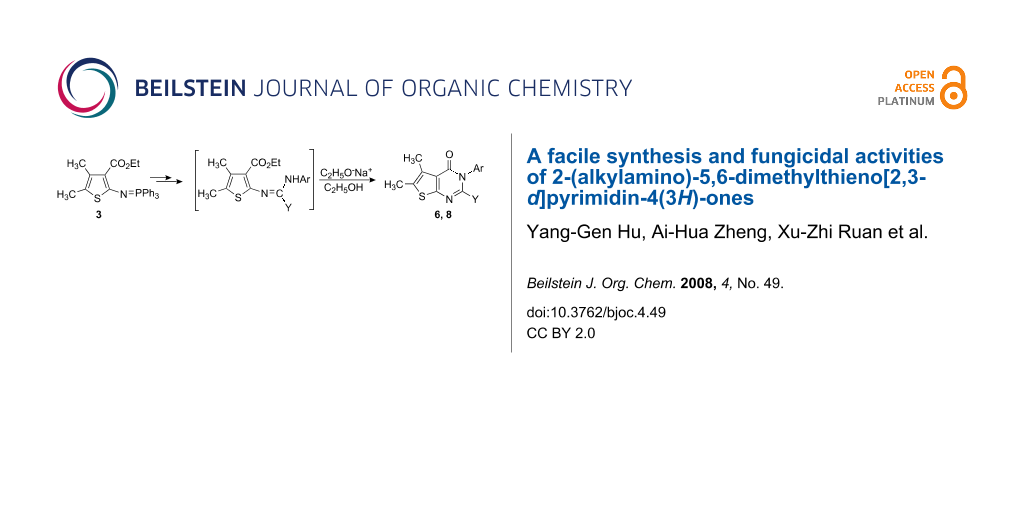
Abstract
The aza-Wittig reactions of iminophosphorane 3 with aromatic isocyanates generated carbodiimides 4, which were reacted with alkylamines under mild conditions to give a series of 2-(alkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 6 and 8 in satisfactory yield. Their structures were confirmed by 1H NMR, EI-MS, IR and elementary analysis, and compound 8c was further analyzed by single-crystal X-ray diffraction. The preliminary bioassays indicated that these compounds showed excellent fungicidal activities against six kinds of fungi.
Graphical Abstract
Introduction
Over the past ten years, aza-Wittig reactions of functionalized iminophosphoranes with isocyanates have been applied to produce carbodiimides, functional groups consisting of the formula N=C=N, able to undergo a plethora of heterocyclization reactions [1-6]. At the same time, many heterocycles containing thienopyrimidine system are of great importance for use as potential drugs because of their remarkable biological activity. For example, some 2-alkylthio- or 2-alkyl-substituted thienopyrimidinones show significant antifungal and antibacterial activities [7,8], whereas others exhibit good anticonvulsant and angiotensin or H1 receptor antagonistic activities [9]. The chemistry of thienopyrimidinones has also received attention because their starting materials, 2-amino-3-carboxythiophenes, can be conveniently synthesized by Gewald reaction [10]. Synthetically useful approaches to thienopyrimidinones starting from easily accessible 2-amino-3-carboxythiophenes are therefore of great importance. Recently we have been interested in the synthesis of a series of new heterocyclic compounds via aza-Wittig reaction of α- or β-(ethoxycarbonyl)-substituted iminophosphoranes with aromatic isocyanates and subsequent reaction with various nucleophiles under mild conditions [11-14]. Herein we wish to report an efficient synthesis of 2-(alkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones via iminophosphorane 3. Bioassays indicated that these compounds showed good to excellent fungicidal activities against six kinds of fungi.
Results and Discussion
Synthesis
The ethyl 2-amino-4,5-dimethylthiophene-3-carboxylate (2), easily obtained by cyclization of 2-butanone (1) with ethyl 2-cyanoacetate and sulfur under basic conditions [10], was converted to iminophosphorane 3 via reaction with triphenylphosphine, hexachloroethane and triethylamine (Scheme 1).
Scheme 1: Preparation of iminophosphorane 3.
Scheme 1: Preparation of iminophosphorane 3.
Iminophosphorane 3 reacted with aromatic isocyanates to give carbodiimides 4, which were allowed to react with secondary amines to provide guanidine intermediates 5. Even in refluxing toluene, compounds 5 did not cyclize. However, in the presence of a catalytic amount of sodium ethoxide, compounds 5 were converted easily to 2-(dialkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 6 in satisfactory yields at room temperature (Scheme 2). The results are listed in Table 1.
Scheme 2: Preparation of 2-(dialkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 6.
Scheme 2: Preparation of 2-(dialkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 6.
Table 1: Preparation of 2-(alkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones.
| Entry | Product | Ar | −NR2 (or −NHR) | Conditions | Yield (%)a |
|---|---|---|---|---|---|
| 1 | 6a | Ph |
|
r.t./4 h | 76 |
| 2 | 6b | Ph |
|
r.t./4 h | 73 |
| 3 | 6c | Ph | –N(n-C4H9)2 | r.t./6 h | 70 |
| 4 | 6d | Ph | –N(n-C6H13)2 | r.t./6 h | 61 |
| 5 | 6e | Ph | –N(Me)Ph | r.t./4 h | 74 |
| 6 | 6f | 4-Me-C6H4 |
|
r.t./4 h | 78 |
| 7 | 6g | 4-Cl-C6H4 |
|
r.t./5 h | 86 |
| 8 | 8a | Ph | –NH(t-C4H9) | r.t./6 h | 71 |
| 9 | 8b | 3-Me-Ph | –NH(n-C4H9) | r.t./5 h | 77 |
| 10 | 8c | Ph |
|
r.t./5 h | 75 |
| 11 | 8d | Ph | –NH(n-C3H7) | r.t./4 h | 73 |
| 12 | 8e | 4-Cl-C6H4 |
|
r.t./6 h | 71 |
aYields of isolated products based on iminophosphorane 3.
The reaction of carbodiimides 4 with primary amine RNH2 in the presence of EtONa provided only 2-(alkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 8 (Scheme 3), one of the possible regioisomers. We obtained only 8 from the reaction mixture after recrystallization; the other isomer 9 was not found by 1H NMR analysis of the reaction mixture.
Scheme 3: Preparation of 2-alkylamino-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 8.
Scheme 3: Preparation of 2-alkylamino-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 8.
The structure of 8 was deduced from its 1H NMR data. For example, the 1H NMR spectrum in 8b (R = n-C4H9) shows the signals of NH at 4.01 ppm as a broad absorption and NCH2 at 3.38–3.31 ppm as a multiple absorption, which strongly suggests the existence of a NHCH2CH2CH2CH3 group in 8b. Furthermore a single crystal of 8c was obtained from a methylene dichloride solution of 8c and X-ray structure analysis verified again the proposed structure [15]. The solitary formation of 8 can be rationalized in terms of a base catalyzed cyclization of the guanidine intermediate 7 to give 8 across the arylamino group rather than the alkylamino one. This may probably be due to the preferential generation of –N−Ar from more acidic –NHAr. The results are also listed in Table 1.
Fungicidal activity
The fungicidal activities of compounds 6 and 8 were screened against six kinds of fungi, Fusarium oxysporum, Rhizoctonia solani, Botrytis cinerea, Gibberella zeae, Dothiorella gregaria, Colletotrichum gossypii at a concentration of 50 mg/L according to the reported method [16]. Bioassays indicated that these compounds showed good to excellent fungicidal activities against six kinds of fungi. For example, 6b, 6d, 8a, 8c, 8d showed 100% inhibition of Botrytis cinerea. See Table 2.
Table 2: The fungicidal activities of compounds 6 and 8 (50 mg/L).
| Compounds | Relative inhibition (%) | |||||
|---|---|---|---|---|---|---|
| Fusarium oxysporum | Rhizoctonia solani | Botrytis cinerea | Gibberella zeae | Dothiorella gregaria | Colletotrichum gossypii | |
| 6a | 56 | 92 | 97 | 61 | 78 | 84 |
| 6b | 74 | 99 | 100 | 86 | 93 | 88 |
| 6c | 63 | 94 | 97 | 67 | 85 | 80 |
| 6d | 74 | 96 | 100 | 69 | 81 | 80 |
| 6e | 56 | 98 | 95 | 72 | 85 | 85 |
| 6f | 70 | 93 | 98 | 64 | 81 | 84 |
| 6g | 67 | 93 | 99 | 64 | 78 | 86 |
| 8a | 70 | 87 | 100 | 82 | 78 | 80 |
| 8b | 74 | 96 | 97 | 65 | 85 | 76 |
| 8c | 52 | 98 | 100 | 68 | 81 | 81 |
| 8d | 66 | 93 | 100 | 82 | 93 | 72 |
| 8e | 71 | 81 | 74 | 44 | 67 | 68 |
In conclusion, we have developed an efficient synthesis of 2-(alkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones via base-catalyzed reaction of functionalized carbodiimides with various amines. Due to the mild reaction conditions, good yields, easily accessible starting material and straightforward product isolation, we think that the versatile synthetic approach discussed here in many cases compares favorably with other existing methods. The preliminary bioassay of the compounds indicated that the 2-amino-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones can be used as lead structure for developing novel fungicides. Further bioassay, optimization and structure-activity relationships of the title compounds are underway.
Experimental
Melting points were uncorrected. MS were measured on a Finnigan Trace MS spectrometer. IR were recorded on a PE-983 infrared spectrometer as KBr pellets with absorption in cm−1. 1H NMR spectra were recorded in CDCl3 on a Varian Mercury 400 spectrometer and resonances are given in ppm (δ) relative to TMS. Elementary analyses were taken on a Perkin-Elmer CHN 2400 elementary analysis instrument.
Preparation of [(3-ethoxycarbonyl-4,5-dimethylthiophen-2-yl)imino]triphenylphosphorane (3)
To a mixture of ethyl 2-amino-4,5-dimethylthiophene-3-carboxylate (2) (2.0 g, 10 mmol), PPh3 (5.24 g, 20 mmol) and C2Cl6 (4.74 g, 20 mmol) in dry CH3CN (50 mL), was added dropwise NEt3 (4.2 mL, 30 mmol) at room temperature. After stirring for 4–5 h, the solvent was removed under reduced pressure and the residue was recrystallized from EtOH to give iminophosphorane 3 as pale yellow crystals (82% yield), mp 131–133 °C; IR (KBr) cm−1 1693 (C=O), 1484, 1196, 1149, 686; 1H NMR (CDCl3, 400 MHz) δ: 7.86–7.46 (m, 15H, Ph-H), 4.28 (q, J = 7.2 Hz, 2H, OCH2), 2.39 (s, 3H, CH3), 2.35 (s, 3H, CH3), 1.11 (t, J = 7.2 Hz, 3H, CH3); MS (70 eV) m/z (%): 459 (M+, 30), 444 (23), 277 (86), 183 (100), 77 (59). Anal. Calcd for C27H26NO2PS: C, 70.57; H, 5.70; N, 3.05; found: C, 70.45; H, 5.55; N, 3.19.
Preparation of 2-(dialkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 6
To a solution of iminophosphorane 3 (0.92 g, 2 mmol) in dry DCM (15 mL) was added the aromatic isocyanate (2 mmol) under nitrogen at room temperature. After the reaction mixture was stirred for 6–12 h at 0–5 °C, the solvent was removed under reduced pressure and ether/petroleum ether (1:2, 20 mL) was added to precipitate triphenylphosphine oxide. After filtration, the solvent was removed to give carbodiimide 4, which was used directly without further purification.
To a solution of 4 (prepared above) in DCM (15 ml) was added the secondary amine (2 mmol). After the reaction mixture was stirred for 2–4 h, the solvent was removed and anhydrous ethanol (10 ml) with several drops of EtONa in EtOH were added. The mixture was stirred for 6–12 h at room temperature. The solution was condensed and the residue was recrystallized from ethanol to give 2-(dialkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 6.
2-Morpholino-3-phenyl-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-one (6a). White crystals (76% yield), mp: 193–194 °C; IR (KBr) cm−1 1694 (C=O), 1535, 1315, 748; 1H NMR (CDCl3, 400 MHz) δ: 7.52–7.33 (m, 5H, Ph-H), 3.40 (t, J = 4.8 Hz, 4H, 2×OCH2), 3.08 (t, J = 4.8 Hz, 4H, 2×NCH2), 2.39 (s, 3H, CH3), 2.35 (s, 3H, CH3); MS (70 eV) m/z (%): 340/341 (M+, 81), 309 (7), 284 (31), 254 (55), 152 (100), 90 (36), 76 (86); Anal. calcd for C18H19N3O2S: C, 63.32; H, 5.61; N, 12.31; found: C, 63.25, H, 5.69; N, 12.45.
2-Piperidino-3-phenyl-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-one (6b). White crystals (73% yield), mp: 140–141 °C; IR (KBr) cm−1 1690 (C=O), 1529, 1319, 745; 1H NMR (CDCl3, 400 MHz) δ: 7.49–7.31 (m, 5H, Ph-H), 3.05 (t, J = 5.6 Hz, 4H, 2×NCH2), 2.39 (s, 3H, CH3), 2.35 (s, 3H, CH3), 1.39–1.18 (m, 6H, 3×CH2); MS (70 eV) m/z (%): 339 (M+, 100), 310 (21), 254 (49), 194 (37), 152 (97), 76 (71); Anal. calcd for C19H21N3OS: C, 67.23; H, 6.24; N, 12.38; found: C, 67.16; H, 6.26; N, 12.42.
2-(Dibutylamino)-3-phenyl-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-one (6c): White crystals (70% yield), mp: 87–89 °C; IR (KBr) cm−1 1688 (C=O), 1530, 1320, 742; 1H NMR (CDCl3, 400 MHz) δ: 7.70–7.26 (m, 5H, Ph-H), 2.97–2.93 (t, J = 7.2, 4H, 2×NCH2), 2.38 (s, 3H, CH3), 2.34 (s, 3H, CH3), 1.22–1.09 (m, 8H, 2×CH2CH2CH2), 0.82 (t, J = 7.2, 6H, 2×CH3); MS (70 eV) m/z (%): 383 (M+, 100), 354 (6), 340 (15), 326 (28), 281/282 (29), 254/255 (34), 90 (11), 76 (32); Anal. calcd for C22H29N3OS: C, 68.89; H, 7.62; N, 10.96; found: C, 68.76; H, 7.68; N, 10.82.
2-(Dihexylamino)-3-phenyl-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-one (6d): White crystals (61% yield), mp: 70–71 °C; IR (KBr) cm−1 1689 (C=O), 1530, 1323, 745; 1H NMR (CDCl3, 400 MHz) δ: 7.48–7.26 (m, 5H, Ph-H), 2.97–2.93 (t, J = 7.2, 4H, 2×NCH2), 2.38 (s, 3H, CH3), 2.34 (s, 3H, CH3), 1.27–1.08 (m, 16H, 2×CH2CH2CH2CH2), 0.86 (t, J = 7.2, 6H, 2×CH3); MS (70 eV) m/z (%): 439 (M+, 100), 368 (6), 354 (10), 281/282 (10), 77/76 (6); Anal. calcd for C26H37N3OS: C, 71.03; H, 8.48; N, 9.56; found: C, 70.94; H, 8.50; N, 9.60.
2-(N-Methyl-N-phenylamino)-3-phenyl-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-one (6e): White crystals (74% yield), mp: 194–196 °C; IR (KBr) cm−1 1684 (C=O), 1530, 1315, 746; 1H NMR (CDCl3, 400 MHz) δ: 7.10–6.57 (m, 10H, Ph-H), 3.25 (s, 3H, NCH3), 2.40 (s, 3H, CH3), 2.38 (s, 3H, CH3); MS (70 eV) m/z (%): 361/360 (M+, 84), 254 (8), 103 (17), 90 (8), 76 (100), 58 (19); Anal. calcd for C21H19N3OS: C, 69.78; H, 5.30; N, 11.62; found: C, 69.70; H, 5.18; N, 11.53.
2-Morpholino-3-(4-methylphenyl)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-one (6f): White crystals (78% yield), mp: 164–166 °C; IR (KBr) cm−1 1685 (C=O), 1524, 1116, 743; 1H NMR (CDCl3, 400 MHz) δ: 7.30–7.20 (m, 4H, Ph-H), 3.42 (t, J = 4.8 Hz, 4H, 2×OCH2), 3.09 (t, J = 4.8 Hz, 4H, 2×NCH2), 2.41 (s, 3H, Ph-CH3), 2.39 (s, 3H, CH3), 2.35 (s, 3H, CH3); MS (70 eV) m/z (%): 355 (M+, 100), 324 (8), 310 (20), 269 (23), 153 (28), 91 (22); Anal. calcd for C19H21N3O2S: C, 64.20; H, 5.95; N, 11.82; found: C, 64.06; H, 5.61; N, 11.75.
2-Morpholino-3-(4-chlorophenyl)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-one (6g): White crystals (86% yield), mp: 173–175 °C; IR (KBr) cm−1 1689 (C=O), 1528, 1320, 741; 1H NMR (CDCl3, 400 MHz) δ: 7.48–7.28 (m, 4H, Ph-H), 3.44 (t, J = 4.8 Hz, 4H, 2×OCH2), 3.08 (t, J = 4.8 Hz, 4H, 2×NCH2), 2.38 (s, 3H, CH3), 2.35 (s, 3H, CH3); MS (70 eV) m/z (%): 375 (M+, 100), 340 (54), 289/290 (19), 162 (24), 110 (78), 90 (29), 77/76 (23); Anal. calcd for C18H18ClN3O2S: C, 57.52; H, 4.83; N, 11.18; found: C, 57.45; H, 4.76; N, 11.20.
Preparation of 2-(alkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 8
To the solution of 4 (2 mmol) prepared above in DCM (10 ml) was added the primary amine (2 mmol). After the reaction mixture was stirred for 5–6 h, the solvent was removed and anhydrous ethanol (10 ml) with several drops of EtONa in EtOH were added. The mixture was stirred for 6–12 h at room temperature. The solution was condensed and the residue was recrystallized from ethanol to give 2-(alkylamino)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-ones 8.
2-(tert-Butylamino)-5,6-dimethyl-3-phenylthieno[2,3-d]pyrimidin-4(3H)-one (8a). White crystals (71% yield), mp: 184–186 °C; IR (KBr) cm−1 1692 (C=O), 1526, 1120, 748; 1H NMR (CDCl3, 400 MHz) δ: 7.56–7.16 (m, 5H, Ph-H), 3.82 (s, 1H, NH), 2.35 (s, 3H, CH3), 2.31 (s, 3H, CH3), 1.34 (s, 9H, 3×CH3); MS (70 eV) m/z (%): 327 (M+, 93), 271 (91), 255 (31), 153 (100), 119 (19), 77 (23); Anal. calcd for C18H21N3OS: C, 66.02; H, 6.46; N, 12.83; found: C, 66.22; H, 6.57; N, 12.97.
2-(Butylamino)-5,6-dimethyl-3-(3-methylphenyl)thieno[2,3-d]pyrimidin-4(3H)-one (8b). White crystals (77% yield), mp: 202–204 °C; IR (KBr) cm−1 1697 (C=O), 1543, 1120, 748; 1H NMR (CDCl3, 400 MHz) δ: 7.48–7.06 (m, 4H, Ph-H), 4.01 (s, 1H, NH), 3.38–3.31 (m, 2H, NCH2), 2.42 (s, 3H, CH3), 2.37 (s, 3H, CH3), 2.32 (s, 3H, CH3), 1.49–1.22 (m, 4H, 2×CH2), 0.88 (t, J = 7.2, 3H, CH3); MS (70 eV) m/z (%): 341.2 (M+, 95), 285 (60), 153 (100), 133 (18), 105 (26), 91 (39); Anal. calcd for C19H23N3OS: C, 66.83; H, 6.79; N, 12.31; found: C, 66.79; H, 6.88; N, 12.42.
2-(Cyclohexylamino)-5,6-dimethyl-3-phenylthieno[2,3-d]pyrimidin-4(3H)-one (8c). White crystals (75% yield), mp: 198–200 °C; IR (KBr) cm−1 1698 (C=O), 1540, 1128, 740; 1H NMR (CDCl3, 400 MHz) δ: 7.48–7.25 (m, 5H, Ph-H), 4.02 (s, 1H, NH), 2.37 (s, 3H, CH3), 2.35 (s, 3H, CH3), 2.00–1.96 (m, 1H, CH), 1.62–1.60 (m, 4H, 2×CH2), 1.44–1.02 (m, 6H, 3×CH2); MS (70 eV) m/z (%): 353 (M+, 44), 270 (34), 153 (100), 133 (24), 98 (19), 91 (52); Anal. calcd for C20H23N3OS: C, 67.96; H, 6.56; N, 11.89; found: C, 68.08; H, 6.71; N, 11.78.
2-(Propylamino)-5,6-dimethyl-3-phenylthieno[2,3-d]pyrimidin-4(3H)-one (8d). White crystals (73% yield), mp: 212–214 °C; IR (KBr) cm−1 1689 (C=O), 1530, 1130, 740; 1H NMR (CDCl3, 400 MHz) δ: 7.61–7.28 (m, 5H, Ph-H), 4.00 (s, 1H, NH), 3.34–3.29 (m, 2H, NCH2), 2.36 (s, 3H, CH3), 2.31 (s, 3H, CH3), 1.52–1.46 (m, 2H, CH2), 0.83 (t, J = 7.2, 3H, CH3); MS (70 eV) m/z (%): 313 (M+, 77), 270 (79), 255 (22), 153 (100), 119 (31), 77 (34); Anal. calcd for C17H19N3OS: C, 65.15; H, 6.11; N, 13.41; found: C, 65.28; H, 6.32; N, 13.60.
2-(Benzylamino)-3-(4-chlorophenyl)-5,6-dimethylthieno[2,3-d]pyrimidin-4(3H)-one (8e). White crystals (71% yield), mp: 248–250 °C; IR (KBr) cm−1 1698 (C=O), 1538, 1130, 747; 1H NMR (CDCl3, 400 MHz) δ: 7.52–7.20 (m, 9H, Ph-H), 4.57 (s, 2H, CH2), 4.32 (s, 1H, NH), 2.36 (s, 3H, CH3), 2.31 (s, 3H, CH3); MS (70 eV) m/z (%): 395 (M+, 100), 330 (48), 270 (12), 201 (74), 153 (24), 91 (31); Anal. calcd for C21H18ClN3OS: C, 63.71; H, 4.58; N, 10.61; found: C, 63.80; H, 4.72; N, 10.44.
References
-
Ulrich, H. Chemistry and Technology of Carbodiimides; John Wiley & Sons, Ltd.: Chichester, 2007.
Return to citation in text: [1] -
Zhao, M.-X.; Wang, M.-X.; Yu, C.-Y.; Huang, Z.-T.; Fleet, G. W. J. J. Org. Chem. 2004, 69, 997. doi:10.1021/jo0351320
Return to citation in text: [1] -
Csámpai, A.; Túrós, G.; Kudar, V.; Simon, K.; Oeynhausen, H.; Wamhoff, H.; Sohár, P. Eur. J. Org. Chem. 2004, 717. doi:10.1002/ejoc.200300511
Return to citation in text: [1] -
Hao, J.; Xia, Y.; Wang, L.; Ruhlmann, L.; Zhu, Y.; Li, Q.; Yin, P.; Wei, Y.; Guo, H. Angew. Chem., Int. Ed. 2008, 47, 2626. doi:10.1002/anie.200704546
Return to citation in text: [1] -
Li, Q.; Wei, Y.; Hao, J.; Zhu, Y.; Wang, L. J. Am. Chem. Soc. 2007, 129, 5810. doi:10.1021/ja070600z
Return to citation in text: [1] -
Wei, Y.; Xu, B.; Barnes, C. L.; Peng, Z. J. Am. Chem. Soc. 2001, 123, 4083. doi:10.1021/ja004033q
Return to citation in text: [1] -
Walter, H. Novel Pyrimidin-4-one and Pyrimidin-4-thione as Fungicide. PCT Int. Pat. Appl. WO 99/14202, March 25, 1999.
Chem. Abstr. 1999, 130, 252368k.
Return to citation in text: [1] -
Chambhare, R. V.; Khadse, B. G.; Bobde, A. S.; Bahekar, R. H. Eur. J. Med. Chem. 2003, 38, 89. doi:10.1016/S0223-5234(02)01442-3
Return to citation in text: [1] -
Shishoo, C. J.; Shirsath, V. S.; Rathod, I. S.; Yande, V. D. Eur. J. Med. Chem. 2000, 35, 351. doi:10.1016/S0223-5234(00)00128-8
Return to citation in text: [1] -
Sabnis, R. W.; Rangnekar, D. W.; Sonawane, N. D. J. Heterocycl. Chem. 1999, 36, 333.
Return to citation in text: [1] [2] -
Sun, Y.; Huang, N. Y.; Ding, M. W. Synth. Commun. 2009, in press.
Return to citation in text: [1] -
Ding, M.-W.; Xu, S.-Z.; Zhao, J.-F. J. Org. Chem. 2004, 69, 8366. doi:10.1021/jo048691v
Return to citation in text: [1] -
Yuan, J.-Z.; Fu, B.-Q.; Ding, M.-W.; Yang, G.-F. Eur. J. Org. Chem. 2006, 4170. doi:10.1002/ejoc.200600201
Return to citation in text: [1] -
Zhao, J.-F.; Xie, C.; Xu, S.-Z.; Ding, M.-W.; Xiao, W.-J. Org. Biomol. Chem. 2006, 4, 130. doi:10.1039/b513715b
Return to citation in text: [1] -
Zheng, A.; Xu, J.; Hu, Y.-G. Acta Crystallogr., Sect. E 2006, 62, o3710. doi:10.1107/S1600536806030248
Return to citation in text: [1] -
Ren, Q.; Cui, Z.; He, H.; Gu, Y. J. Fluorine Chem. 2007, 128, 1369. doi:10.1016/j.jfluchem.2007.06.007
Return to citation in text: [1]
| 1. | Ulrich, H. Chemistry and Technology of Carbodiimides; John Wiley & Sons, Ltd.: Chichester, 2007. |
| 2. | Zhao, M.-X.; Wang, M.-X.; Yu, C.-Y.; Huang, Z.-T.; Fleet, G. W. J. J. Org. Chem. 2004, 69, 997. doi:10.1021/jo0351320 |
| 3. | Csámpai, A.; Túrós, G.; Kudar, V.; Simon, K.; Oeynhausen, H.; Wamhoff, H.; Sohár, P. Eur. J. Org. Chem. 2004, 717. doi:10.1002/ejoc.200300511 |
| 4. | Hao, J.; Xia, Y.; Wang, L.; Ruhlmann, L.; Zhu, Y.; Li, Q.; Yin, P.; Wei, Y.; Guo, H. Angew. Chem., Int. Ed. 2008, 47, 2626. doi:10.1002/anie.200704546 |
| 5. | Li, Q.; Wei, Y.; Hao, J.; Zhu, Y.; Wang, L. J. Am. Chem. Soc. 2007, 129, 5810. doi:10.1021/ja070600z |
| 6. | Wei, Y.; Xu, B.; Barnes, C. L.; Peng, Z. J. Am. Chem. Soc. 2001, 123, 4083. doi:10.1021/ja004033q |
| 11. | Sun, Y.; Huang, N. Y.; Ding, M. W. Synth. Commun. 2009, in press. |
| 12. | Ding, M.-W.; Xu, S.-Z.; Zhao, J.-F. J. Org. Chem. 2004, 69, 8366. doi:10.1021/jo048691v |
| 13. | Yuan, J.-Z.; Fu, B.-Q.; Ding, M.-W.; Yang, G.-F. Eur. J. Org. Chem. 2006, 4170. doi:10.1002/ejoc.200600201 |
| 14. | Zhao, J.-F.; Xie, C.; Xu, S.-Z.; Ding, M.-W.; Xiao, W.-J. Org. Biomol. Chem. 2006, 4, 130. doi:10.1039/b513715b |
| 10. | Sabnis, R. W.; Rangnekar, D. W.; Sonawane, N. D. J. Heterocycl. Chem. 1999, 36, 333. |
| 9. | Shishoo, C. J.; Shirsath, V. S.; Rathod, I. S.; Yande, V. D. Eur. J. Med. Chem. 2000, 35, 351. doi:10.1016/S0223-5234(00)00128-8 |
| 7. |
Walter, H. Novel Pyrimidin-4-one and Pyrimidin-4-thione as Fungicide. PCT Int. Pat. Appl. WO 99/14202, March 25, 1999.
Chem. Abstr. 1999, 130, 252368k. |
| 8. | Chambhare, R. V.; Khadse, B. G.; Bobde, A. S.; Bahekar, R. H. Eur. J. Med. Chem. 2003, 38, 89. doi:10.1016/S0223-5234(02)01442-3 |
| 16. | Ren, Q.; Cui, Z.; He, H.; Gu, Y. J. Fluorine Chem. 2007, 128, 1369. doi:10.1016/j.jfluchem.2007.06.007 |
| 15. | Zheng, A.; Xu, J.; Hu, Y.-G. Acta Crystallogr., Sect. E 2006, 62, o3710. doi:10.1107/S1600536806030248 |
| 10. | Sabnis, R. W.; Rangnekar, D. W.; Sonawane, N. D. J. Heterocycl. Chem. 1999, 36, 333. |
© 2008 Hu et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)