Abstract
The coupling of arylboroxines with a variety of amines, amides, imides and sulfonamides catalyzed by a copper salt/EtOH system has been developed. In the absence of a base or additive the corresponding N-arylation products were obtained in moderate to excellent yields.
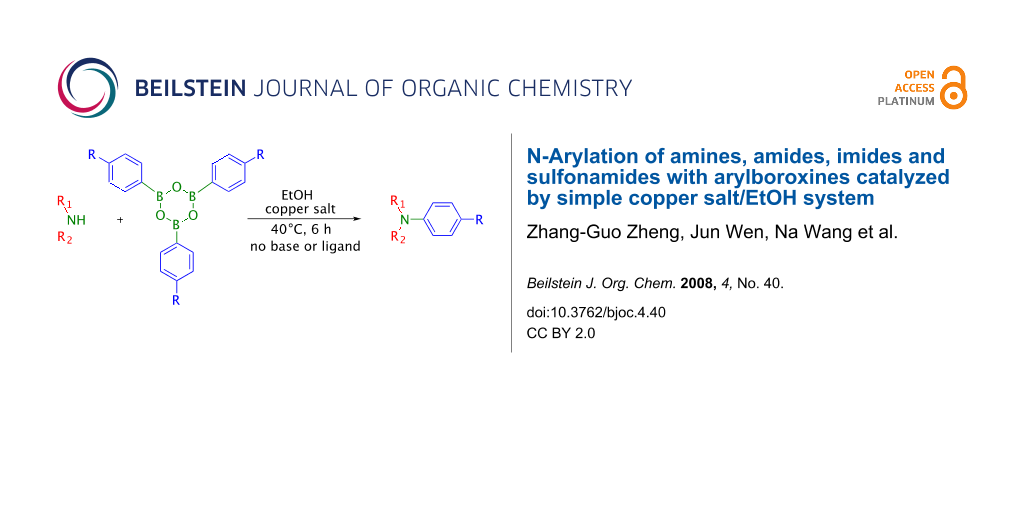
Graphical Abstract
Introduction
The copper-mediated N-arylation reaction plays an important role in organic synthesis since the resultant products, arylamines and N-arylheterocyclic compounds, are ubiquitous compounds in pharmaceuticals, crop-protection chemicals and material science [1-4]. In 1997, the copper-mediated heteroatom arylation reaction using arylboronic acids as aryl donors was discovered by Chan, Evans and Lam independently [5-7]. Based on these studies, further improvements to the catalytic variation of organoboron compounds cross coupling have been reported. Among these organoboron compounds, arylboronic acids are the most used aryl donors in the cross-coupling reaction. However, these reactions were carried out with Et3N [8-10], pyridine [10-13], or TMEDA [14] as base, or addition of ligand [15]. These procedures usually also used a halogenated hydrocarbon as solvent [8-12]. Moreover, the reaction rates of these reactions were generally slow, even requiring 3 d for completion [5-7].
An attractive alternative to this approach is to develop a simple and efficient catalytic system under mild reaction conditions. Thus, a simple copper salt-catalyzed N-arylation of imides with arylboronic acids in protic solvent system had been developed in our previous reports [16,17]. Similar catalytic systems using non-halogenated hydrocarbon solvents have also been reported by Kantam and Prakash [18,19]. However, these procedures require an atmosphere of air or O2 and the use of high temperature, even reflux conditions. It is very dangerous to introduce oxygen or air into a reactor to regenerate the Cu catalyst under reflux condition because of possible explosion and fire hazards especially on large scale. Recently, Kantam et al. reported the N-arylation of imidazoles and amines with arylboronic acids in good yields in methanol at room temperature using copper-exchanged fluorapatite [20]. However, copper-exchanged fluorapatite is a composite salt prepared by a complex, elaborate procedure and has several limitations for the further application.
There has been considerable interest recently in the mechanism of the cross-coupling reaction based on boronic acid. The group of Chan has reported the dynamic behavior of boronic acid in the copper salt catalytic system. The results implied that the active arylating agent such as arylboronic acid in the cross-coupling reaction is indeed its anhydride form (boroxine) and not the free acid [21]. This result prompted us to study arylboroxines as aryl donors instead of arylboronic acids in the cross-coupling reaction, since arylboroxine is more active and may remarkably accelerate the reaction. In this paper we found that N-arylation reaction can be more efficiently promoted under milder reaction conditions when an arylboroxine was used. After the reaction conditions had been optimized, a more general and efficient catalytic system for the cross-coupling reaction of N-arylation was developed in presence of only simple copper salt (Scheme 1). Furthermore, we expand the substrate scope of this reaction: a variety of amines, amides, imides and sulfonamides with arylboroxine can also participate in this catalytic system to give the corresponding N-arylation products in moderate to excellent yields. To the best of our knowledge, N-arylation of NH-containing substrates with arylboroxine conducted in protic solvent only with the use of copper salts has not been explored previously.
Scheme 1: Simple copper salt-catalyzed N-arylation reaction of amines, amides, imides and sulfonamides.
Scheme 1: Simple copper salt-catalyzed N-arylation reaction of amines, amides, imides and sulfonamides.
Results and Discussion
Firstly, we chose phthalimide and phenylboroxine as model substrates to optimize the catalytic conditions (copper source, temperature, solvent, amount of catalyst) to achieve the best results in the cross-coupling reactions (Scheme 2). Several simple copper salts were tested as copper sources to promote the coupling reaction with methanol as solvent. As shown in Table 1, most copper salts (20 mol %) that were used gave the desired products in high yields (Table 1, entries 1–10), and the counterion did not play a significant role. This result is much better than previously reported [10] for a similar catalytic system. However, the time required to accomplish this coupling reaction is quite different with these copper salts. In the case of Cu(OTf)2, which was chosen as copper source, this coupling reaction took only 6 h to accomplish.
Scheme 2: N-arylation reaction of phthalimide with arylboroxine.
Scheme 2: N-arylation reaction of phthalimide with arylboroxine.
Table 1: Optimization of the copper salt for the coupling reactiona.
| Entry | Copper Salt | Timeb (h) | Yieldc (%) |
|---|---|---|---|
| 1 | CuCO3·Cu(OH)2·H2O | 24 | 93 |
| 2 | Cu(NO3)2·2H2O | 11 | 86 |
| 3 | Cu(ClO4)2·7H2O | 6 | 75 |
| 4 | CuSO4·5H2O | 48 | 90 |
| 5 | Cu(OAc)2·H2O | 48 | 86 |
| 6 | CuCl2·2H2O | 12 | 84 |
| 7 | Cu(OTf)2 | 6 | 98 |
| 8 | CuCl | 8 | 98 |
| 9 | CuBr | 20 | 98 |
| 10 | CuI | 20 | 98 |
aReaction conditions: 1a (0.5 mmol), 1b (0.5 mmol), copper salt (0.1 mmol), methanol (5 ml); temperature 40 °C; bMonitored by TLC; cIsolated yield, purity confirmed by MS and 1H NMR.
Reaction temperature plays a crucial role in the cross-coupling reaction. It is reported that reaction time may sometimes be dramatically affected by changing the reaction temperature, which creates opportunities for the activation of the catalytic system. We found that increasing the temperature remarkably accelerated the reaction (Table 2, entries 1–7). A high yield was obtained when the coupling reaction was carried out in methanol at 40 °C within 6 h (Table 2, entry 5). The coupling reaction at 0 °C for almost 2 d gave the same yield of desired product. However, for this cross-coupling reaction, higher temperature was unfavorable as more by-product biphenyl was obtained.
Table 2: Optimization of the temperature for the coupling reactiona.
| Entry | Temperature (°C) | Timeb (h) | Yieldc (%) |
|---|---|---|---|
| 1 | 0 | 48 | 98 |
| 2 | 10 | 30 | 98 |
| 3 | 20 | 20 | 98 |
| 4 | 30 | 15 | 98 |
| 5 | 40 | 6 | 98 |
| 6 | 50 | 18 | 98 |
| 7 | 60 | 72 | 43 |
aReaction conditions: 1a (0.5 mmol), 1b (0.5 mmol), Cu(OTf)2 (0.1 mmol), methanol (5 ml); bMonitored by TLC; cIsolated yield, purity confirmed by MS and 1H NMR.
The effect of solvent on chemical yield was also examined (Table 3, entries 1–9). We firstly selected PhCH3, CH2Cl2, CH3CN as reaction solvents (Table 3, entries 4–6). None of desired products was obtained in the catalytic system of a simple copper salt. However, when protic solvents such as CH3OH and EtOH were employed (Table 3, entries 1 and 2), the desired product was obtained in almost quantitative yields in the simple copper salt system. Being of lower toxicity, easier to process and environmentally benign, EtOH was chosen as reaction medium for this coupling reaction.
Table 3: Optimization of the solvent for the coupling reactiona.
| Entry | Solvent (5 ml) | Timeb (h) | Yieldc (%) |
|---|---|---|---|
| 1 | CH3OH | 6 | 98 |
| 2 | EtOH | 6 | 98 |
| 3 | CH3COOEt | 12 | 90 |
| 4 | CH3CN | >48 | trace |
| 5 | PhCH3 | >48 | trace |
| 6 | CH2Cl2 | >48 | trace |
| 7 | THF | 10 | 36 |
| 8 | DMSO | 23 | 44 |
| 9 | DMF | 5 | 38 |
aReaction conditions: 1a (0.5 mmol), 1b (0.5 mmol), Cu(OTf)2 (0.1 mmol); temperature 40 °C; bMonitored by TLC; cIsolated yield, purity confirmed by MS and 1H NMR.
The ratio of phthalimide to arylboroxine and the amount of Cu(OTf)2 are both important factors for this coupling reaction (Table 4, entries 1–7). We found that when the ratio is more than 1 : 0.5, the arylated product was obtained in almost quantitative yield (Table 4, entries 1–3). Decreasing the ratio to 1 : 0.3, the arylated product was obtained in only 92% yield (Table 4, entry 4). A decrease of the amount of Cu(OTf)2 loading from 20% to 10% had hardly any effect (Table 4, entries 3 and 5), but it took more time to accomplish the reaction when 5 mol % Cu(OTf)2 was used (Table 4, entry 6). Decreasing the amount to 2% (Table 4, entry 7), the product was obtained in only 68% within 48 h.
Table 4: Optimization of the ratio for the coupling reactiona.
| Entry | Ratio (1a : 1b) | Cu(OTf)2 (mol %) | Yieldb (%) |
|---|---|---|---|
| 1 | 1 : 1 | 20 | 98 |
| 2 | 1 : 0.75 | 20 | 98 |
| 3 | 1 : 0.5 | 20 | 98 |
| 4 | 1 : 0.3 | 20 | 92 |
| 5 | 1 : 0.5 | 10 | 98 |
| 6 | 1 : 0.5 | 5 | 98c |
| 7 | 1 : 0.5 | 2 | 68d |
aReaction conditions: 1a (0.5 mmol), EtOH (5 ml); temperature 40 °C, 6 h; bIsolated yield, purity confirmed by MS and 1H NMR; cReaction stirred for 20 h; dReaction stirred for 48 h.
Good yields of cross-coupled products were also obtained with a variety of substrates bearing methyl- and bromophenylboroxines using phthalimide under our generalized conditions (Table 5, entries 2–3). The results demonstrated that there was little difference between the effect of an electron-rich aryl ring and an electron-deficient aryl ring in this cross-coupling reaction.
Table 5: N-Arylation of amines, amides, imides, and sulfonamide with phenylboroxine using copper salt/EtOH systema.
| Entry | Substrate a | Product | Yieldb (%) |
|---|---|---|---|
| 1 |
|
1c |
98 |
| 2 |
|
2c |
96c |
| 3 |
|
3c |
98d |
| 4 |
|
4c |
98 |
| 5 |
|
5c |
92 |
| 6 |
|
6c |
99 |
| 7 |
|
7c |
93 |
| 8 |
|
8c |
92 |
| 9 |
|
9c |
40 |
| 10 |
|
10c |
42 |
| 11 |
|
11c |
62 |
| 12 |
|
12c |
60 |
| 13 |
|
13c |
58 |
| 14 |
|
14c |
55 |
| 15 |
|
15c |
63 |
| 16 |
|
16c |
40 |
| 17 |
|
17c |
56 |
| 18 |
|
18c |
41 |
| 19 |
|
19c |
30 |
aReaction conditions: a (0.5 mmol), 1b (0.25 mmol), Cu(OTf)2 (0.05 mmol), anhyd EtOH (5 ml), 40 °C; bIsolated yield, purity confirmed by MS and 1H NMR; c2b (4-CH3PhBO)3 (0.25 mmol); d3b (4-BrPhBO)3 (0.25 mmol).
In an endeavor to expand the scope of the above methodology, the catalytic system was also applied to imides, amines, amides and sulfonamides. Such coupling was found to give the desired N-arylation products in moderate yields, as shown in Table 5, except for sulfonamide, which afforded the corresponding products in lower yield (Table 5, entry 19). A series of substituted imidazoles (Table 5, entries 5–8) and imides (Table 5, entries 1–4) were coupled with arylboroxine under the generalized reaction conditions to afford the corresponding products in excellent yields, which are comparable to the literature values using a similar catalyst [19]. It is interesting that bis-arylated products were never detected during the course of the coupling reactions, and the result indicated that this catalytic system had high selectivity for different N-nucleophiles, which is consistent with previous work [16].
In general, the reactions are facile, mild and clean for the synthesis of a variety of N-arylated products. Several functional groups, such as Cl-, Br-, CF3- groups remain unaffected under the present reaction conditions.
Conclusion
In summary, a facile and efficient method for the N-arylation of phthalimide with arylboroxine catalyzed by simple copper salt in EtOH was developed in this paper. The catalytic system is base-free, economical, easy to handle and does not need addition of oxygen. Different reaction conditions such as copper salt, temperature, solvent were systematically optimized. The N-arylation reaction of a variety of amines, amides, imides and sulfonamides with arylboroxine can also occur in this catalytic system to give corresponding N-arylation products in moderate to excellent yields.
Supporting Information
| Supporting Information File 1: N-Arylation of amines, amides, imides and sulfonamides with arylboroxine catalyzed by simple copper salt/EtOH system | ||
| Format: DOC | Size: 6.7 MB | Download |
References
-
Ley, S. V.; Thomas, A. W. Angew. Chem., Int. Ed. 2003, 42, 5400–5449. doi:10.1002/anie.200300594
Return to citation in text: [1] -
Lam, P. Y. S.; Vincent, G.; Clark, C. G.; Deudon, S.; Jadhav, P. K. Tetrahedron Lett. 2001, 42, 3415–3418. doi:10.1016/S0040-4039(01)00510-X
Return to citation in text: [1] -
Schlummer, B.; Scholz, U. Adv. Synth. Catal. 2004, 346, 1599–1626. doi:10.1002/adsc.200404216
Return to citation in text: [1] -
Muci, A. R.; Buchwald, S. L. Top. Curr. Chem. 2002, 219, 131–209. doi:10.1007/3-540-45313-X_5
Return to citation in text: [1] -
Chan, D. M. T.; Monaco, K. L.; Wang, R.-P.; Winters, M. P. Tetrahedron Lett. 1998, 39, 2933–2936. doi:10.1016/S0040-4039(98)00503-6
Return to citation in text: [1] [2] -
Evans, D. A.; Katz, J. L.; West, T. R. Tetrahedron Lett. 1998, 39, 2937–2940. doi:10.1016/S0040-4039(98)00502-4
Return to citation in text: [1] [2] -
Lam, P. Y. S.; Clark, C. G.; Saubern, S.; Adams, J.; Winters, M. P.; Chan, D. M. T.; Combs, A. Tetrahedron Lett. 1998, 39, 2941–2944. doi:10.1016/S0040-4039(98)00504-8
Return to citation in text: [1] [2] -
Lam, P. Y. S.; Bonne, D.; Vincent, G.; Clark, C. G.; Combs, A. P. Tetrahedron Lett. 2003, 44, 1691–1694. doi:10.1016/S0040-4039(02)02882-4
Return to citation in text: [1] [2] -
Chernick, E. T.; Ahrens, M. J.; Scheidt, K. A.; Wasielewski, M. R. J. Org. Chem. 2005, 70, 1486–1489. doi:10.1021/jo0481351
Return to citation in text: [1] [2] -
Singh, B. K.; Appukkuttan, P.; Claerhout, S.; Parmar, V. S.; Van der Eycken, E. Org. Lett. 2006, 8, 1863–1866. doi:10.1021/ol060422z
Return to citation in text: [1] [2] [3] [4] -
Rossiter, S.; Woo, C. K.; Hartzoulakis, B.; Wishart, G.; Stanyer, L.; Labadie, J. W.; Selwood, D. L. J. Comb. Chem. 2004, 6, 385–390. doi:10.1021/cc034065k
Return to citation in text: [1] [2] -
Jacobsen, M. F.; Knudsen, M. M.; Gothelf, K. V. J. Org. Chem. 2006, 71, 9183–9190. doi:10.1021/jo061694i
Return to citation in text: [1] [2] -
Nishiura, K.; Urawa, Y.; Soda, S. Adv. Synth. Catal. 2004, 346, 1679–1684. doi:10.1002/adsc.200404193
Return to citation in text: [1] -
Yue, Y.; Zheng, Z.-G.; Wu, B.; Xia, C.-Q.; Yu, X.-Q. Eur. J. Org. Chem. 2005, 5154–5157. doi:10.1002/ejoc.200500589
Return to citation in text: [1] -
Antilla, J. C.; Buchwald, S. L. Org. Lett. 2001, 3, 2077–2079. doi:10.1021/ol0160396
Return to citation in text: [1] -
Lan, J.-B.; Chen, L.; Yu, X.-Q.; You, J.-S.; Xie, R.-G. Chem. Commun. 2004, 188–189. doi:10.1039/b307734a
Return to citation in text: [1] [2] -
Lan, J.-B.; Zhang, G.-L.; Yu, X.-Q.; You, J.-S.; Chen, L.; Yan, M.; Xie, R.-G. Synlett 2004, 1095–1097. doi:10.1055/s-2004-820059
Return to citation in text: [1] -
Kantam, M. L.; Prakash, B. V.; Reddy, C. V. J. Mol. Catal. A 2005, 241, 162–165. doi:10.1016/j.molcata.2005.07.015
Return to citation in text: [1] -
Kantam, M. L.; Neelima, B.; Reddy, C. V.; Neeraja, V. J. Mol. Catal. A 2006, 249, 201–206. doi:10.1016/j.molcata.2006.01.012
Return to citation in text: [1] [2] -
Kantam, M. L.; Venkanna, G. T.; Sridhar, C.; Sreedhar, B.; Choudary, B. M. J. Org. Chem. 2006, 71, 9522–9524. doi:10.1021/jo0614036
Return to citation in text: [1] -
Chan, D. M. T.; Monaco, K. L.; Li, R.; Bonne, D.; Clark, C. G.; Lam, P. Y. S. Tetrahedron Lett. 2003, 44, 3863–3865. doi:10.1016/S0040-4039(03)00739-1
Return to citation in text: [1]
| 1. | Ley, S. V.; Thomas, A. W. Angew. Chem., Int. Ed. 2003, 42, 5400–5449. doi:10.1002/anie.200300594 |
| 2. | Lam, P. Y. S.; Vincent, G.; Clark, C. G.; Deudon, S.; Jadhav, P. K. Tetrahedron Lett. 2001, 42, 3415–3418. doi:10.1016/S0040-4039(01)00510-X |
| 3. | Schlummer, B.; Scholz, U. Adv. Synth. Catal. 2004, 346, 1599–1626. doi:10.1002/adsc.200404216 |
| 4. | Muci, A. R.; Buchwald, S. L. Top. Curr. Chem. 2002, 219, 131–209. doi:10.1007/3-540-45313-X_5 |
| 14. | Yue, Y.; Zheng, Z.-G.; Wu, B.; Xia, C.-Q.; Yu, X.-Q. Eur. J. Org. Chem. 2005, 5154–5157. doi:10.1002/ejoc.200500589 |
| 16. | Lan, J.-B.; Chen, L.; Yu, X.-Q.; You, J.-S.; Xie, R.-G. Chem. Commun. 2004, 188–189. doi:10.1039/b307734a |
| 10. | Singh, B. K.; Appukkuttan, P.; Claerhout, S.; Parmar, V. S.; Van der Eycken, E. Org. Lett. 2006, 8, 1863–1866. doi:10.1021/ol060422z |
| 11. | Rossiter, S.; Woo, C. K.; Hartzoulakis, B.; Wishart, G.; Stanyer, L.; Labadie, J. W.; Selwood, D. L. J. Comb. Chem. 2004, 6, 385–390. doi:10.1021/cc034065k |
| 12. | Jacobsen, M. F.; Knudsen, M. M.; Gothelf, K. V. J. Org. Chem. 2006, 71, 9183–9190. doi:10.1021/jo061694i |
| 13. | Nishiura, K.; Urawa, Y.; Soda, S. Adv. Synth. Catal. 2004, 346, 1679–1684. doi:10.1002/adsc.200404193 |
| 8. | Lam, P. Y. S.; Bonne, D.; Vincent, G.; Clark, C. G.; Combs, A. P. Tetrahedron Lett. 2003, 44, 1691–1694. doi:10.1016/S0040-4039(02)02882-4 |
| 9. | Chernick, E. T.; Ahrens, M. J.; Scheidt, K. A.; Wasielewski, M. R. J. Org. Chem. 2005, 70, 1486–1489. doi:10.1021/jo0481351 |
| 10. | Singh, B. K.; Appukkuttan, P.; Claerhout, S.; Parmar, V. S.; Van der Eycken, E. Org. Lett. 2006, 8, 1863–1866. doi:10.1021/ol060422z |
| 10. | Singh, B. K.; Appukkuttan, P.; Claerhout, S.; Parmar, V. S.; Van der Eycken, E. Org. Lett. 2006, 8, 1863–1866. doi:10.1021/ol060422z |
| 5. | Chan, D. M. T.; Monaco, K. L.; Wang, R.-P.; Winters, M. P. Tetrahedron Lett. 1998, 39, 2933–2936. doi:10.1016/S0040-4039(98)00503-6 |
| 6. | Evans, D. A.; Katz, J. L.; West, T. R. Tetrahedron Lett. 1998, 39, 2937–2940. doi:10.1016/S0040-4039(98)00502-4 |
| 7. | Lam, P. Y. S.; Clark, C. G.; Saubern, S.; Adams, J.; Winters, M. P.; Chan, D. M. T.; Combs, A. Tetrahedron Lett. 1998, 39, 2941–2944. doi:10.1016/S0040-4039(98)00504-8 |
| 19. | Kantam, M. L.; Neelima, B.; Reddy, C. V.; Neeraja, V. J. Mol. Catal. A 2006, 249, 201–206. doi:10.1016/j.molcata.2006.01.012 |
| 16. | Lan, J.-B.; Chen, L.; Yu, X.-Q.; You, J.-S.; Xie, R.-G. Chem. Commun. 2004, 188–189. doi:10.1039/b307734a |
| 17. | Lan, J.-B.; Zhang, G.-L.; Yu, X.-Q.; You, J.-S.; Chen, L.; Yan, M.; Xie, R.-G. Synlett 2004, 1095–1097. doi:10.1055/s-2004-820059 |
| 20. | Kantam, M. L.; Venkanna, G. T.; Sridhar, C.; Sreedhar, B.; Choudary, B. M. J. Org. Chem. 2006, 71, 9522–9524. doi:10.1021/jo0614036 |
| 5. | Chan, D. M. T.; Monaco, K. L.; Wang, R.-P.; Winters, M. P. Tetrahedron Lett. 1998, 39, 2933–2936. doi:10.1016/S0040-4039(98)00503-6 |
| 6. | Evans, D. A.; Katz, J. L.; West, T. R. Tetrahedron Lett. 1998, 39, 2937–2940. doi:10.1016/S0040-4039(98)00502-4 |
| 7. | Lam, P. Y. S.; Clark, C. G.; Saubern, S.; Adams, J.; Winters, M. P.; Chan, D. M. T.; Combs, A. Tetrahedron Lett. 1998, 39, 2941–2944. doi:10.1016/S0040-4039(98)00504-8 |
| 21. | Chan, D. M. T.; Monaco, K. L.; Li, R.; Bonne, D.; Clark, C. G.; Lam, P. Y. S. Tetrahedron Lett. 2003, 44, 3863–3865. doi:10.1016/S0040-4039(03)00739-1 |
| 8. | Lam, P. Y. S.; Bonne, D.; Vincent, G.; Clark, C. G.; Combs, A. P. Tetrahedron Lett. 2003, 44, 1691–1694. doi:10.1016/S0040-4039(02)02882-4 |
| 9. | Chernick, E. T.; Ahrens, M. J.; Scheidt, K. A.; Wasielewski, M. R. J. Org. Chem. 2005, 70, 1486–1489. doi:10.1021/jo0481351 |
| 10. | Singh, B. K.; Appukkuttan, P.; Claerhout, S.; Parmar, V. S.; Van der Eycken, E. Org. Lett. 2006, 8, 1863–1866. doi:10.1021/ol060422z |
| 11. | Rossiter, S.; Woo, C. K.; Hartzoulakis, B.; Wishart, G.; Stanyer, L.; Labadie, J. W.; Selwood, D. L. J. Comb. Chem. 2004, 6, 385–390. doi:10.1021/cc034065k |
| 12. | Jacobsen, M. F.; Knudsen, M. M.; Gothelf, K. V. J. Org. Chem. 2006, 71, 9183–9190. doi:10.1021/jo061694i |
| 15. | Antilla, J. C.; Buchwald, S. L. Org. Lett. 2001, 3, 2077–2079. doi:10.1021/ol0160396 |
| 18. | Kantam, M. L.; Prakash, B. V.; Reddy, C. V. J. Mol. Catal. A 2005, 241, 162–165. doi:10.1016/j.molcata.2005.07.015 |
| 19. | Kantam, M. L.; Neelima, B.; Reddy, C. V.; Neeraja, V. J. Mol. Catal. A 2006, 249, 201–206. doi:10.1016/j.molcata.2006.01.012 |
© 2008 Zheng et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)