Abstract
End game synthetic strategy studies towards the total synthesis of the vibsanin type diterpenes, vibsanin E, 3-hydroxyvibsanin E, furanovibsanin A, and 3-O-methylfuranovibsanin A are discussed, with focus on construction of the side chain and peripheral functionality associated with this group of natural products is the current focus of this report.
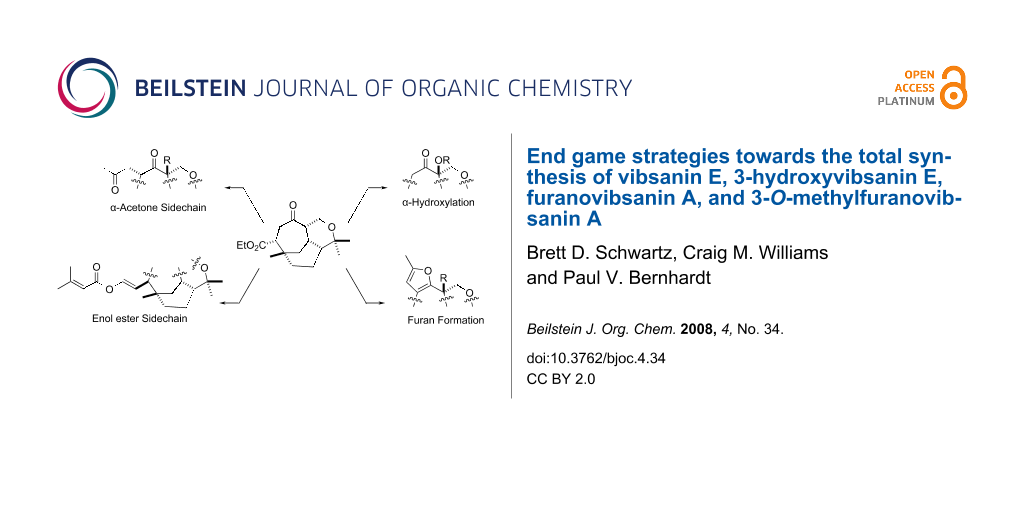
Graphical Abstract
Introduction
Vibsane-type diterpenes occur exclusively in Viburnum species such as V. awabuki [1], V. odoratissimum [2] and V. suspensum [3], and can be regarded as quite rare natural products. Nine structure subtypes have so far been isolated from this family, for example, vibsanin B (1) [1], vibsanin C (2) [1], vibsanin E (3) [1], vibsanin O (4) [4], cyclovibsanin A (5) [5], furanovibsanin D (6) [6], spirovibsanin A (7) [7], aldolvibsanin B (8) [8], and neovibsanin A (9) [9] (Figure 1).
Figure 1: A collection of the structural diversity seen in the vibsanin type diterpene family.
Figure 1: A collection of the structural diversity seen in the vibsanin type diterpene family.
In previous reports our group detailed biogenetically modelled approaches to rapidly access the central core of family members of type 3 [10,11], 5 [10,12] and 7 [13-15] (Figure 1). We now detail end game synthetic strategy studies towards the total synthesis of the vibsanin type diterpenes, vibsanin E (3), 3-hydroxyvibsanin E (13), furanovibsanin A (14), and 3-O-methylfuranovibsanin A (15) (Figure 2) building on core structures 10–12 (Figure 2).
Figure 2: Vibsanin type diterpene synthetic targets.
Figure 2: Vibsanin type diterpene synthetic targets.
Results and Discussion
As shown in the first generation retrosynthesis (Scheme 1) a [4+2] cycloaddition to install the required functionality was envisaged. All attempts, however, to procure this transformation (i.e. 16), that is reaction of isoprene and oxygenated derivatives, with enone 12 completely failed. Davies [16,17], however, demonstrated that a photochemical assisted thermal [4+2] cycloaddition does proceed but with incorrect relative stereochemistry and limited regiocontrol (i.e. 18). Nevertheless, Davies [16] pursued and completed an elegant synthesis of (±)-5,10-bis-epi-vibsanin E based on their cycloaddition methodology.
Scheme 1: Retrosynthesis of vibsanin type targets.
Scheme 1: Retrosynthesis of vibsanin type targets.
With this knowledge in hand, and the availability of racemic 10 [10,11], attention was directed towards stepwise introduction of the required sidechain and corresponding α-oxo functionality depicted in Scheme 2. Essentially four areas were identified for study; 1) regio- and stereospecific α-hydroxylation (methoxylation) 19, 2) furan formation i.e. 20, 3) installing the acetone sidechain i.e. 21, and 4) building the enol ester function i.e. 22 (Scheme 2). The results of each area of investigation allow end game strategies to be postulated based on combinations of these results. For example, success with α-hydroxylation (methoxylation) 19 could flow into furan formation (i.e. 20), installing the acetone sidechain i.e. 21, or building the enol ester function i.e. 22, with subsequent flow into each area to attempt total synthesis (Scheme 2).
Scheme 2: The four functional group areas identified for investigation.
Scheme 2: The four functional group areas identified for investigation.
The first area of study [18] concentrated on implementation of the acetone sidechain. Enolate chemistry was the only viable option in this regard and as such two electrophiles were investigated. Initially the lithium enolate of 23 (best generated with LDA) was reacted with bromoacetone but this afforded only trace amounts of product. Switching to the more active electrophile methallyl bromide gave the desired methallylated product 25 in an optimized yield of 37% along with the undesired regioisomer 24. Temperature was critical to the outcome of the reaction. At −78 °C only undesired regioisomer 24 was obtained in low yield (11%). However, when the enolate was quenched at 0 °C the desired regioisomer 25 was obtained in 15% yield along with the undesired isomer 24 in 17% (Scheme 3, Figure 3). The ratio and yield could be further improved [25 (37%) : 24 (25%)] if the enolate solution was heated to 50 °C before addition of the electrophile. The difficulty in overcoming a significant preference for the undesired regioisomer 24 could be attributed to a number of combined, or individual, factors. For example, the first formed enolate could be stabilised by overlap of the π orbital with the σ*C–O orbital [19], or because the tertiary bridgehead hydrogen is a longer C-H bond than the secondary hydrogen C-H bond, which is kinetically favoured. Conversion of the undesired isomer 24 into the desired (i.e. 25) by a Claisen rearrangment (via the silyl enol ether) was not high yielding and produced many side products.
Figure 3: ORTEP diagrams of compounds 24 and 23 (30% probability elipsoids).
Figure 3: ORTEP diagrams of compounds 24 and 23 (30% probability elipsoids).
Ozonolysis of 25 afforded the acetone sidechain (i.e. 26) in acceptable yield (50%). Other methods to unmask the ketone functionality failed, for example, dihydroxylation followed by oxidative cleavage. Nevertheless, the acetone sidechain could be introduced in ~20% overall yield allowing end game functionalisation (as discussed below).
α-Hydroxylation was next investigated. Considering the observed preference for regiospecific enolate formation in our system we devised a simple two pot procedure based on the epoxidation of silyl enol ethers. Ketone 23 was smoothly converted into the TBS enol ether 27 (85% yield) with TBS triflate, which was then treated with dimethyldioxirane (DMDO). When work up was restricted to a simple 1 M hydrochloric acid wash (i.e. separatory funnel) only the epoxide ring opened product (i.e. 28) was isolated (via epoxide 29). Subsequent treatment of the crude material (i.e. 28) with sodium hydride gave as the sole product the TBS protected α-hydroxy ketone 30 in 80% yield over two steps, via a 1,2-Brook rearrangement. The unprotected derivative 31 could be obtained in 93% yield from 27, via 29, if hydrogen fluoride was used. Unfortunately, methylation of the hydroxy group in compound 31 was unsuccessful since unavoidable C-methylation also occurred to afford 32 (Scheme 4, Figure 4).
Scheme 4: α-Hydroxylation investigations.
Scheme 4: α-Hydroxylation investigations.
Figure 4: ORTEP diagram of compound 32 (30% probability ellipsoids).
Figure 4: ORTEP diagram of compound 32 (30% probability ellipsoids).
In the view that α-hydroxylation, in the form of TBS protection, proceeded so efficiently furan ring formation was investigated with ketone 30. Three general protocols were identified as suitable for attempting fused furan formation with substrate 30; 1) Padwa [20] and Mukaiyama [21] furan synthesis, 2) Nishizawa furan synthesis [22], and 3) classical acid catalysed diketone dehydration (i.e. phosphorus pentaoxide [23]). For Padwa’s protocol the TMS enol ether 33 was required, which was obtained in 75% from sequential treatment of 30 with LDA and TMSCl. Subsequent reaction of 33 with Padwa’s electrophile 34 [24] and silver tetrafluoroborate gave a complex mixture with no identifiable trace of desired product 35, a precusor to desired furan 36 (Scheme 5). The lack of reactivity was without doubt substrate specific (i.e. 33), as model studies on the TMS enol ether of cycloheptanone gave the expected furan product using Padwa’s protocol. Mukaiyama reported [21] the use of electrophile 37 to access the furan ring system using similar conditions to that of Padwa, however, this returned mostly starting material and traces of the tertiary hydroxy compound 38 (Scheme 5).
Scheme 5: Investigating literature methods to install the furan ring system.
Scheme 5: Investigating literature methods to install the furan ring system.
Nishizawa reported [22] the conversion of α-propargyl substituted ketones directly into methylated furans using catalytic amounts of mercury triflate. Although verification of this protocol was undertaken on a cycloheptanone derivative, substrate 39 failed to give the desired furan 42 (Scheme 6). Instead, hydration was observed as the major reaction pathway (i.e. 40) with furan 41 being obtained as the minor component. Furan 41 is an interesting molecule in that it contains a bridgehead double bond, presumably formed due to the ease of carbocation formation at the benzylic (tertiary) centre. Unfortunately, the bridgehead double bond contained within 41 could not be hydrated. Conversion of diketone 40, which could be accessed from 43 (and 44) via a Wacker oxidation (64%), also failed to yield furan functionality using classical conditions (i.e. phosphorus pentaoxide and amberlyst resin) (Scheme 6).
Scheme 6: Installation of the furan ring system continued.
Scheme 6: Installation of the furan ring system continued.
Enol ester sidechain construction: although Davies [16] has reported the construction of the enol ester sidechain (3,3-dimethylacrylic anhydride, 4-pyrrolidinopyridine) associated with the vibsanin family members this functionality was derived from a two carbon chain aldehyde (i.e. CH2CHO). In the current case (i.e. 23) the ester function would require homologation or new methodology to install the enol ester sidechain from one carbon unit (i.e. aldehyde). Considering one carbon homologation would demand multiple steps we opted to develop new methodology. A literature search revealed the work of Anders [25-30], which utilized methyleneoxy ylids of type 45. Our modification [31] introduced 3-methylcrotonate functionality (i.e. 45), which gave similar yields to that reported for the benzoate and related studies [25-30]. For example, treating 23 with lithium aluminium hydride followed by Swern oxidation gave 46 (77% over two steps) which when treated with 45 gave the desired material 47 in 21% yield with an E/Z ratio of 3.4:1 respectively (Scheme 7). This could be improved if the reduction/oxidation [32] sequence was performed on the TBS enol ether 27, which gave 48 in 88% yield and subsequently gave 49 in 32% yield E (2.4) : Z (1)]. Enol ether 49 could be conveniently converted in 92% yield to 47 by treating 49 with hydrogen fluoride pyridine complex at −78 °C (Scheme 7).
Scheme 7: Installation of the enol sidechain utilizing Wittig chemistry.
Scheme 7: Installation of the enol sidechain utilizing Wittig chemistry.
With the four areas of study complete [i.e. α-hydroxylation, furan formation, acetone sidechain, and enol ester function (Scheme 2)] formulation of suitable end game stategies could now be undertaken. In summary, these studies showed that α-hydroxylation was viable and high yielding, the incorporation of the acetone and enol ether sidechains were possible but moderately yielding, and furan formation was not viable. On this basis only two targets seemed approachable: 1) bis-epi-vibsanin E 50, and 2) bis-epi-3-hydroxyvibsanin E 51.
Initial studies concentrated on 26, in that tricarbonyl reduction followed by oxidation was envisaged to give aldehyde 52, which could then undergo reaction with ylid 45 in the hope of gaining access to bis-epi-vibsanin E 50. Reduction with lithium aluminium hydride proceded smoothly, however, global oxidation caused significant problems yielding only very low amounts of aldehyde 52, which was not enough to attempt the Wittig reaction with 45 (Scheme 8).
Scheme 8: Attempts to gain access to targets 50 and 51.
Scheme 8: Attempts to gain access to targets 50 and 51.
In the view of the diasppointing results obtained above (Scheme 8) all attention was directed towards bis-epi-3-hydroxyvibsanin E 51. This manoeuvre was further justified by the fact that diketone 40 was readily available via the allylation/Wacker protocol as described in Scheme 6.
Considering the knowledge gained in Scheme 8, it was perceived best not to perform tricarbonyl reduction then oxidation on diketone 40, but to first protect the ketone functionality as silyl enol ethers as was undertaken in Scheme 7 (i.e. 27–48). Treating diketone 40 with t-butyldimethylsilyl trifluoromethanesulfonate afforded only the monoprotected product 53 (crude yield 55%), which smoothly underwent reduction with diisobutylaluminium hydride, but all attempts to oxidise the diol to 54 failed (Scheme 9). Oxidation and reduction problems occurred also when working with ketone 43, for example, ketone 43 gave only partial reduction and subsequent oxidation of diol 56 gave the aldehyde 55 only in 5% yield (Scheme 9).
Scheme 9: Further attempts to gain access to target compound 51.
Scheme 9: Further attempts to gain access to target compound 51.
Conclusion
In conclusion, we have investigated the construction of four different functionality types [i.e. α-hydroxylation, furan formation, acetone and enol ester sidechain functions (Scheme 2)] associated with the vibsanin family of natural products. These studies were vital for investigating end game strategies for attempting total syntheses of vibsanin E, 3-hydroxyvibsanin E, furanovibsanin A, and 3-O-methylfuranovibsanin A. Unfortunately, the optimum combination of functional group installation could not be found. Nevertheless, valuable insights into the scope and limitations of some literature methods called upon for the attempted total synthesis of this family of natural products were gained.
References
-
Kawazu, K. Agric. Biol. Chem. 1980, 44, 1367–1372.
Return to citation in text: [1] [2] [3] [4] -
Fukuyama, Y.; Minami, H.; Takeuchi, K.; Kodama, M.; Kawazu, K. Tetrahedron Lett. 1996, 37, 6767–6770. doi:10.1016/S0040-4039(96)01463-3
Return to citation in text: [1] -
Fukuyama, Y.; Minami, H.; Fujii, H.; Tajima, M. Phytochemistry 2002, 60, 765–768. doi:10.1016/S0031-9422(02)00153-X
Return to citation in text: [1] -
Duh, C.-Y.; El-Gamal, A. A. H.; Wang, S.-K. Tetrahedron Lett. 2003, 44, 9321–9322. doi:10.1016/j.tetlet.2003.10.084
Return to citation in text: [1] -
Fukuyama, Y.; Morisaki, M.; Minoshima, Y.; Minami, H.; Takahashi, H.; Asakawa, Y. Lett. Org. Chem. 2004, 1, 189–193. doi:10.2174/1570178043488473
Return to citation in text: [1] -
Fukuyama, Y.; Kubo, M.; Fujii, T.; Matsuo, A.; Minoshima, Y.; Minami, H.; Morisaki, M. Tetrahedron 2002, 58, 10033–10041. doi:10.1016/S0040-4020(02)01330-3
Return to citation in text: [1] -
Kubo, M.; Fujii, T.; Hioki, H.; Tanaka, M.; Kawazu, K.; Fukuyama, Y. Tetrahedron Lett. 2001, 42, 1081–1083. doi:10.1016/S0040-4039(00)02181-X
Return to citation in text: [1] -
Kubo, M.; Chen, I.-S.; Minami, H.; Fukuyama, Y. Chem. Pharm. Bull. 1999, 47, 295–296.
Return to citation in text: [1] -
Fukuyama, Y.; Minami, H.; Takeuchi, K.; Kodama, M.; Kawazu, K. Tetrahedron Lett. 1996, 37, 6767–6770. doi:10.1016/S0040-4039(96)01463-3
Return to citation in text: [1] -
Schwartz, B. D.; Tilly, D. P.; Heim, R.; Wiedemann, S.; Williams, C. M.; Bernhardt, P. V. Eur. J. Org. Chem. 2006, 3181–3192. doi:10.1002/ejoc.200600246
Return to citation in text: [1] [2] [3] -
Heim, R.; Wiedemann, S.; Williams, C. M.; Bernhardt, P. V. Org. Lett. 2005, 7, 1327–1329. doi:10.1021/ol0501222
Return to citation in text: [1] [2] -
Tilly, D. P.; Williams, C. M.; Bernhardt, P. V. Org. Lett. 2005, 7, 5155–5157. doi:10.1021/ol051897d
Return to citation in text: [1] -
Gallen, M. J.; Goumont, R.; Clark, T.; Terrier, F.; Williams, C. M. Angew. Chem., Int. Ed. 2006, 45, 2929–2934. doi:10.1002/anie.200504156
Return to citation in text: [1] -
Gallen, M. J.; Williams, C. M. Org. Lett. 2008, 10, 713–715. doi:10.1021/ol702827x
Return to citation in text: [1] -
Gallen, M. J.; Williams, C. M. Eur. J. Org. Chem. 2008, 4697–4705. doi:10.1002/ejoc.200800574
Return to citation in text: [1] -
Davies, H. M. L.; Loe, Ø.; Stafford, D. G. Org. Lett. 2005, 7, 5561–5563. doi:10.1021/ol052005c
Return to citation in text: [1] [2] [3] -
Nikolai, J.; Loe, Ø.; Dominiak, P. M.; Gerlitz, O. O.; Autschbach, J.; Davies, H. M. L. J. Am. Chem. Soc. 2007, 129, 10763–10772. doi:10.1021/ja072090e
Return to citation in text: [1] -
Midway through this investigation an X-ray crystal structure of 10 was obtained which showed the ethoxycarbonyl stereochemistry to be α not β as first predicted. Therefore 10 is referred to the reassigned structure 23. All attempts to epimerise 23 to give 10 failed.
Return to citation in text: [1] -
Murray, L. M.; O’Brien, P.; Taylor, R. J. K.; Wünnemann, S. Tetrahedron Lett. 2004, 45, 2597–2601. doi:10.1016/j.tetlet.2004.01.148
Return to citation in text: [1] -
Padwa, A.; Ishida, M. Tetrahedron Lett. 1991, 32, 5673–5676. doi:10.1016/S0040-4039(00)93526-3
Return to citation in text: [1] -
Mukaiyama, T.; Ishihara, H.; Inomata, K. Chem. Lett. 1975, 527–530. doi:10.1246/cl.1975.527
Return to citation in text: [1] [2] -
Imagawa, H.; Kurisaki, T.; Nishizawa, M. Org. Lett. 2004, 6, 3679–3681. doi:10.1021/ol048730p
Return to citation in text: [1] [2] -
Lai, Y.-H.; Chen, P. Tetrahedron Lett. 1988, 29, 3483–3486. doi:10.1016/0040-4039(88)85196-7
Return to citation in text: [1] -
Padwa, A.; Austin, D. J.; Ishida, M.; Muller, C. L.; Murphree, S. S.; Yeske, P. E. J. Org. Chem. 1992, 57, 1161–1169. doi:10.1021/jo00030a024
Return to citation in text: [1] -
Anders, E.; Gassner, T. Angew. Chem., Int. Ed. Engl. 1982, 21, 289–290. doi:10.1002/anie.198202891
Return to citation in text: [1] [2] -
Anders, E.; Gaßner, T. Angew. Chem. Suppl. 1982, 675–685. doi:10.1002/anie.198206750
Return to citation in text: [1] [2] -
Anders, E.; Gassner, T. Angew. Chem., Int. Ed. Engl. 1983, 22, 619–620. doi:10.1002/anie.198306191
Return to citation in text: [1] [2] -
Anders, E.; Gaßner, T. Chem. Ber. 1984, 117, 1034–1038. doi:10.1002/cber.19841170317
Return to citation in text: [1] [2] -
Anders, E.; Gaßner, T.; Stankowiak, A. Chem. Ber. 1985, 118, 124–131. doi:10.1002/cber.19851180113
Return to citation in text: [1] [2] -
Anders, E.; Clark, T.; Gaßner, T. Chem. Ber. 1986, 119, 1350–1360. doi:10.1002/cber.19861190422
Return to citation in text: [1] [2] -
Schwartz, B. D.; Williams, C. M.; Anders, E.; Bernhardt, P. V. Tetrahedron 2008, 64, 6482–6487. doi:10.1016/j.tet.2008.04.068
Return to citation in text: [1] -
Ley, S. V.; Norman, J.; Griffith, W. P.; Marsden, S. P. Synthesis 1994, 639–666. doi:10.1055/s-1994-25538
Return to citation in text: [1]
| 16. | Davies, H. M. L.; Loe, Ø.; Stafford, D. G. Org. Lett. 2005, 7, 5561–5563. doi:10.1021/ol052005c |
| 25. | Anders, E.; Gassner, T. Angew. Chem., Int. Ed. Engl. 1982, 21, 289–290. doi:10.1002/anie.198202891 |
| 26. | Anders, E.; Gaßner, T. Angew. Chem. Suppl. 1982, 675–685. doi:10.1002/anie.198206750 |
| 27. | Anders, E.; Gassner, T. Angew. Chem., Int. Ed. Engl. 1983, 22, 619–620. doi:10.1002/anie.198306191 |
| 28. | Anders, E.; Gaßner, T. Chem. Ber. 1984, 117, 1034–1038. doi:10.1002/cber.19841170317 |
| 29. | Anders, E.; Gaßner, T.; Stankowiak, A. Chem. Ber. 1985, 118, 124–131. doi:10.1002/cber.19851180113 |
| 30. | Anders, E.; Clark, T.; Gaßner, T. Chem. Ber. 1986, 119, 1350–1360. doi:10.1002/cber.19861190422 |
| 31. | Schwartz, B. D.; Williams, C. M.; Anders, E.; Bernhardt, P. V. Tetrahedron 2008, 64, 6482–6487. doi:10.1016/j.tet.2008.04.068 |
| 13. | Gallen, M. J.; Goumont, R.; Clark, T.; Terrier, F.; Williams, C. M. Angew. Chem., Int. Ed. 2006, 45, 2929–2934. doi:10.1002/anie.200504156 |
| 14. | Gallen, M. J.; Williams, C. M. Org. Lett. 2008, 10, 713–715. doi:10.1021/ol702827x |
| 15. | Gallen, M. J.; Williams, C. M. Eur. J. Org. Chem. 2008, 4697–4705. doi:10.1002/ejoc.200800574 |
| 16. | Davies, H. M. L.; Loe, Ø.; Stafford, D. G. Org. Lett. 2005, 7, 5561–5563. doi:10.1021/ol052005c |
| 17. | Nikolai, J.; Loe, Ø.; Dominiak, P. M.; Gerlitz, O. O.; Autschbach, J.; Davies, H. M. L. J. Am. Chem. Soc. 2007, 129, 10763–10772. doi:10.1021/ja072090e |
| 3. | Fukuyama, Y.; Minami, H.; Fujii, H.; Tajima, M. Phytochemistry 2002, 60, 765–768. doi:10.1016/S0031-9422(02)00153-X |
| 10. | Schwartz, B. D.; Tilly, D. P.; Heim, R.; Wiedemann, S.; Williams, C. M.; Bernhardt, P. V. Eur. J. Org. Chem. 2006, 3181–3192. doi:10.1002/ejoc.200600246 |
| 11. | Heim, R.; Wiedemann, S.; Williams, C. M.; Bernhardt, P. V. Org. Lett. 2005, 7, 1327–1329. doi:10.1021/ol0501222 |
| 2. | Fukuyama, Y.; Minami, H.; Takeuchi, K.; Kodama, M.; Kawazu, K. Tetrahedron Lett. 1996, 37, 6767–6770. doi:10.1016/S0040-4039(96)01463-3 |
| 10. | Schwartz, B. D.; Tilly, D. P.; Heim, R.; Wiedemann, S.; Williams, C. M.; Bernhardt, P. V. Eur. J. Org. Chem. 2006, 3181–3192. doi:10.1002/ejoc.200600246 |
| 12. | Tilly, D. P.; Williams, C. M.; Bernhardt, P. V. Org. Lett. 2005, 7, 5155–5157. doi:10.1021/ol051897d |
| 6. | Fukuyama, Y.; Kubo, M.; Fujii, T.; Matsuo, A.; Minoshima, Y.; Minami, H.; Morisaki, M. Tetrahedron 2002, 58, 10033–10041. doi:10.1016/S0040-4020(02)01330-3 |
| 8. | Kubo, M.; Chen, I.-S.; Minami, H.; Fukuyama, Y. Chem. Pharm. Bull. 1999, 47, 295–296. |
| 5. | Fukuyama, Y.; Morisaki, M.; Minoshima, Y.; Minami, H.; Takahashi, H.; Asakawa, Y. Lett. Org. Chem. 2004, 1, 189–193. doi:10.2174/1570178043488473 |
| 9. | Fukuyama, Y.; Minami, H.; Takeuchi, K.; Kodama, M.; Kawazu, K. Tetrahedron Lett. 1996, 37, 6767–6770. doi:10.1016/S0040-4039(96)01463-3 |
| 4. | Duh, C.-Y.; El-Gamal, A. A. H.; Wang, S.-K. Tetrahedron Lett. 2003, 44, 9321–9322. doi:10.1016/j.tetlet.2003.10.084 |
| 25. | Anders, E.; Gassner, T. Angew. Chem., Int. Ed. Engl. 1982, 21, 289–290. doi:10.1002/anie.198202891 |
| 26. | Anders, E.; Gaßner, T. Angew. Chem. Suppl. 1982, 675–685. doi:10.1002/anie.198206750 |
| 27. | Anders, E.; Gassner, T. Angew. Chem., Int. Ed. Engl. 1983, 22, 619–620. doi:10.1002/anie.198306191 |
| 28. | Anders, E.; Gaßner, T. Chem. Ber. 1984, 117, 1034–1038. doi:10.1002/cber.19841170317 |
| 29. | Anders, E.; Gaßner, T.; Stankowiak, A. Chem. Ber. 1985, 118, 124–131. doi:10.1002/cber.19851180113 |
| 30. | Anders, E.; Clark, T.; Gaßner, T. Chem. Ber. 1986, 119, 1350–1360. doi:10.1002/cber.19861190422 |
| 7. | Kubo, M.; Fujii, T.; Hioki, H.; Tanaka, M.; Kawazu, K.; Fukuyama, Y. Tetrahedron Lett. 2001, 42, 1081–1083. doi:10.1016/S0040-4039(00)02181-X |
| 32. | Ley, S. V.; Norman, J.; Griffith, W. P.; Marsden, S. P. Synthesis 1994, 639–666. doi:10.1055/s-1994-25538 |
| 18. | Midway through this investigation an X-ray crystal structure of 10 was obtained which showed the ethoxycarbonyl stereochemistry to be α not β as first predicted. Therefore 10 is referred to the reassigned structure 23. All attempts to epimerise 23 to give 10 failed. |
| 16. | Davies, H. M. L.; Loe, Ø.; Stafford, D. G. Org. Lett. 2005, 7, 5561–5563. doi:10.1021/ol052005c |
| 10. | Schwartz, B. D.; Tilly, D. P.; Heim, R.; Wiedemann, S.; Williams, C. M.; Bernhardt, P. V. Eur. J. Org. Chem. 2006, 3181–3192. doi:10.1002/ejoc.200600246 |
| 11. | Heim, R.; Wiedemann, S.; Williams, C. M.; Bernhardt, P. V. Org. Lett. 2005, 7, 1327–1329. doi:10.1021/ol0501222 |
| 21. | Mukaiyama, T.; Ishihara, H.; Inomata, K. Chem. Lett. 1975, 527–530. doi:10.1246/cl.1975.527 |
| 22. | Imagawa, H.; Kurisaki, T.; Nishizawa, M. Org. Lett. 2004, 6, 3679–3681. doi:10.1021/ol048730p |
| 23. | Lai, Y.-H.; Chen, P. Tetrahedron Lett. 1988, 29, 3483–3486. doi:10.1016/0040-4039(88)85196-7 |
| 24. | Padwa, A.; Austin, D. J.; Ishida, M.; Muller, C. L.; Murphree, S. S.; Yeske, P. E. J. Org. Chem. 1992, 57, 1161–1169. doi:10.1021/jo00030a024 |
| 21. | Mukaiyama, T.; Ishihara, H.; Inomata, K. Chem. Lett. 1975, 527–530. doi:10.1246/cl.1975.527 |
| 22. | Imagawa, H.; Kurisaki, T.; Nishizawa, M. Org. Lett. 2004, 6, 3679–3681. doi:10.1021/ol048730p |
| 19. | Murray, L. M.; O’Brien, P.; Taylor, R. J. K.; Wünnemann, S. Tetrahedron Lett. 2004, 45, 2597–2601. doi:10.1016/j.tetlet.2004.01.148 |
| 20. | Padwa, A.; Ishida, M. Tetrahedron Lett. 1991, 32, 5673–5676. doi:10.1016/S0040-4039(00)93526-3 |
© 2008 Schwartz et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)