Abstract
Reactions of O,O'-dialkyl thiophosphoric acids with alkyl halides, in the presence of a base, provide a direct synthetic route to phosphorothioates via O,O'-dialkyl thiophosphate anion formation. Studies on the reaction of ambident nucleophile ammonium O,O'-diethyl thiophosphate with benzyl halides and tosylate in different solvents show that only S-alkylation is obtained. Reaction of this ambident nucleophile with benzoyl chloride (a hard electrophile), gave the O-acylation product. A simple, efficient, and general method has been developed for the synthesis of phosphorothioates through a one-pot reaction of alkyl halides with the mixture of diethyl phosphite in the presence of triethylamine/sulfur/and acidic alumina under solvent-free conditions using microwave irradiation.
Graphical Abstract
Introduction
Organophosphorus compounds have found a wide range of application in the areas of industrial, agricultural, and medicinal chemistry owing to their biological and physical properties as well as their utility as synthetic intermediates. [1] The synthesis of phosphate esters is an important objective in organic synthesis, since they have found use in the preparation of biologically active molecules, and also versatile intermediate in synthesis of amides and esters. [2-4] Among the phosphate esters, phosphorothioate derivatives are of interest as effective pesticides. [5-8] In recent years a number of phosphorothioates have been introduced as potential chemotherapeutic agent. [9-12] Despite their wide range of pharmacological activity, industrial and synthetic applications, the synthesis of phosphorothioates has received little attention. The following methods, not generally applicable, have been reported in the literature: (i) reaction of dialkyl phosphites with sulfenyl chlorides,[13] sulfenyl cyanides,[14] thiosulfonates,[15,16] disulfides,[17] and sulfur, [18-21] (ii) condensation of phosphorchloridate with thiols [22-26] and (iii) redox-type reactions of phosphorus triesters with thiols in the presence of tellurium (IV) chloride. [27,28] However, all of these methods have problems, including drastic reaction conditions and also some severe side reactions. Surface-mediated solid phase reactions are of growing interest [29-35] because of their ease of set-up, work-up, mild reaction conditions, rate of the reaction, selectivity, high yields, lack of solvent and the low cost of the reactions in comparison with their homogeneous counterparts. The application of microwave energy to accelerate organic reactions is of increasing interest and offers several advantages over conventional techniques. [36] Synthesis of molecules that normally require long reaction times, can be achieved conveniently and very rapidly in a microwave oven. As a part of our efforts to explore the utility of surface-mediated reactions for the synthesis of organophosphorus compounds, [37-48] we report a new method for the preparation of phosphorothioates by reaction of diethyl phosphite with alkyl halides in the presence of a mixture of ammonium acetate/sulfur/alumina under solvent-free conditions using microwave irradiation which produces high yields of phosphorothioates (Scheme 1).
Scheme 1: Synthesis of phosphorothioates using microwave irradiation
Scheme 1: Synthesis of phosphorothioates using microwave irradiation
Results and Discussion
Recently we have found that ammonium O,O'-diethyl thiophosphate can be obtained by reaction of diethylphosphite in the presence of a mixture of ammonium acetate/sulfur/acidic alumina under solvent-free conditions using microwave irradiation. [49] This reagent can be used as an efficient reagent for the conversion of epoxides to thiiranes. This ambident nucleophile has two potentially attacking atoms (S or O) and can attack with either of them, depending on conditions, and mixtures are often obtained in the reaction with electrophilic centers (Scheme 2). [50]
Scheme 2: Ambident nucleophile ammonium O,O'-diethylthiophosphate
Scheme 2: Ambident nucleophile ammonium O,O'-diethylthiophosphate
We have found that the reaction of diethyl phosphite with alkyl halides in the presence of a mixture of ammonium acetate/sulfur/alumina under solvent-free conditions using microwave irradiation produces high yields of phosphothioates (S-alkylation, Scheme 1). [44] We decided to investigate the reaction of this ambident nuclophile under different conditions (different leaving groups and solvents).
Firstly, we introduce a novel method for large-scale synthesis of ammonium O,O'-diethyl thiophosphate. The reaction of sulfur with diethylphosphite in the presence of ammonium hydrogen carbonate under reflux condition in a solvent mixture of ethyl acetate and diethyl ether (1:1) gave ammonium O,O'-diethyl thiophosphate in quantitative yield (Scheme 3).
Scheme 3: Synthesis of ammonium O,O'-diethyl thiophosphate
Scheme 3: Synthesis of ammonium O,O'-diethyl thiophosphate
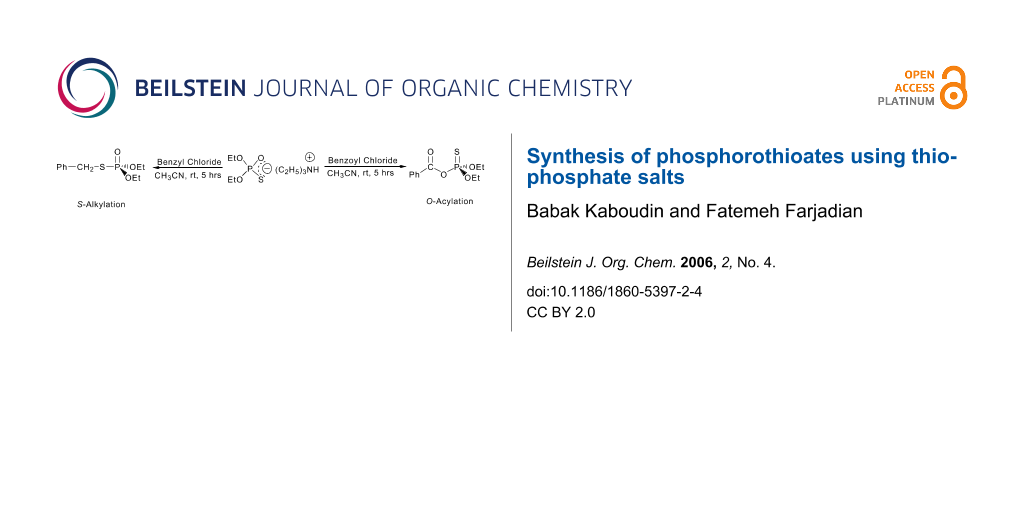
The results of the reaction of this reagent with benzyl bromide, chloride and tosylate in different aporotic and protic solvents show that S-benzyl O,O'-diethyl phosphorothioate (S-alkylation) was formed as sole product (Scheme 4).
Scheme 4: Solvent and leaving group effects on the synthesis phosphorothioates
Scheme 4: Solvent and leaving group effects on the synthesis phosphorothioates
We conclude here that changing of leaving group and use of different media gives no O-alkylation product (i.e. changing from soft to hard leaving group and aprotic to protic solvent). Although ammonium O,O'-diethyl thiophosphate is a potential ambident nucleophile, only its soft center is reactive in this case.
Recently the synthesis of S-thioacyl dithiophosphates has been reported as an efficient and chemoselective thioacylating agent using the reaction of acyl chlorides with dithiophosphoric acid in the presence of pyridine or triethylamine. [51-53] In another study we decided to investigate the reaction of the ambident nucleophile ammonium O,O'-diethyl thiophosphate salt with acyl chlorides. Reaction of ammonium O,O'-diethyl thiophosphate with benzoyl chloride, as a model compound, in acetonitrile gave benzamide as the major product (Scheme 5).
Scheme 5: Reaction of ammonium O,O'-diethyl thiophosphate with benzoyl chloride
Scheme 5: Reaction of ammonium O,O'-diethyl thiophosphate with benzoyl chloride
Benzoyl chloride reacts with ammonia (from ammonium O,O'-diethyl thiophosphate) faster than anion O,O'-diethyl thiophosphate to give benzamide. All efforts for solving this problem failed and in all cases benzamide was obtained as the major product.
We decided to replace this ammonium ion with a triethyl ammonium ion and then to study the reaction of new salt with benzoyl chloride. Triethylammonium O,O'-diethyl thiophosphate was obtained by reaction of diethylphosphite, sulfur and triethyl amine. [54-57] We found that reaction of triethylammonium O,O'-diethyl thiophosphate with benzoyl chloride gave benzoyl O,O'-diethyl phosphorothioate with O-acylation product (Scheme 6).
Scheme 6: Reaction of triethylammonium O,O'-diethyl thiophosphate with benzoyl chloride
Scheme 6: Reaction of triethylammonium O,O'-diethyl thiophosphate with benzoyl chloride
We conclude that replacement of benzyl with benzoyl group (hard electrophilic center) gives the O-acylation product.
As a part of our efforts to explore the utility of surface-mediated reactions for the synthesis of organophosphorus compounds, [16-18] herein we report a new method for the preparation of phosphorothioates by reaction of diethyl phosphite with alkyl halides in the presence of a mixture of triethylamine/sulfur/alumina under solvent-free conditions using microwave irradiation. We found that a mixture of alumina, sulfur, diethylphosphite and triethylamine under microwave irradiation gave triethylammonium O,O'-diethyl thiophosphate that can be used for the synthesis of phosphorothioates under solvent free conditions (Scheme 7, Table 1). As shown in Table 1, a wide range of alkyl halides in the presence triethylamine/sulfur/alumina reacted with diethyl phosphite, giving the required products 2 in moderate to good yields.
Scheme 7: Synthesis of phosphorothioates using triethylammonium O,O'-diethyl thiophosphate using microwave irradiation.
Scheme 7: Synthesis of phosphorothioates using triethylammonium O,O'-diethyl thiophosphate using microwave ir...
Table 1: Reaction of alkyl halides and tosylates in the presence of a mixture of triethyl amine/sulfure/alumina with diethylphosphite under solvent-free conditions
| 2 | R | X | Reaction Time (min) | Yield %a |
|---|---|---|---|---|
| a | PhCH2 | Br | 3 | 62 |
| a | PhCH2 | OTs | 3 | 67 |
| b | PhCH2CH2 | Br | 2 | 72 |
| c | p-NO2C6H4CH2 | Br | 4 | 70 |
| c | p-NO2C6H4CH2 | OTs | 5 | 83 |
| d | o-MeC6H4CH2 | Br | 3 | 65 |
| d | o-MeC6H4CH2 | Cl | 3 | 65 |
| e | p-ClC6H4CH2 | OTs | 2 | 67 |
| f | m-ClC6H4CH2 | OTs | 2 | 55 |
| g | p-MeC6H4CH2 | Br | 4 | 62 |
| h | 1-Butyl | Br | 2 | 76 |
| I | 1-Hexyl | Cl | 5 | 75 |
a: Isolated Yields
In summary, a simple work-up, low consumption of solvent, fast reaction rates, mild reaction conditions, good to excellent yields, relatively clean reactions with no tar formation make these methods an attractive and a useful contribution to present methods for the preparation of phosphorothioates. Studies on the reaction of ambident nucleophile ammonium O,O'-diethyl thiophosphate with benzyl halides and tosylate in different solvents show that only S-alkylation will be obtained as sole product. Reaction of this ambident nucleophile with benzoyl chloride (hard electrophilic center), gave the O-acylation product.
Supporting Information
| Supporting Information File 1: The additional file contains full experimental details | ||
| Format: DOC | Size: 33.5 KB | Download |
References
-
Engel, R. Chem. Rev. 1977, 77, 349. doi:10.1021/cr60307a003
Return to citation in text: [1] -
Deloude, L.; Laszlo, P. J. Org. Chem. 1996, 61, 6360. doi:10.1021/jo960633p
Return to citation in text: [1] -
Varma, R. S.; Meshram, H. M. Tetrahedron Lett. 1997, 38, 7973. doi:10.1016/S0040-4039(97)10143-5
Return to citation in text: [1] -
Smyth, M. S.; Ford, H., Jr.; Burke, T. R., Jr. Tetrahedron Lett. 1992, 33, 4137–4140. doi:10.1016/S0040-4039(00)74672-7
Return to citation in text: [1] -
Burke, T. R.; Smyth, M. S.; Nomizu, M.; Otaka, A.; Roller, P. P. J. Org. Chem. 1993, 58, 1336. doi:10.1021/jo00058a009
Return to citation in text: [1] -
Burke, T. R.; Smyth, M. S.; Otaka, A.; Roller, P. P. Tetrahedron Lett. 1993, 34, 4125. doi:10.1016/S0040-4039(00)60508-7
Return to citation in text: [1] -
Smyth, M. S.; Burke, T. R. Tetrahedron Lett. 1994, 35, 551. doi:10.1016/S0040-4039(00)75835-7
Return to citation in text: [1] -
Benayound, F.; Hammond, G. B. Chem. Commun. 1996, 1447. doi:10.1039/cc9960001447
Return to citation in text: [1] -
Fest, C.; Schmidt, K.-J. The Chemistry of Organophosphorus Pesticides; Springer Verlag: Berlin Heidelberg New York, 1982.
Return to citation in text: [1] -
Uhlman, E.; Peyman, A. Chem. Rev. 1990, 90, 543. doi:10.1021/cr00102a001
Return to citation in text: [1] -
Stein, C. A.; Cheng, Y. C. Science 1993, 261, 1004. doi:10.1126/science.8351515
Return to citation in text: [1] -
Crooke, S. T.; Bennett, C. F. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 107. doi:10.1146/annurev.pa.36.040196.000543
Return to citation in text: [1] -
Elzagheid, M. I.; Mattila, K.; Oivanen, M.; Jones, B. C. N. M.; Cosstick, R.; Lonnberg, H. Eur. J. Org. Chem. 2000, 1987. doi:10.1002/(SICI)1099-0690(200005)2000:10<1987::AID-EJOC1987>3.0.CO;2-X
Return to citation in text: [1] -
Yoshido, M.; Maeda, T.; Sugiyama, H. Chem. Abstr. 1967, 66, 115455g.
Return to citation in text: [1] -
Fokin, A. V.; Kolomiets, A. F.; Iznoskova, M. G. Izv. Akad. Nauk. SSSR, Ser. Khim. 1974, 2837–2840.
Chem. Abstr., 1975, 82, 97323m.
Return to citation in text: [1] -
Schrader, G. Neutral esters of thiolphosphoric acid. US Patent 2,597,534, May 20, 1952.
Chem. Abstr., 1953, 47, 4357g.
Return to citation in text: [1] [2] -
Michalski, J.; Modro, T.; Wieczorkowski, J. J. Chem. Soc. 1960, 1665. doi:10.1039/jr9600001665
Return to citation in text: [1] [2] -
Michalski, J.; Wasiak, J. J. Chem. Soc. 1962, 5056. doi:10.1039/jr9620005056
Return to citation in text: [1] [2] -
Michalski, J.; Wieczorkowski, J.; Wasiak, J.; Pliszka, B. Rocz. Chem. 1959, 33, 247.
Chem. Abstr. 1959, 53, 17884i.
Return to citation in text: [1] -
Harvey, R. G.; Jacobson, H. I.; Jensen, E. V. J. Am. Chem. Soc. 1963, 85, 1618. doi:10.1021/ja00894a018
Return to citation in text: [1] -
Torii, S.; Tanaka, H.; Sayo, N. J. Org. Chem. 1979, 44, 1938.
Return to citation in text: [1] -
Sato, Z.; Shimizu, S.; Shimizu, F.; Kusano, S.; Takagi, K.; Imamiya, Y. Verfahren zur Herstellung von O,O-Dialkyl-S-benzyl-thiophosphaten. Ger. Offen. 26 01 532, Aug 5, 1976.
Chem. Abstr. 1976, 85, 123628y.
Return to citation in text: [1] -
Hashimoto, T.; Ohkubo, T. Chem. Abstr. 1977, 87, 134503w.
Return to citation in text: [1] -
Schrader, G.; Lorenz, W. Benzyl esters of thiophosphoric acids and their production. US Patent 2,862,017, Nov 25, 1958.
Chem. Abstr. 1960, 54, 1437i.
Return to citation in text: [1] -
Farbenfabriken Bayer Akt.-Ges. Process for the production of Thiol-Phosphoric Acid Esters. British Patent 814332, June 3, 1959.
Chem. Abstr. 1960, 54, 17330b.
Return to citation in text: [1] -
Kabachnik, M. I.; Mastrykova, T. A. Zh. Obshch. Khim. 1955, 25, 1924.
Chem. Abstr. 1956, 50, 8499d.
Return to citation in text: [1] -
Schrader, G.; Lorenz, W. Schädlingsbekämpfungsmittel. German Patent 817 057, Aug 23, 1951.
Chem. Abstr. 1954, 48, 6643c.
Return to citation in text: [1] -
Sallmann, R. Verfahren zur Herstellung von Phosphor enthaltenden organischen Verbindungen. Swiss Patent 324980, Nov 30, 1957.
Chem. Abstr. 1958, 52, 14960a.
Return to citation in text: [1] -
Fadel, A.; Yefash, R.; Saluan, J. Synthesis 1987, 37. doi:10.1055/s-1987-27833
Return to citation in text: [1] -
Rosini, G.; Galarini, R.; Marotta, E.; Righi, R. J. Org. Chem. 1990, 55, 781. doi:10.1021/jo00290a001
Return to citation in text: [1] -
Kodomari, M.; Sakamoto, T.; Yoshitomi, S. J. Chem. Soc., Chem. Commun. 1990, 701. doi:10.1039/c39900000701
Return to citation in text: [1] -
Kropp, P. J.; Daus, K. A.; Crawford, S. D.; Tubergren, M. W.; Kepler, K. D.; Craig, S. L.; Wilson, V. P. J. Am. Chem. Soc. 1990, 112, 7433. doi:10.1021/ja00176a075
Return to citation in text: [1] -
Hondrogiannis, G.; Pagni, R. M.; Kabalka, G. W.; Anisoki, P.; Kurt, R. Tetrahedron Lett. 1990, 31, 5433. doi:10.1016/S0040-4039(00)97865-1
Return to citation in text: [1] -
Pantney, H. K. Tetrahedron Lett. 1991, 32, 2259. doi:10.1016/S0040-4039(00)79696-1
Return to citation in text: [1] -
Pauter, F.; Daudon, M. Tetrahedron Lett. 1991, 32, 1457. doi:10.1016/0040-4039(91)80357-C
Return to citation in text: [1] -
Caddick, S. Tetrahedron 1995, 55, 10403. doi:10.1016/0040-4020(95)00662-R
Return to citation in text: [1] -
Sardarian, A. R.; Kaboudin, B. Synth. Commun. 1997, 27, 543.
Return to citation in text: [1] -
Sardarian, A. R.; Kaboudin, B. Tetrahedron Lett. 1997, 38, 2543. doi:10.1016/S0040-4039(97)00396-1
Return to citation in text: [1] -
Kaboudin, B. Tetrahedron Lett. 2000, 41, 3169. doi:10.1016/S0040-4039(00)00323-3
Return to citation in text: [1] -
Kaboudin, B. Chem. Lett. 2001, 880. doi:10.1246/cl.2001.880
Return to citation in text: [1] -
Kaboudin, B.; Nazari, R. Tetrahedron Lett. 2001, 42, 8211. doi:10.1016/S0040-4039(01)01627-6
Return to citation in text: [1] -
Kaboudin, B.; Nazari, R. Synth. Commun. 2001, 31, 2245. doi:10.1081/SCC-100104822
Return to citation in text: [1] -
Kaboudin, B.; Balakrishna, M. S. Synth. Commun. 2001, 31, 2773. doi:10.1081/SCC-100105324
Return to citation in text: [1] -
Kaboudin, B. Tetrahedron Lett. 2002, 43, 8713. doi:10.1016/S0040-4039(02)02136-6
Return to citation in text: [1] [2] -
Kaboudin, B. Tetrahedron Lett. 2003, 44, 1051. doi:10.1016/S0040-4039(02)02727-2
Return to citation in text: [1] -
Kaboudin, B.; Rahmani, A. Synthesis 2003, 2705. doi:10.1055/s-2003-42427
Return to citation in text: [1] -
Kaboudin, B.; Norouzi, H. Synthesis 2004, 2035. doi:10.1055/s-2004-829176
Return to citation in text: [1] -
Kaboudin, B.; Saadati, F. Synthesis 2004, 1249. doi:10.1055/s-2004-822350
Return to citation in text: [1] -
Kaboudin, B.; Norouzi, H. Tetrahedron Lett. 2004, 45, 1283. doi:10.1016/j.tetlet.2003.11.099
Return to citation in text: [1] -
Frey, P. A.; Sammons, R. D. Science 1985, 228, 541. doi:10.1126/science.2984773
Return to citation in text: [1] -
Doszczak, L.; Rachon, J. Chem. Commun. 2000, 2093. doi:10.1039/b005911k
Return to citation in text: [1] -
Doszczak, L.; Rachon, J. J. Chem. Soc., Perkin Trans. 1 2002, 1271. doi:10.1039/b201233b
Return to citation in text: [1] -
Doszczak, L.; Rachon, J. Synthesis 2002, 1047. doi:10.1055/s-2002-31957
Return to citation in text: [1] -
Chojnowski, J.; Cypryk, M.; Fortuniak, W.; Michalski, J. Synthesis 1977, 683. doi:10.1055/s-1977-24532
Return to citation in text: [1] -
Pogosyan, A. S.; Torgomyan, A. M.; Indzhikyan, M. G. J. Gen. Chem. USSR 1990, 60, 1383.
Return to citation in text: [1] -
Nasser, F. A. K.; Mastrukova, T. A. J. Organomet. Chem. 1983, 244, 17. doi:10.1016/S0022-328X(00)98630-3
Return to citation in text: [1] -
Desforges, E.; Grysan, A.; Oget, N.; Sindt, M.; Mieloszynski, J. L. Tetrahedron Lett. 2003, 44, 6273–6276.
Return to citation in text: [1]
| 54. | Chojnowski, J.; Cypryk, M.; Fortuniak, W.; Michalski, J. Synthesis 1977, 683. doi:10.1055/s-1977-24532 |
| 55. | Pogosyan, A. S.; Torgomyan, A. M.; Indzhikyan, M. G. J. Gen. Chem. USSR 1990, 60, 1383. |
| 56. | Nasser, F. A. K.; Mastrukova, T. A. J. Organomet. Chem. 1983, 244, 17. doi:10.1016/S0022-328X(00)98630-3 |
| 57. | Desforges, E.; Grysan, A.; Oget, N.; Sindt, M.; Mieloszynski, J. L. Tetrahedron Lett. 2003, 44, 6273–6276. |
| 44. | Kaboudin, B. Tetrahedron Lett. 2002, 43, 8713. doi:10.1016/S0040-4039(02)02136-6 |
| 51. | Doszczak, L.; Rachon, J. Chem. Commun. 2000, 2093. doi:10.1039/b005911k |
| 52. | Doszczak, L.; Rachon, J. J. Chem. Soc., Perkin Trans. 1 2002, 1271. doi:10.1039/b201233b |
| 53. | Doszczak, L.; Rachon, J. Synthesis 2002, 1047. doi:10.1055/s-2002-31957 |
| 13. | Elzagheid, M. I.; Mattila, K.; Oivanen, M.; Jones, B. C. N. M.; Cosstick, R.; Lonnberg, H. Eur. J. Org. Chem. 2000, 1987. doi:10.1002/(SICI)1099-0690(200005)2000:10<1987::AID-EJOC1987>3.0.CO;2-X |
| 49. | Kaboudin, B.; Norouzi, H. Tetrahedron Lett. 2004, 45, 1283. doi:10.1016/j.tetlet.2003.11.099 |
| 9. | Fest, C.; Schmidt, K.-J. The Chemistry of Organophosphorus Pesticides; Springer Verlag: Berlin Heidelberg New York, 1982. |
| 10. | Uhlman, E.; Peyman, A. Chem. Rev. 1990, 90, 543. doi:10.1021/cr00102a001 |
| 11. | Stein, C. A.; Cheng, Y. C. Science 1993, 261, 1004. doi:10.1126/science.8351515 |
| 12. | Crooke, S. T.; Bennett, C. F. Annu. Rev. Pharmacol. Toxicol. 1996, 36, 107. doi:10.1146/annurev.pa.36.040196.000543 |
| 50. | Frey, P. A.; Sammons, R. D. Science 1985, 228, 541. doi:10.1126/science.2984773 |
| 5. | Burke, T. R.; Smyth, M. S.; Nomizu, M.; Otaka, A.; Roller, P. P. J. Org. Chem. 1993, 58, 1336. doi:10.1021/jo00058a009 |
| 6. | Burke, T. R.; Smyth, M. S.; Otaka, A.; Roller, P. P. Tetrahedron Lett. 1993, 34, 4125. doi:10.1016/S0040-4039(00)60508-7 |
| 7. | Smyth, M. S.; Burke, T. R. Tetrahedron Lett. 1994, 35, 551. doi:10.1016/S0040-4039(00)75835-7 |
| 8. | Benayound, F.; Hammond, G. B. Chem. Commun. 1996, 1447. doi:10.1039/cc9960001447 |
| 2. | Deloude, L.; Laszlo, P. J. Org. Chem. 1996, 61, 6360. doi:10.1021/jo960633p |
| 3. | Varma, R. S.; Meshram, H. M. Tetrahedron Lett. 1997, 38, 7973. doi:10.1016/S0040-4039(97)10143-5 |
| 4. | Smyth, M. S.; Ford, H., Jr.; Burke, T. R., Jr. Tetrahedron Lett. 1992, 33, 4137–4140. doi:10.1016/S0040-4039(00)74672-7 |
| 37. | Sardarian, A. R.; Kaboudin, B. Synth. Commun. 1997, 27, 543. |
| 38. | Sardarian, A. R.; Kaboudin, B. Tetrahedron Lett. 1997, 38, 2543. doi:10.1016/S0040-4039(97)00396-1 |
| 39. | Kaboudin, B. Tetrahedron Lett. 2000, 41, 3169. doi:10.1016/S0040-4039(00)00323-3 |
| 40. | Kaboudin, B. Chem. Lett. 2001, 880. doi:10.1246/cl.2001.880 |
| 41. | Kaboudin, B.; Nazari, R. Tetrahedron Lett. 2001, 42, 8211. doi:10.1016/S0040-4039(01)01627-6 |
| 42. | Kaboudin, B.; Nazari, R. Synth. Commun. 2001, 31, 2245. doi:10.1081/SCC-100104822 |
| 43. | Kaboudin, B.; Balakrishna, M. S. Synth. Commun. 2001, 31, 2773. doi:10.1081/SCC-100105324 |
| 44. | Kaboudin, B. Tetrahedron Lett. 2002, 43, 8713. doi:10.1016/S0040-4039(02)02136-6 |
| 45. | Kaboudin, B. Tetrahedron Lett. 2003, 44, 1051. doi:10.1016/S0040-4039(02)02727-2 |
| 46. | Kaboudin, B.; Rahmani, A. Synthesis 2003, 2705. doi:10.1055/s-2003-42427 |
| 47. | Kaboudin, B.; Norouzi, H. Synthesis 2004, 2035. doi:10.1055/s-2004-829176 |
| 48. | Kaboudin, B.; Saadati, F. Synthesis 2004, 1249. doi:10.1055/s-2004-822350 |
| 18. | Michalski, J.; Wasiak, J. J. Chem. Soc. 1962, 5056. doi:10.1039/jr9620005056 |
| 19. |
Michalski, J.; Wieczorkowski, J.; Wasiak, J.; Pliszka, B. Rocz. Chem. 1959, 33, 247.
Chem. Abstr. 1959, 53, 17884i. |
| 20. | Harvey, R. G.; Jacobson, H. I.; Jensen, E. V. J. Am. Chem. Soc. 1963, 85, 1618. doi:10.1021/ja00894a018 |
| 21. | Torii, S.; Tanaka, H.; Sayo, N. J. Org. Chem. 1979, 44, 1938. |
| 27. |
Schrader, G.; Lorenz, W. Schädlingsbekämpfungsmittel. German Patent 817 057, Aug 23, 1951.
Chem. Abstr. 1954, 48, 6643c. |
| 28. |
Sallmann, R. Verfahren zur Herstellung von Phosphor enthaltenden organischen Verbindungen. Swiss Patent 324980, Nov 30, 1957.
Chem. Abstr. 1958, 52, 14960a. |
| 17. | Michalski, J.; Modro, T.; Wieczorkowski, J. J. Chem. Soc. 1960, 1665. doi:10.1039/jr9600001665 |
| 29. | Fadel, A.; Yefash, R.; Saluan, J. Synthesis 1987, 37. doi:10.1055/s-1987-27833 |
| 30. | Rosini, G.; Galarini, R.; Marotta, E.; Righi, R. J. Org. Chem. 1990, 55, 781. doi:10.1021/jo00290a001 |
| 31. | Kodomari, M.; Sakamoto, T.; Yoshitomi, S. J. Chem. Soc., Chem. Commun. 1990, 701. doi:10.1039/c39900000701 |
| 32. | Kropp, P. J.; Daus, K. A.; Crawford, S. D.; Tubergren, M. W.; Kepler, K. D.; Craig, S. L.; Wilson, V. P. J. Am. Chem. Soc. 1990, 112, 7433. doi:10.1021/ja00176a075 |
| 33. | Hondrogiannis, G.; Pagni, R. M.; Kabalka, G. W.; Anisoki, P.; Kurt, R. Tetrahedron Lett. 1990, 31, 5433. doi:10.1016/S0040-4039(00)97865-1 |
| 34. | Pantney, H. K. Tetrahedron Lett. 1991, 32, 2259. doi:10.1016/S0040-4039(00)79696-1 |
| 35. | Pauter, F.; Daudon, M. Tetrahedron Lett. 1991, 32, 1457. doi:10.1016/0040-4039(91)80357-C |
| 15. |
Fokin, A. V.; Kolomiets, A. F.; Iznoskova, M. G. Izv. Akad. Nauk. SSSR, Ser. Khim. 1974, 2837–2840.
Chem. Abstr., 1975, 82, 97323m. |
| 16. |
Schrader, G. Neutral esters of thiolphosphoric acid. US Patent 2,597,534, May 20, 1952.
Chem. Abstr., 1953, 47, 4357g. |
| 16. |
Schrader, G. Neutral esters of thiolphosphoric acid. US Patent 2,597,534, May 20, 1952.
Chem. Abstr., 1953, 47, 4357g. |
| 17. | Michalski, J.; Modro, T.; Wieczorkowski, J. J. Chem. Soc. 1960, 1665. doi:10.1039/jr9600001665 |
| 18. | Michalski, J.; Wasiak, J. J. Chem. Soc. 1962, 5056. doi:10.1039/jr9620005056 |
| 22. |
Sato, Z.; Shimizu, S.; Shimizu, F.; Kusano, S.; Takagi, K.; Imamiya, Y. Verfahren zur Herstellung von O,O-Dialkyl-S-benzyl-thiophosphaten. Ger. Offen. 26 01 532, Aug 5, 1976.
Chem. Abstr. 1976, 85, 123628y. |
| 23. | Hashimoto, T.; Ohkubo, T. Chem. Abstr. 1977, 87, 134503w. |
| 24. |
Schrader, G.; Lorenz, W. Benzyl esters of thiophosphoric acids and their production. US Patent 2,862,017, Nov 25, 1958.
Chem. Abstr. 1960, 54, 1437i. |
| 25. |
Farbenfabriken Bayer Akt.-Ges. Process for the production of Thiol-Phosphoric Acid Esters. British Patent 814332, June 3, 1959.
Chem. Abstr. 1960, 54, 17330b. |
| 26. |
Kabachnik, M. I.; Mastrykova, T. A. Zh. Obshch. Khim. 1955, 25, 1924.
Chem. Abstr. 1956, 50, 8499d. |
© 2006 Kaboudin and Farjadian; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)