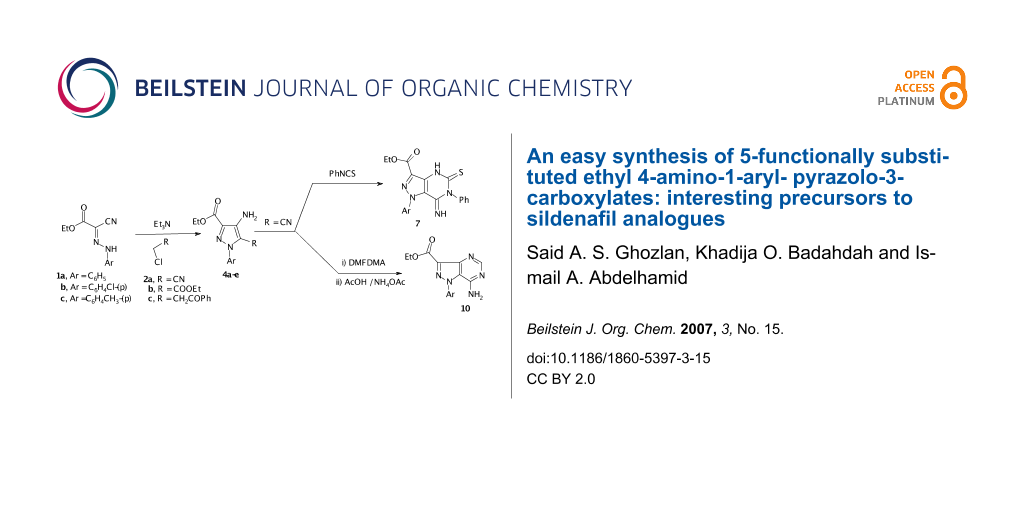
Abstract
3-Oxo-2-arylhydrazononitriles 1a-c react readily with chloroacetonitrile, ethyl chloroacetate, and with phenacyl chloride to give 4-aminopyrazoles 4a-e. The pyrazolo[4,3-d]pyrimidine derivatives 7 and 10 are synthesized via reaction of the aminopyrazole 4b with phenylisothiocyanate and DMFDMA/NH4OAc respectively.
Graphical Abstract
Background
Interest in the chemistry of 4-aminopyrazole carboxylic acid derivatives has recently been recognized as their derivatives are ideal precursors for the synthesis of biologically active pyrazolo[4,3-d]pyrimidine ring systems [1-6]. The reported synthetic approaches to these derivatives are also multistep, non atom economical and non eco friendly [1,5,6]. Recently however a route to 4-aminopyrazole-5-carboxylic acid derivatives via reacting 2-arylhydrazononitriles with α-haloacid derivatives has been reported by Elnagdi et al [7,8] as well as other researchers [9]. In the present article we report results of our work aimed at exploring this synthetic methodology and adoption of products for the synthesis of pyrazolo[4.3-d]pyrimidines. Thus, compounds 1a-c, were prepared according to literature procedures via coupling of ethyl cyanoacetate with aromatic diazonium salts [10]. It has been found that 1a-c react with α-chloroacetonitrile 2a to yield 4a-c, most likely via acyclic intermediates 3a-c that could not be isolated. The structure of 4a-c was confirmed based on 1H NMR spectra that revealed the presence of amino signals and also 13C NMR which revealed the presence of only one CN signal. Similarly reacting 1b with ethyl chloroacetate 2b and with phenacyl chloride 2c afforded 4d,e. The structure of 4d,e was also confirmed based on IR and 13C NMR, which revealed the absence of CN bands and signals (cf. Scheme 1).
Scheme 1: synthesis of Ethyl 4-amino-5-substituted-1-aryl-1H-pyrazole-3-carboxylates (4)
Scheme 1: synthesis of Ethyl 4-amino-5-substituted-1-aryl-1H-pyrazole-3-carboxylates (4)
Compound 4b reacted readily with phenylisothiocyanate to yield a 1:1 adduct. The IR and 13C NMR spectra of the product revealed the absence of CN bands and signals. Thus structure 6 or 7 is suggested. 1H NMR showed two NH signals at δ 8.33 and 10.3 ppm, thus structure 7 is assigned for the reaction product. Acetylation of 4b in acetic anhydride afforded monoacetyl derivative 8. (cf. Scheme 2)
Scheme 2: Reactivity of pyrazole 4b with phenylisothiocyanate and acetic anhydride
Scheme 2: Reactivity of pyrazole 4b with phenylisothiocyanate and acetic anhydride
Compound 4b condensed with dimethylformamide dimethylacetal (DMFDMA) to yield the enamine 9. The 1H NMR spectrum indicated two distinct singlets at ä 2.97 and 3.05 ppm for the N,N-dimethylamino protons which mean that the two methyl groups are magnetically nonequivalent, as to be expected. Compound 9 could be readily converted into pyrazolo[4,3-d]pyrimidine 10 on treatment with AcOH/NH4OAc mixture. (cf. Scheme 3)
Scheme 3: Conversion of pyrazole 4b Ethyl into pyrazolo[4,3-d]pyrimidine-3-carboxylate 10
Scheme 3: Conversion of pyrazole 4b Ethyl into pyrazolo[4,3-d]pyrimidine-3-carboxylate 10
Compound 1 reacted with hydroxylamine hydrochloride in ethanol/sodium acetate solution to yield amidooxime 11 as in the literature [10]. Trials to cyclize the amidooxime into 1,2,3-triazole 12 utilizing the reaction conditions described earlier in literature [11] failed. However, the amidooxime 11 cyclizes smoothly via loss of ethanol in DMF and in presence of anhydrous sodium acetate into isoxazolone 13. (cf. Scheme 4)
Scheme 4: Conversion of arylhydrazononitriles 1 into 3-Amino-4-arylhydrazono-4H-isoxazol-5-one
Scheme 4: Conversion of arylhydrazononitriles 1 into 3-Amino-4-arylhydrazono-4H-isoxazol-5-one
Conclusion
We could show that arylhydrazononitriles 1a-c are valuable precursors to 4-amino-5-substituted-1-aryl-1H-pyrazole-3-carboxylic acid ethyl ester which can be used for preparation of sildenafil analogues.
Supporting Information
| Supporting Information File 1: The experimental section. The experimental data and the results of analysis | ||
| Format: DOC | Size: 46.0 KB | Download |
References
-
Haning, H.; Niewöhner, U.; Schenke, T.; Lampe, T.; Hillisch, A.; Bischoff, E. Bioorg. Med. Chem. Lett. 2005, 15, 3900–3907. doi:10.1016/j.bmcl.2005.05.090
Return to citation in text: [1] [2] -
Kim, D.-K.; Lee, J. Y.; Lee, N.; Ryu, D. H.; Kim, J.-S.; Lee, S.; Choi, J.-Y.; Ryu, J.-H.; Kim, N.-H.; Im, G.-J.; Choi, W.-S.; Kim, T.-K. Bioorg. Med. Chem. 2001, 9, 3013–3021. doi:10.1016/S0968-0896(01)00200-0
Return to citation in text: [1] -
El Haddad, M.; Soukri, M.; Lazar, S.; Bennamara, A.; Guillaumet, G.; Akssira, M. J. Heterocycl. Chem. 2000, 37, 1247–1252.
Return to citation in text: [1] -
Holla, B. S.; Mahalinga, M.; Karthikeyan, M. S.; Akberali, P. M.; Shetty, N. S. Bioorg. Med. Chem. 2006, 14, 2040–2047. doi:10.1016/j.bmc.2005.10.053
Return to citation in text: [1] -
Zhao, Y.-f.; Zhai, X.; Chen, J.-y.; Guo, S.-c.; Gong, P. Chem. Res. Chin. Univ. 2006, 22, 468–473. doi:10.1016/S1005-9040(06)60144-X
Return to citation in text: [1] [2] -
Carpino, P. A.; Griffith, D. A.; Sakya, S.; Dow, R. L.; Black, S. C.; Hadcock, J. R.; Iredale, P. A.; Scott, D. O.; Fichtner, M. W.; Rose, C. R.; Day, R.; Dibrino, J.; Butler, M.; DeBartolo, D. B.; Dutcher, D.; Gautreau, D.; Lizano, J. S.; O'Connor, R. E.; Sands, M. A.; Kelly-Sullivan, D.; Ward, K. M. Bioorg. Med. Chem. 2006, 16, 731–736. doi:10.1016/j.bmcl.2005.10.019
Return to citation in text: [1] [2] -
Abdel-Motaleb, R. M.; Makhloof, A.-M. A.; Ibrahim, H. M.; Elnagdi, M. H. J. Heterocycl. Chem. 2006, 43, 931–934.
Return to citation in text: [1] -
Abdel-Motaleb, R. M.; Makhloof, A.-M. A.-S.; Ibrahim, H. M.; Elnagdi, M. H. J. Heterocycl. Chem. 2007, 44, 109–114.
Return to citation in text: [1] -
Salaheldin, A. M.; Abdallah, T. A.; Radwan, N. F.; Hassaneen, H. M. Z. Naturforsch. 2007, in press.
Return to citation in text: [1] -
Elnagdi, M. H.; Elmoghayar, M. R. H.; Hafez, E. A. A.; Alnima, H. H. J. Org. Chem. 1975, 40, 2604–2607. doi:10.1021/jo00906a007
Return to citation in text: [1] [2] -
Ghozlan, S. A. S.; Abdelhamid, I. A.; Ibrahim, H. M.; Elnagdi, M. H. ARKIVOC 2007, No. xv, 53–60.
Return to citation in text: [1]
| 1. | Haning, H.; Niewöhner, U.; Schenke, T.; Lampe, T.; Hillisch, A.; Bischoff, E. Bioorg. Med. Chem. Lett. 2005, 15, 3900–3907. doi:10.1016/j.bmcl.2005.05.090 |
| 2. | Kim, D.-K.; Lee, J. Y.; Lee, N.; Ryu, D. H.; Kim, J.-S.; Lee, S.; Choi, J.-Y.; Ryu, J.-H.; Kim, N.-H.; Im, G.-J.; Choi, W.-S.; Kim, T.-K. Bioorg. Med. Chem. 2001, 9, 3013–3021. doi:10.1016/S0968-0896(01)00200-0 |
| 3. | El Haddad, M.; Soukri, M.; Lazar, S.; Bennamara, A.; Guillaumet, G.; Akssira, M. J. Heterocycl. Chem. 2000, 37, 1247–1252. |
| 4. | Holla, B. S.; Mahalinga, M.; Karthikeyan, M. S.; Akberali, P. M.; Shetty, N. S. Bioorg. Med. Chem. 2006, 14, 2040–2047. doi:10.1016/j.bmc.2005.10.053 |
| 5. | Zhao, Y.-f.; Zhai, X.; Chen, J.-y.; Guo, S.-c.; Gong, P. Chem. Res. Chin. Univ. 2006, 22, 468–473. doi:10.1016/S1005-9040(06)60144-X |
| 6. | Carpino, P. A.; Griffith, D. A.; Sakya, S.; Dow, R. L.; Black, S. C.; Hadcock, J. R.; Iredale, P. A.; Scott, D. O.; Fichtner, M. W.; Rose, C. R.; Day, R.; Dibrino, J.; Butler, M.; DeBartolo, D. B.; Dutcher, D.; Gautreau, D.; Lizano, J. S.; O'Connor, R. E.; Sands, M. A.; Kelly-Sullivan, D.; Ward, K. M. Bioorg. Med. Chem. 2006, 16, 731–736. doi:10.1016/j.bmcl.2005.10.019 |
| 10. | Elnagdi, M. H.; Elmoghayar, M. R. H.; Hafez, E. A. A.; Alnima, H. H. J. Org. Chem. 1975, 40, 2604–2607. doi:10.1021/jo00906a007 |
| 9. | Salaheldin, A. M.; Abdallah, T. A.; Radwan, N. F.; Hassaneen, H. M. Z. Naturforsch. 2007, in press. |
| 7. | Abdel-Motaleb, R. M.; Makhloof, A.-M. A.; Ibrahim, H. M.; Elnagdi, M. H. J. Heterocycl. Chem. 2006, 43, 931–934. |
| 8. | Abdel-Motaleb, R. M.; Makhloof, A.-M. A.-S.; Ibrahim, H. M.; Elnagdi, M. H. J. Heterocycl. Chem. 2007, 44, 109–114. |
| 1. | Haning, H.; Niewöhner, U.; Schenke, T.; Lampe, T.; Hillisch, A.; Bischoff, E. Bioorg. Med. Chem. Lett. 2005, 15, 3900–3907. doi:10.1016/j.bmcl.2005.05.090 |
| 5. | Zhao, Y.-f.; Zhai, X.; Chen, J.-y.; Guo, S.-c.; Gong, P. Chem. Res. Chin. Univ. 2006, 22, 468–473. doi:10.1016/S1005-9040(06)60144-X |
| 6. | Carpino, P. A.; Griffith, D. A.; Sakya, S.; Dow, R. L.; Black, S. C.; Hadcock, J. R.; Iredale, P. A.; Scott, D. O.; Fichtner, M. W.; Rose, C. R.; Day, R.; Dibrino, J.; Butler, M.; DeBartolo, D. B.; Dutcher, D.; Gautreau, D.; Lizano, J. S.; O'Connor, R. E.; Sands, M. A.; Kelly-Sullivan, D.; Ward, K. M. Bioorg. Med. Chem. 2006, 16, 731–736. doi:10.1016/j.bmcl.2005.10.019 |
| 11. | Ghozlan, S. A. S.; Abdelhamid, I. A.; Ibrahim, H. M.; Elnagdi, M. H. ARKIVOC 2007, No. xv, 53–60. |
| 10. | Elnagdi, M. H.; Elmoghayar, M. R. H.; Hafez, E. A. A.; Alnima, H. H. J. Org. Chem. 1975, 40, 2604–2607. doi:10.1021/jo00906a007 |
© 2007 Ghozlan et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)