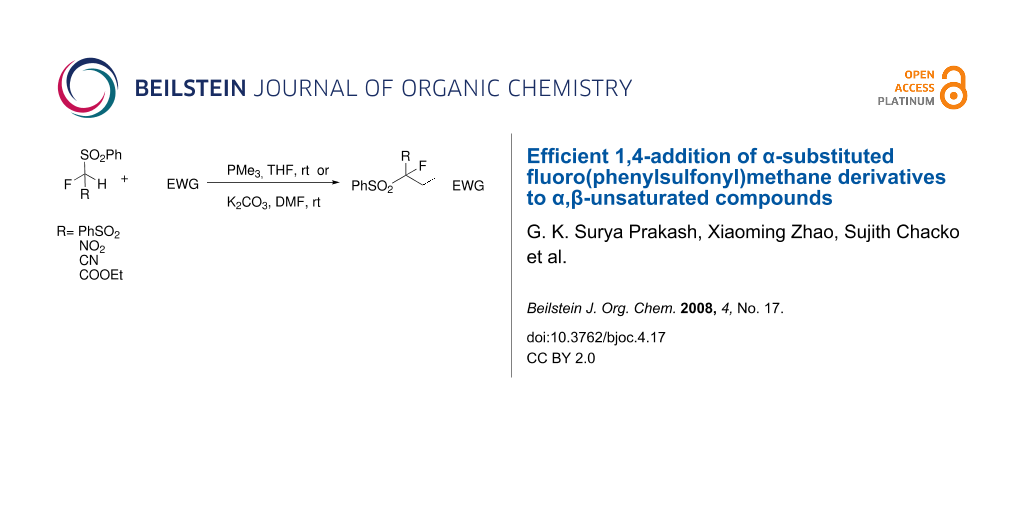
Abstract
The 1,4-addition of a monofluoromethyl nucleophile to a variety of α,β-unsaturated compounds has been achieved under mild conditions using either phosphines or potassium carbonate at room temperature. α-Substituted fluoro(phenylsulfonyl)methane easily undergoes Michael addition to α,β-unsaturated ketones, esters, nitriles, sulfones, as well as propynoates at room temperature to yield the corresponding adducts in moderate to excellent yields.
Graphical Abstract
Background
Compounds with a monofluoromethyl moiety are of great importance with regards to isostere-based drug design [1-4]. Consequently, synthesis of new functionalized α-monofluorine-substituted active methylene derivatives has attracted considerable attention particularly in the field of medicinal chemistry [5,6]. One of the major interests in our group has focused on developing new fluorination reagents or fluorinated building blocks for preparation of fluorine-substituted compounds [7-13]. As part of our ongoing effort to extend the applications of fluorine-containing (phenylsulfonyl)methane derivatives, we envisaged that fluoro(phenylsulfonyl)methane, α-substituted by nitro, cyano, ester, or acetyl would be useful for the synthesis of functionalized monofluoromethylated compounds, which would undergo various transformations. New synthetic methods for the synthesis of α-substituted fluoro(phenylsulfonyl)methane derivatives under mild reaction conditions, using convenient starting materials, are still desirable.
Fluorinated carbanions are in principle "hard" nucleophiles that readily undergo 1,2-addition with Michael type acceptors instead of 1,4-addition [14-16]. Different strategies have been employed to achieve 1,4-addition, which is still percieved to be a challenge. For instance, Yamamoto [17] and Röschenthaler [18,19] have made use of bulky aluminum Lewis acids to protect the carbonyl of Michael acceptors and thus sucessfully transferred the trifluoromethyl anion generated from the "Ruppert-Prakash reagent" (TMS-CF3) in a 1,4-manner rather than the favored 1,2-addition. Portella et al. [20] have shown that 1,4-addition of difluoroenoxysilanes to enones can be used to introduce difluoromethylene moiety while Kumadaki and coworkers [21,22] have used bromodifluoroacetate with a copper catalyst to introduce the CF2 functionality. There also exist few reports on the 1,4-addition of monofluoromethylene moieties to α,β-unsaturated compounds [23,24]. Takeuchi and coworkers [25] have shown that α-fluoronitroalkanes can undergo 1,4-addition to methyl vinyl ketone and acrylonitrile to afford the dialkylated products.
Results and Discussion
Herein, we disclose the facile reaction of fluoro(phenylsulfonyl)methane derivatives and various Michael acceptors. We first began with the preparation of nitro, cyano, ester, or acetyl-substituted (phenylsulfonyl)methanes from the corresponding (phenylthio)methane derivatives, the precursors of α-substituted fluoro(phenylsulfonyl)methane derivatives. Oxidation of (nitromethyl)(phenyl)sulfide with aqueous hydrogen peroxide [H2O2, 30% (wt)] was attempted in acetic acid at room temperature. Tuning the conditions by using 4-fold excess of H2O2 afforded 90% yield of (nitromethylsulfonyl)benzene overnight (Table 1, entry 1) [26-28]. 2a–c and 2e were prepared in 76–91% yields under the optimized condition and used without further purification [30-34]. Interestingly, the oxidation of 1d gave a mixture of 2d and methylsulfonylbenzene in a ratio of 2:1. Hence, compound 2d was synthesized from another known procedure [29]. Fluorobis(phenylsulfonyl)methane 3f was prepared following a literature procedure by fluorinating bis(phenylsulfonyl)methane, which is commercially available [11].
Table 1: Oxidation of sulfides and monofluorination of sulfones.
|
|
|||||
| Entry | R | 2 | Yielda (%) | 3 | Yielda (%) |
|---|---|---|---|---|---|
| 1 | NO2 |
2a |
90 |
3a |
62 |
| 2 | CN |
2b |
76 |
3b |
45 |
| 3 | CO2Et |
2c |
83 |
3c |
56 |
| 4 | COMe |
2d |
b |
3d |
61 |
| 5 | CH=CH2 |
2e |
91 |
3e |
c |
aisolated yield, b2d was prepared according to ref. [29], cno product obtained
Our strategy for the preparation of monofluoro methanes was to use commercially available Selectfluor® [35] as the electrophilic fluorine source. The monofluorination of (nitromethylsulfonyl)benzene with Selectfluor® under the improved conditions [36] [treatment of (nitromethylsulfonyl)benzene (6.75 mmol) with NaH (6.75 mmol) in THF (25 mL) followed by Selectfluor® (6.75 mmol) in 15 mL of DMF at 0 °C] gave [fluoro(nitro)methylsulfonyl]benzene 3a in 62% isolated yield (Table 1, entry 1). A doublet was observed at δ −142.16 ppm in the 19F NMR spectrum of 3a, which matched our previously reported result [11]. Other fluoro(phenylsulfonyl)methane derivatives were synthesized in 45–61% yields under similar conditions (Table 1, entries 2–5).
Alkylation of 3a–d, 3f is reported to be a challenge because the combined carbanion-stabilizing abilities of the two strong electron-withdrawing groups are not sufficient to overcome the well-known carbanion destabilization by the adjacent fluorine [25]. With this in mind, we first opted to use phosphines as nucleophilic catalysts. Concerning applications of 3a–d, and 3f in constructing the carbon-carbon bond, a reaction of [fluoro(nitro)methylsulfonyl]benzene with methyl vinyl ketone was first tested in the presence of PPh3 (50 mol%) in THF at room temperature under argon. Interestingly, 5-fluoro-5-nitro-5-(phenylsulfonyl)pentan-2-one (5a) was obtained in 93% yield as the sole product, which was characterized by 1H, 13C, 19F NMR, and HRMS. In the 19F NMR spectrum of 5a, two doublets at δ −125.94 ppm were observed. As it is known, fluoro substitution can cause problems in transformations, since fluoride can also act as a leaving group. Notably, fluorine-free products were not detected in the course of above-mentioned Michael reactions, under the reaction conditions.
We then screened various electronically and sterically different phosphines such as PPh3, Bu3P, (iPr)3P and PMe3. Initial experiments revealed that the catalytic activity of phosphines and phosphine loading (varying from 20% to 50%) efficiently promoted the Michael reaction. With 50 mol% catalyst loading, the reaction rates were approximately 2- to 3-fold faster than the corresponding 20 mol% PPh3 catalyzed reactions. A dramatic increase in the efficiency of the reaction came from the usage of less bulkier phosphine, PMe3 (20 mol%), which led to the best result (Table 2, entry 1).
Table 2: PMe3-catalyzed reaction of fluoro(phenylsulfonyl)-substituted methane derivatives with methyl vinyl ketone and ethyl acrylate.
|
|
|||||
| Entry | R1 | R2 | Product | Time (h) | Yielda (%) |
|---|---|---|---|---|---|
| 1 | NO2 | Me |
5a |
40 | 93 |
| 2 | CN | Me |
5b |
22 | 90 |
| 3 | CO2Et | Me |
5c |
24 | 75 |
| 4 | PhSO2 | Me |
5d |
17 | 91 |
| 5 | NO2 | OEt |
5e |
72 | 64 |
| 6 | CN | OEt |
5f |
72 | 60 |
| 7 | CO2Et | OEt |
5g |
116 | 71 |
| 8 | PhSO2 | OEt |
5h |
67 | 88 |
aisolated yield
Having established optimal conditions, we then investigated the scope of various substrates in the reaction (Table 2). Methyl vinyl ketone reacted with 3a, 3b, 3c, or 3f to furnish the corresponding products in good to excellent yields, respectively, except in the case of 1-fluoro-1-(phenylsulfonyl)propane-2-one (3d) (Table 2, entries 1–4). Compound 3d gave complex results due to the reactions of the two types of active acidic α-Hs adjacent to the carbonyl group in the molecule.
When ethyl acrylate was subjected to similar reaction conditions, the yields of the products were 60–88% after prolonged reaction time. Compared to methyl vinyl ketone, the reaction rates were slow due to the somewhat lower reactivity of ethyl acrylate (Table 2, entries 5–8). All products obtained were characterised by 19F, 1H, 13C NMR spectra, as well as HRMS. In addition, base-sensitive functional groups such as cyano, nitro, and ester were well tolerated during the course of the reaction. The failure of (E)-pent-3-en-2-one to undergo the Michael reaction under these conditions demonstrates that the reaction is not tolerant of substituents at the terminal position of the double bond due to steric effects.
On the basis of the above mentioned results, a proposed mechanism for the formation of 5a–h is outlined in Scheme 1. Trialkylphosphine catalysed Morita-Baylis-Hillman reaction is well studied by a number of groups [37-39]. Addition of PMe3 to alkene 4 generates the dipolar intermediate. The latter abstracts a proton from the α-fluoro-substituted methylene derivative 3, followed by an intermolecular SN2 reaction to furnish the desired product 5 and the release of PMe3 (Scheme 1). The mechanism supports the fact that the less steric hindered catalyst PMe3 is more efficient than PPh3, Bu3P or (iPr)3P.
Scheme 1: Reaction mechanism for phosphine catalyzed 1,4-addition to α,β-unsaturated compounds.
Scheme 1: Reaction mechanism for phosphine catalyzed 1,4-addition to α,β-unsaturated compounds.
In addition, the presence of electron withdrawing groups such as the phenylsulfonyl group can be exploited to generate a carbanion that can act as a "soft" nucleophile [40,41]. The phenylsulfonyl group delocalises the electron density on the fluorinated carbanion center, which makes the resulting nucleophile softer and more suitable for 1,4-addition with Michael acceptors. Hence, we explored the possibility of a base induced Michael addition reaction. Among the various bases and solvent combinations that we explored, the K2CO3/DMF system was found to be very efficient both in terms of conversions as well as reaction times.
The reactions were carried out at room temperature and the completion observed within 2 h. The reaction was found to be versatile for various α,β-unsaturated compounds such as ketones, esters, nitriles and sulfones (Table 3). In case of α,β-unsaturated aldehydes the reaction was found to be not clean and too many fluorine peaks appeared in the 19F NMR even when the reaction was carried out at low temperatures. On the other hand, α,β-unsaturated nitriles underwent a second Michael addition of the product 6h. Both fluoro(bisphenylsulfonyl)methane and fluoronitro(phenylsulfonyl)methane were added to propynoates under similar conditions. As expected, a mixture of both cis and trans products were obtained as shown in Table 3 (entries 10–11). Interestingly, in the case of fluoronitro(phenylsulfonyl)methane only the trans isomer 6l was obtained in an appreciable amount while the cis product was observed only in traces.
Table 3: K2CO3/DMF catalyzed 1,4-addition to α,β-unsaturated esters, ketones, sulfone, nitriles and propynoates.
|
|
||||
| Entry | R | Substrate | Product (5/6) | Yielda |
|---|---|---|---|---|
| 1 | PhSO2 |
|
|
70 |
| 2 | PhSO2 |
|
|
70 |
| 3 | PhSO2 |
|
|
71 |
| 4 | PhSO2 |
|
|
90 |
| 5 | PhSO2 |
|
|
79 |
| 6 | PhSO2 |
|
|
60 |
| 7 | PhSO2 |
|
|
54 (1:2) |
| 8 | NO2 |
|
|
45 |
| 9 | COOEt |
|
|
65b |
| 10 | PhSO2 |
|
|
76 (2:3)c |
| 11 | PhSO2 |
|
|
60 (1:1)c |
| 12 | NO2 |
|
|
46d |
aisolated yield, bNMR yield (based on 19F NMR), ccis : trans ratio, donly trace amount of cis isomer observed
During our study, we observed that the steric factor affects the addition of the pronucleophile to the Michael acceptor. Substitution at the α-position of the Michael acceptor affects the reactivity of the nucleophile generated by the K2CO3/DMF system. The reaction of fluoro(bisphenylsulfonyl)methane with methyl crotonate was found to give only 50% conversion based on 19F NMR after 36 h and methyl cinnamate did not react at all at room temperature using the K2CO3/DMF system. These observations are consistent with what was observed in the phosphine case discussed earlier. Attempted reductive desulfonylation on compound 6d using Mg/CH3OH [11] was not selective as simultaneous reduction of the carbonyl group was also observed.
Conclusion
In summary, a convenient protocol for the preparation of α-substituted fluoro(phenylsulfonyl)methane derivatives has been described and its subsequent use in 1,4-addition to a variety of Michael acceptors has also been demonstrated. Further applications of fluoro(phenylsulfonyl)methane including stereocontrolled synthesis will be reported in due course.
References
-
Smart, B. E. Characteristics of C-F Systems. In Organofluorine Chemistry: Principles and Commercial Applications; Banks, R. E.; Smart, B. E.; Tatlow, J. C., Eds.; Plenum Press: New York, 1994; Chapter 3.
Return to citation in text: [1] -
Smart, B. E. J. Fluorine Chem. 2001, 109, 3–11. doi:10.1016/S0022-1139(01)00375-X
Return to citation in text: [1] -
Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004.
Return to citation in text: [1] -
Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013–1029. doi:10.1016/j.jfluchem.2006.06.007
Return to citation in text: [1] -
Ojima, I.; McCarthy, J. R.; Welch, J. T., Eds. Biomedical Frontiers of Fluorine Chemistry; ACS Symposium Series 639; American Chemical Society: Washington, DC, 1996.
Return to citation in text: [1] -
Filler, R.; Kobayashi, Y.; Yagupolskii, L. M., Eds. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications; Elsevier: Amsterdam, 1993.
Return to citation in text: [1] -
Prakash, G. K. S.; Hu, J.; Olah, G. A. J. Org. Chem. 2003, 68, 4457–4463. doi:10.1021/jo030110z
Return to citation in text: [1] -
Prakash, G. K. S.; Mandal, M. J. Am. Chem. Soc. 2002, 124, 6538–6539. doi:10.1021/ja020482+
Return to citation in text: [1] -
Prakash, G. K. S.; Hu, J.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2003, 42, 5216–5219. doi:10.1002/anie.200352172
Return to citation in text: [1] -
Prakash, G. K. S.; Mandal, M.; Olah, G. A. Angew. Chem., Int. Ed. 2001, 40, 589–590. doi:10.1002/1521-3773(20010202)40:3<589::AID-ANIE589>3.0.CO;2-9
Return to citation in text: [1] -
Prakash, G. K. S.; Chacko, S.; Alconcel, S.; Stewart, T.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2007, 46, 4933–4936. doi:10.1002/anie.200700834
Return to citation in text: [1] [2] [3] [4] -
Prakash, G. K. S.; Weber, C.; Chacko, S.; Olah, G. A. J. Comb. Chem. 2007, 9, 920–923. doi:10.1021/cc700148z
Return to citation in text: [1] -
Prakash, G. K. S.; Weber, C.; Chacko, S.; Olah, G. A. Org. Lett. 2007, 9, 1863–1866. doi:10.1021/ol070195g
Return to citation in text: [1] -
Prakash, G. K. S.; Hu, J. New Nucleophilic Fluoroalkylation Chemistry. In Fluorine-Containing Synthons; Soloshonok, V. A., Ed.; ACS Symposium Series 911; American Chemical Society: Washington, DC, 2005.
Return to citation in text: [1] -
Prakash, G. K. S.; Yudin, A. K. Chem. Rev. 1997, 97, 757–786. doi:10.1021/cr9408991
Return to citation in text: [1] -
Prakash, G. K. S.; Mandal, M. J. Fluorine Chem. 2001, 112, 123–131. doi:10.1016/S0022-1139(01)00477-8
Return to citation in text: [1] -
Maruoka, K.; Shimada, I.; Akakura, M.; Yamamoto, H. Synlett 1994, 847–848. doi:10.1055/s-1994-23027
Return to citation in text: [1] -
Sevenard, D. V.; Sosnovskikh, V. Y.; Kolomeitsev, A. A.; Königsmann, M. H.; Röschenthaler, G.-V. Tetrahedron Lett. 2003, 44, 7623–7627. doi:10.1016/j.tetlet.2003.08.050
Return to citation in text: [1] -
Sosnovskikh, V. Y.; Usachev, B. I.; Sevenard, D. V.; Röschenthaler, G.-V. J. Org. Chem. 2003, 68, 7747–7754. doi:10.1021/jo034591y
Return to citation in text: [1] -
Lefebvre, O.; Brigaud, T.; Portella, C. Tetrahedron 1998, 54, 5939–5948. doi:10.1016/S0040-4020(98)00224-5
Return to citation in text: [1] -
Sato, K.; Nakazato, S.; Enko, H.; Tsujita, H.; Fujita, K.; Yamamoto, T.; Omote, M.; Ando, A.; Kumadaki, I. J. Fluorine Chem. 2003, 121, 105–107. doi:10.1016/S0022-1139(03)00003-4
Return to citation in text: [1] -
Sato, K.; Omote, M.; Ando, A.; Kumadaki, I. J. Fluorine Chem. 2004, 125, 509–515. doi:10.1016/j.jfluchem.2003.11.023
Return to citation in text: [1] -
Kitazume, T.; Nakayama, Y. J. Org. Chem. 1986, 51, 2795–2799. doi:10.1021/jo00364a034
Return to citation in text: [1] -
Bridge, C. F.; O'Hagan, D. J. Fluorine Chem. 1997, 82, 21–24. doi:10.1016/S0022-1139(96)03535-X
Return to citation in text: [1] -
Takeuchi, Y.; Nagata, K.; Koizumi, T. J. Org. Chem. 1989, 54, 5453–5459. doi:10.1021/jo00284a017
Return to citation in text: [1] [2] -
Wade, P. A.; Hinney, H. R.; Amin, N. V.; Vail, P. D.; Morrow, S. D.; Hardinger, S. A.; Saft, M. S. J. Org. Chem. 1981, 46, 765–770. doi:10.1021/jo00317a023
Return to citation in text: [1] -
Truce, W. E.; Klingler, T. C.; Paar, J. E.; Feuer, H.; Wu, D. K. J. Org. Chem. 1969, 34, 3104–3107. doi:10.1021/jo01262a068
Return to citation in text: [1] -
Peng, W.; Shreeve, J. M. Tetrahedron Lett. 2005, 46, 4905–4909. doi:10.1016/j.tetlet.2005.05.056
Return to citation in text: [1] -
Fargeas, V.; Baalouch, M.; Metay, E.; Baffreau, J.; Ménard, D.; Gosselin, P.; Bergé, J. P.; Barthomeuf, C.; Lebreton, J. Tetrahedron 2004, 60, 10359–10364. doi:10.1016/j.tet.2004.07.087
Return to citation in text: [1] [2] -
Walker, D. J. Org. Chem. 1966, 31, 835–837. doi:10.1021/jo01341a045
Return to citation in text: [1] -
Doyle, K. J.; Moody, C. J. Tetrahedron 1994, 50, 3761–3772. doi:10.1016/S0040-4020(01)90396-5
Return to citation in text: [1] -
Takeuchi, Y.; Asahina, M.; Hori, K.; Koizumi, T. J. Chem. Soc., Perkin Trans. 1 1988, 1149–1153. doi:10.1039/P19880001149
Return to citation in text: [1] -
Ohta, H.; Kato, Y.; Tsuchihashi, G. J. Org. Chem. 1987, 52, 2735–2739. doi:10.1021/jo00389a018
Return to citation in text: [1] -
Takeuchi, Y.; Ogura, H.; Kanada, A.; Koizumi, T. J. Org. Chem. 1992, 57, 2196–2199. doi:10.1021/jo00033a058
Return to citation in text: [1] -
Nyffeler, P. T.; Gonzalez Durón, S.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648
For a review.
Return to citation in text: [1] -
Fukuzumi, T.; Shibata, N.; Sugiura, M.; Yasui, H.; Nakamura, S.; Toru, T. Angew. Chem., Int. Ed. 2006, 45, 4973–4977. doi:10.1002/anie.200600625
Return to citation in text: [1] -
McDougal, N. T.; Schaus, S. E. Angew. Chem., Int. Ed. 2006, 45, 3117–3119. doi:10.1002/anie.200600126
Return to citation in text: [1] -
Kraft, M. E.; Seibert, K. A.; Haxell, T. F. N.; Hirosawa, C. Chem. Commun. 2005, 5772–5774. doi:10.1039/b512665g
Return to citation in text: [1] -
Palmelund, A.; Myers, E. L.; Tai, L. R.; Tisserand, S.; Butts, C. P.; Aggarwal, V. K. Chem. Commun. 2007, 4128–4130. doi:10.1039/b709157e
Return to citation in text: [1] -
Ni, C.; Li, Y.; Hu, J. J. Org. Chem. 2006, 71, 6829–6833. doi:10.1021/jo060955l
Return to citation in text: [1] -
Prakash, G. K. S.; Hu, J. Acc. Chem. Res. 2007, 40, 921–930. doi:10.1021/ar700149s
Return to citation in text: [1]
| 25. | Takeuchi, Y.; Nagata, K.; Koizumi, T. J. Org. Chem. 1989, 54, 5453–5459. doi:10.1021/jo00284a017 |
| 36. | Fukuzumi, T.; Shibata, N.; Sugiura, M.; Yasui, H.; Nakamura, S.; Toru, T. Angew. Chem., Int. Ed. 2006, 45, 4973–4977. doi:10.1002/anie.200600625 |
| 11. | Prakash, G. K. S.; Chacko, S.; Alconcel, S.; Stewart, T.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2007, 46, 4933–4936. doi:10.1002/anie.200700834 |
| 1. | Smart, B. E. Characteristics of C-F Systems. In Organofluorine Chemistry: Principles and Commercial Applications; Banks, R. E.; Smart, B. E.; Tatlow, J. C., Eds.; Plenum Press: New York, 1994; Chapter 3. |
| 2. | Smart, B. E. J. Fluorine Chem. 2001, 109, 3–11. doi:10.1016/S0022-1139(01)00375-X |
| 3. | Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2004. |
| 4. | Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013–1029. doi:10.1016/j.jfluchem.2006.06.007 |
| 17. | Maruoka, K.; Shimada, I.; Akakura, M.; Yamamoto, H. Synlett 1994, 847–848. doi:10.1055/s-1994-23027 |
| 29. | Fargeas, V.; Baalouch, M.; Metay, E.; Baffreau, J.; Ménard, D.; Gosselin, P.; Bergé, J. P.; Barthomeuf, C.; Lebreton, J. Tetrahedron 2004, 60, 10359–10364. doi:10.1016/j.tet.2004.07.087 |
| 14. | Prakash, G. K. S.; Hu, J. New Nucleophilic Fluoroalkylation Chemistry. In Fluorine-Containing Synthons; Soloshonok, V. A., Ed.; ACS Symposium Series 911; American Chemical Society: Washington, DC, 2005. |
| 15. | Prakash, G. K. S.; Yudin, A. K. Chem. Rev. 1997, 97, 757–786. doi:10.1021/cr9408991 |
| 16. | Prakash, G. K. S.; Mandal, M. J. Fluorine Chem. 2001, 112, 123–131. doi:10.1016/S0022-1139(01)00477-8 |
| 35. |
Nyffeler, P. T.; Gonzalez Durón, S.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648
For a review. |
| 7. | Prakash, G. K. S.; Hu, J.; Olah, G. A. J. Org. Chem. 2003, 68, 4457–4463. doi:10.1021/jo030110z |
| 8. | Prakash, G. K. S.; Mandal, M. J. Am. Chem. Soc. 2002, 124, 6538–6539. doi:10.1021/ja020482+ |
| 9. | Prakash, G. K. S.; Hu, J.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2003, 42, 5216–5219. doi:10.1002/anie.200352172 |
| 10. | Prakash, G. K. S.; Mandal, M.; Olah, G. A. Angew. Chem., Int. Ed. 2001, 40, 589–590. doi:10.1002/1521-3773(20010202)40:3<589::AID-ANIE589>3.0.CO;2-9 |
| 11. | Prakash, G. K. S.; Chacko, S.; Alconcel, S.; Stewart, T.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2007, 46, 4933–4936. doi:10.1002/anie.200700834 |
| 12. | Prakash, G. K. S.; Weber, C.; Chacko, S.; Olah, G. A. J. Comb. Chem. 2007, 9, 920–923. doi:10.1021/cc700148z |
| 13. | Prakash, G. K. S.; Weber, C.; Chacko, S.; Olah, G. A. Org. Lett. 2007, 9, 1863–1866. doi:10.1021/ol070195g |
| 29. | Fargeas, V.; Baalouch, M.; Metay, E.; Baffreau, J.; Ménard, D.; Gosselin, P.; Bergé, J. P.; Barthomeuf, C.; Lebreton, J. Tetrahedron 2004, 60, 10359–10364. doi:10.1016/j.tet.2004.07.087 |
| 5. | Ojima, I.; McCarthy, J. R.; Welch, J. T., Eds. Biomedical Frontiers of Fluorine Chemistry; ACS Symposium Series 639; American Chemical Society: Washington, DC, 1996. |
| 6. | Filler, R.; Kobayashi, Y.; Yagupolskii, L. M., Eds. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications; Elsevier: Amsterdam, 1993. |
| 11. | Prakash, G. K. S.; Chacko, S.; Alconcel, S.; Stewart, T.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2007, 46, 4933–4936. doi:10.1002/anie.200700834 |
| 23. | Kitazume, T.; Nakayama, Y. J. Org. Chem. 1986, 51, 2795–2799. doi:10.1021/jo00364a034 |
| 24. | Bridge, C. F.; O'Hagan, D. J. Fluorine Chem. 1997, 82, 21–24. doi:10.1016/S0022-1139(96)03535-X |
| 26. | Wade, P. A.; Hinney, H. R.; Amin, N. V.; Vail, P. D.; Morrow, S. D.; Hardinger, S. A.; Saft, M. S. J. Org. Chem. 1981, 46, 765–770. doi:10.1021/jo00317a023 |
| 27. | Truce, W. E.; Klingler, T. C.; Paar, J. E.; Feuer, H.; Wu, D. K. J. Org. Chem. 1969, 34, 3104–3107. doi:10.1021/jo01262a068 |
| 28. | Peng, W.; Shreeve, J. M. Tetrahedron Lett. 2005, 46, 4905–4909. doi:10.1016/j.tetlet.2005.05.056 |
| 11. | Prakash, G. K. S.; Chacko, S.; Alconcel, S.; Stewart, T.; Mathew, T.; Olah, G. A. Angew. Chem., Int. Ed. 2007, 46, 4933–4936. doi:10.1002/anie.200700834 |
| 21. | Sato, K.; Nakazato, S.; Enko, H.; Tsujita, H.; Fujita, K.; Yamamoto, T.; Omote, M.; Ando, A.; Kumadaki, I. J. Fluorine Chem. 2003, 121, 105–107. doi:10.1016/S0022-1139(03)00003-4 |
| 22. | Sato, K.; Omote, M.; Ando, A.; Kumadaki, I. J. Fluorine Chem. 2004, 125, 509–515. doi:10.1016/j.jfluchem.2003.11.023 |
| 30. | Walker, D. J. Org. Chem. 1966, 31, 835–837. doi:10.1021/jo01341a045 |
| 31. | Doyle, K. J.; Moody, C. J. Tetrahedron 1994, 50, 3761–3772. doi:10.1016/S0040-4020(01)90396-5 |
| 32. | Takeuchi, Y.; Asahina, M.; Hori, K.; Koizumi, T. J. Chem. Soc., Perkin Trans. 1 1988, 1149–1153. doi:10.1039/P19880001149 |
| 33. | Ohta, H.; Kato, Y.; Tsuchihashi, G. J. Org. Chem. 1987, 52, 2735–2739. doi:10.1021/jo00389a018 |
| 34. | Takeuchi, Y.; Ogura, H.; Kanada, A.; Koizumi, T. J. Org. Chem. 1992, 57, 2196–2199. doi:10.1021/jo00033a058 |
| 20. | Lefebvre, O.; Brigaud, T.; Portella, C. Tetrahedron 1998, 54, 5939–5948. doi:10.1016/S0040-4020(98)00224-5 |
| 37. | McDougal, N. T.; Schaus, S. E. Angew. Chem., Int. Ed. 2006, 45, 3117–3119. doi:10.1002/anie.200600126 |
| 38. | Kraft, M. E.; Seibert, K. A.; Haxell, T. F. N.; Hirosawa, C. Chem. Commun. 2005, 5772–5774. doi:10.1039/b512665g |
| 39. | Palmelund, A.; Myers, E. L.; Tai, L. R.; Tisserand, S.; Butts, C. P.; Aggarwal, V. K. Chem. Commun. 2007, 4128–4130. doi:10.1039/b709157e |
| 18. | Sevenard, D. V.; Sosnovskikh, V. Y.; Kolomeitsev, A. A.; Königsmann, M. H.; Röschenthaler, G.-V. Tetrahedron Lett. 2003, 44, 7623–7627. doi:10.1016/j.tetlet.2003.08.050 |
| 19. | Sosnovskikh, V. Y.; Usachev, B. I.; Sevenard, D. V.; Röschenthaler, G.-V. J. Org. Chem. 2003, 68, 7747–7754. doi:10.1021/jo034591y |
| 25. | Takeuchi, Y.; Nagata, K.; Koizumi, T. J. Org. Chem. 1989, 54, 5453–5459. doi:10.1021/jo00284a017 |
| 40. | Ni, C.; Li, Y.; Hu, J. J. Org. Chem. 2006, 71, 6829–6833. doi:10.1021/jo060955l |
| 41. | Prakash, G. K. S.; Hu, J. Acc. Chem. Res. 2007, 40, 921–930. doi:10.1021/ar700149s |
© 2008 Prakash et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)