.-C.; Liu, C.; Chen, Q.-Y. Angew. Chem., Int. Ed. 2015, 54, 4070–4074. doi:10.1002/anie.201411953 |
| 41. | Saravanan, P.; Anbarasan, P. Chem. Commun. 2019, 55, 4639–4642. doi:10.1039/c9cc00815b |
| 9. | Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004 |
| 10. | Hansch, C.; Leo, A.; Unger, S. H.; Kim, K. H.; Nikaitani, D.; Lien, E. J. J. Med. Chem. 1973, 16, 1207–1216. doi:10.1021/jm00269a003 |
| 36. | Xiao, Z.; Liu, Y.; Zheng, L.; Liu, C.; Guo, Y.; Chen, Q.-Y. J. Org. Chem. 2018, 83, 5836–5843. doi:10.1021/acs.joc.8b00650 |
| 37. | Yin, F.; Wang, X.-S. Org. Lett. 2014, 16, 1128–1131. doi:10.1021/ol403739w |
| 38. | Dagousset, G.; Simon, C.; Anselmi, E.; Tuccio, B.; Billard, T.; Magnier, E. Chem. – Eur. J. 2017, 23, 4282–4286. doi:10.1002/chem.201700734 |
| 2. | Wang, J.; Sánchez-Roselló, M.; Aceña, J. L.; del Pozo, C.; Sorochinsky, A. E.; Fustero, S.; Soloshonok, V. A.; Liu, H. Chem. Rev. 2014, 114, 2432–2506. doi:10.1021/cr4002879 |
| 7. | Guo, S.; Cong, F.; Guo, R.; Wang, L.; Tang, P. Nat. Chem. 2017, 9, 546–551. doi:10.1038/nchem.2711 |
| 8. | Shao, X.; Hong, X.; Lu, L.; Shen, Q. Tetrahedron 2019, 75, 4156–4166. doi:10.1016/j.tet.2019.05.061 |
| 39. | Liu, Y.-L.; Qing, F.-L.; Xu, X.-H. Eur. J. Org. Chem. 2020, 1015–1018. doi:10.1002/ejoc.201901836 |
| 31. | Jia, Y.; Qin, H.; Wang, N.; Jiang, Z.-X.; Yang, Z. J. Org. Chem. 2018, 83, 2808–2817. doi:10.1021/acs.joc.7b03261 |
| 32. | Jiang, L.; Ding, T.; Yi, W.-b.; Zeng, X.; Zhang, W. Org. Lett. 2018, 20, 2236–2240. doi:10.1021/acs.orglett.8b00581 |
| 34. | Fang, J.; Wang, Z.-K.; Wu, S.-W.; Shen, W.-G.; Ao, G.-Z.; Liu, F. Chem. Commun. 2017, 53, 7638–7641. doi:10.1039/c7cc01903c |
| 28. | Luo, J.; Liu, Y.; Zhao, X. Org. Lett. 2017, 19, 3434–3437. doi:10.1021/acs.orglett.7b01392 |
| 29. | Xiao, Q.; He, Q.; Li, J.; Wang, J. Org. Lett. 2015, 17, 6090–6093. doi:10.1021/acs.orglett.5b03116 |
| 30. | Luo, J.; Zhu, Z.; Liu, Y.; Zhao, X. Org. Lett. 2015, 17, 3620–3623. doi:10.1021/acs.orglett.5b01727 |
| 35. | Xiao, Z.; Liu, Y.; Zheng, L.; Zhou, X.; Xie, Y.; Liu, C.; Guo, Y.; Chen, Q.-Y. Tetrahedron 2018, 74, 6213–6219. doi:10.1016/j.tet.2018.09.019 |
| 24. | Xiang, H.; Yang, C. Org. Lett. 2014, 16, 5686–5689. doi:10.1021/ol502751k |
| 25. | Zhu, L.; Wang, G.; Guo, Q.; Xu, Z.; Zhang, D.; Wang, R. Org. Lett. 2014, 16, 5390–5393. doi:10.1021/ol502624z |
| 26. | Xu, C.; Shen, Q. Org. Lett. 2015, 17, 4561–4563. doi:10.1021/acs.orglett.5b02315 |
| 27. | Zhu, Z.; Luo, J.; Zhao, X. Org. Lett. 2017, 19, 4940–4943. doi:10.1021/acs.orglett.7b02406 |
| 23. | Ji, M.; Wu, Z.; Yu, J.; Wan, X.; Zhu, C. Adv. Synth. Catal. 2017, 359, 1959–1962. doi:10.1002/adsc.201700218 |
| 33. | Yang, T.; Lu, L.; Shen, Q. Chem. Commun. 2015, 51, 5479–5481. doi:10.1039/c4cc08655d |
| 42. | Li, H.; Shan, C.; Tung, C.-H.; Xu, Z. Chem. Sci. 2017, 8, 2610–2615. doi:10.1039/c6sc05093j |
| 38. | Dagousset, G.; Simon, C.; Anselmi, E.; Tuccio, B.; Billard, T.; Magnier, E. Chem. – Eur. J. 2017, 23, 4282–4286. doi:10.1002/chem.201700734 |
| 34. | Fang, J.; Wang, Z.-K.; Wu, S.-W.; Shen, W.-G.; Ao, G.-Z.; Liu, F. Chem. Commun. 2017, 53, 7638–7641. doi:10.1039/c7cc01903c |
| 50. | Rahman, M.; Buchspies, J.; Szostak, M. Catalysts 2019, 9, 129. doi:10.3390/catal9020129 |
| 33. | Yang, T.; Lu, L.; Shen, Q. Chem. Commun. 2015, 51, 5479–5481. doi:10.1039/c4cc08655d |
| 38. | Dagousset, G.; Simon, C.; Anselmi, E.; Tuccio, B.; Billard, T.; Magnier, E. Chem. – Eur. J. 2017, 23, 4282–4286. doi:10.1002/chem.201700734 |
| 48. | Xu, W.; Ma, J.; Yuan, X.-A.; Dai, J.; Xie, J.; Zhu, C. Angew. Chem., Int. Ed. 2018, 57, 10357–10361. doi:10.1002/anie.201805927 |
| 49. | Pluta, R.; Nikolaienko, P.; Rueping, M. Angew. Chem., Int. Ed. 2014, 53, 1650–1653. doi:10.1002/anie.201307484 |
| 42. | Li, H.; Shan, C.; Tung, C.-H.; Xu, Z. Chem. Sci. 2017, 8, 2610–2615. doi:10.1039/c6sc05093j |
| 46. | Li, H.; Liu, S.; Huang, Y.; Xu, X.-H.; Qing, F.-L. Chem. Commun. 2017, 53, 10136–10139. doi:10.1039/c7cc06232j |
| 47. | Honeker, R.; Garza-Sanchez, R. A.; Hopkinson, M. N.; Glorius, F. Chem. – Eur. J. 2016, 22, 4395–4399. doi:10.1002/chem.201600190 |
| 43. | Ouyang, Y.; Xu, X.-H.; Qing, F.-L. Angew. Chem., Int. Ed. 2019, 58, 18508–18512. doi:10.1002/anie.201911323 |
| 44. | Beletskaya, I. P.; Ananikov, V. P. Chem. Rev. 2011, 111, 1596–1636. doi:10.1021/cr100347k |
| 45. | Otsuka, S.; Nogi, K.; Yorimitsu, H. Top. Curr. Chem. 2018, 376, 13. doi:10.1007/s41061-018-0190-7 |
© 2021 Li et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the author(s) and source are credited and that individual graphics may be subject to special legal provisions.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc/terms)