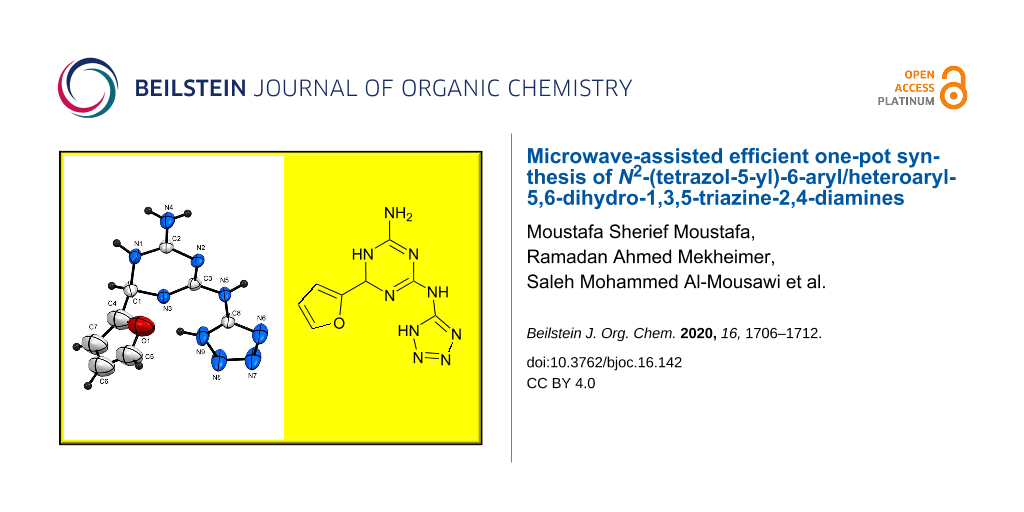
Abstract
An efficient one-pot synthesis of N2-(tetrazol-5-yl)-6-aryl/heteroaryl-1,3,5-triazine-2,4-diamine derivatives was developed by reacting 5-amino-1,2,3,4-tetrazole with aromatic aldehydes and cyanamide in pyridine under controlled microwave heating with high yields. X-ray crystallography confirmed the structure of the obtained products.
Graphical Abstract
Introduction
The family of triazines is of considerable interest in fields related to organic and medicinal chemistry. 2,4-Diaminotriazines are privileged scaffolds exhibiting diverse biological activities such as antibacterial [1], anti-HSV-1 [2], antitumor [3], anti-HIV [4], inhibitor of Trypanosoma brucei [5], angiogenesis inhibitor [6], antiplasmodial antifolates [7], and antimicrobial [8]. Moreover, and in particular N2,6-disubstituted-1,3,5-triazine-2,4-diamines possess a wide range of chemotherapeutic activities [8-11].
Tetrazole derivatives are a potent class of heterocyclic compounds with a wide range of biological activities owing to their unique structure. They play an important role not only as a bioisostere of the carboxylic acid group but also as flexible ligands which easily adopt to different binding modes [12,13]. Tetrazole derivatives exhibit a wide spectrum of biological activities as antibacterial [14], anticancer [15], anti-inflammatory [16], antidiabetic [17], antitubercular [18], and analgesic [19] agents.
It is well established that many medical disorders can be caused as a result of defects at more than one specific biological target such as a receptor or an enzyme. A promising strategy that overcomes the classical one-target, one-molecule approach is the design of stable chemical hybrid molecules which are a combination of two biologically active scaffolds acting at different targets [20-24]. Accordingly, we reasoned that heterocycles incorporating both an N2-(tetrazol-5-yl) ring system and a 1,3,5-triazine-2,4-diamine scaffold could be very effective biologically relevant heterocycles.
Little attention has been paid to the synthesis of N2,6-disubstituted-1,3,5-triazine-2,4-diamines which requires a multistep synthesis route. A first approach relied on the nucleophilic substitution of chlorine in cyanuric chloride with Grignard reagents, ammonia or amines [25,26], which suffered from the high reactivity of the Grignard reagents that prevents further functionalization. Moreover, this protocol required temperature control and showed dependence on the amine nucleophile reactivity [27]. Another route involved the reaction of substituted biguanidines with acetic anhydrides, chlorides or carboxylates [11,28-31]. Liu et al. [32] reported a one-pot synthesis of N2,6-disubstituted-1,3,5-triazine-2,4-diamines in 44–72% yields that employed the reaction of isothiocyanates with sodium hydrogen cyanamide and amidines in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and heating at 75 °C for 3 h. Recently, Ma et al. [33] described a one-pot two step procedure for the synthesis of 6-substituted-N2-aryl-1,6-dihydro-1,3,5-triazine-2,4-diamines via the reaction of aromatic amines, cyanoguanidine, and ketones which afforded the corresponding 1-aryl-1,6-dihydro-6-substituted-1,3,5-triazine-2,4-diamines in 21–56% yields followed by Dimroth rearrangement utilizing sodium hydroxide (50%) in aqueous ethanol (Scheme 1).
Scheme 1: Previously reported methods for the synthesis of 1,3,5-triazine-2,4-diamine derivatives.
Scheme 1: Previously reported methods for the synthesis of 1,3,5-triazine-2,4-diamine derivatives.
Although, these methods have specific merits, they sometimes suffer from drawbacks such as extended reaction temperatures, lengthy procedures, low yields and atom economy, which consume excess reagents. Extensive efforts have been devoted to adopting green methodologies in synthetic heterocyclic chemistry. The utilization of microwave heating as an energy source has several advantages including operational simplicity, high reaction yields, enhanced rates, and increased energy efficiency [34-40].
In continuation of our efforts in performing green methodologies in the synthesis of biologically relevant heterocycles from simple starting materials [41-44], we developed an efficient synthesis of N2-(tetrazol-5-yl)-6-aryl/heteroaryl-1,3,5-triazine-2,4-diamines through the one-pot reaction of cyanamide 1, aromatic aldehydes 2, and 5-aminotetrazole (3) in pyridine under controlled microwave heating (Scheme 2).
Scheme 2: One-pot synthesis of N2-(tetrazol-5-yl)-6-aryl/heteroaryl-5,6-dihydro-1,3,5-triazine-2,4-diamines 4a–j.
Scheme 2: One-pot synthesis of N2-(tetrazol-5-yl)-6-aryl/heteroaryl-5,6-dihydro-1,3,5-triazine-2,4-diamines 4a...
Results and Discussion
With the initial aim of optimizing the reaction conditions, we began our study by reacting equimolar amounts of cyanamide 1, aromatic aldehydes 2, and 5-aminotetrazole (3) in pyridine and the reaction was promoted by microwave heating at 120 °C over 12 min. After cooling the reaction mixture to room temperature and work-up, a solid product was obtained in low yield (40%) and confirmed to be 6-(4-chlorophenyl)-N2-(1H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4a) based on analytical and spectral data. The mass spectrum of the reaction product showed a molecular ion peak at m/z = 290.1 [M − 1]+. The 1H NMR revealed four singlet signals at δ = 11.22, 10.81, 8.72, and 6.17 ppm each integrated for one proton which were assigned to the triazine-NH, NH at N2-(tetrazole-5-yl), tetrazole NH, and triazine CH-2 protons in addition to two broad singlet signals at δ = 8.59 and 7.32 ppm for NH2 function as well as signals for the aromatic protons. The 13C NMR spectrum was in support of the proposed structure. Based on the established product, we revealed that two molecules of cyanamide 1 participated in the reaction course and the yield was increased to 92% when the molar ratio of the reactants 1, 2, and 3 was set at 2:1:1. We next surveyed a structurally diverse group of aromatic aldehydes 2 with cyanamide 1 and 5-aminotetrazole (3) under the same experimental conditions and the results are summarized in (Table 1 and Scheme 2). Irrespective of the aryl group either electron-donating or electron-withdrawing, the reaction proceeded smoothly and gave a variety of 1,3,5-triazine-2,4-diamines 4 in high yields.
The effect of the solvent was also examined. Other solvents were screened under the same experimental conditions and the results revealed that performing the reaction in dioxane, CH3CN, THF, or catalyst-free ethanol resulted in no product formation. However, the same products were obtained with lower yields (≈60%) when performing the reaction under conventional heating utilizing pyridine as the solvent for 3 hours. These results demonstrated the advantage of microwave heating as an efficient energy source.
The structure proposed for the reaction products was established on the bases of analytical and spectral data (MS, 1H NMR, 13C NMR, and elemental analyses). Moreover, the structure of 4i was unequivocally supported by single-crystal X-ray diffraction (Figure 1 and Table 2). A plausible mechanism for the formation of products 4 is postulated in Scheme 3.
Table 2: Selected bond lengths and bond angles for compound 4i.
| bond lengths | bond angles | ||
| atom numbers | geometric parameter (A°) | atom numbers | geometric parameter (°) |
| O1–C5 | 1.360 (9) | C1–N1–C2 | 121.6 (4) |
| C4–C7 | 1.326 (11) | C2–N2–C3 | 115.0 (4) |
| C1–C4 | 1.465 (9) | N1–C2–N4 | 118.2 (4) |
| N6–N7 | 1.336 (7) | C1–C4–C7 | 136.2 (8) |
| N5–N3 | 1.339 (6) | C4–O1–C5 | 109.8 (6) |
| N1–C1 | 1.453 (6) | N6–N7–N8 | 109.9 (5) |
| C1–C4 | 1.465 (12) | N8–N9–C8 | 104.4 (4) |
| N3–C1 | 1.459 (6) | N2–C3–N5 | 116.1 (4) |
| O1–C4–C1 | 116.6 (6) | ||
| O1–C5–C6 | 106.2 (7) | ||
Scheme 3: Plausible different routes to account for the formation of products 4.
Scheme 3: Plausible different routes to account for the formation of products 4.
The dimerization of cyanamide 1 in basic medium to cyanoguanidine 5 and subsequent reaction with 5-aminotetrazole (3) yielded tetrazolylbiguanidine 6 which undergoes a condensation reaction with aromatic aldehydes 2 to afford 7. The nucleophilic attack of the secondary amine in 7 to the arylidene carbon gives rise to the formation of 6-aryl-1-(1H-tetrazol-5-yl)-1,2-dihydro-1,3,5-triazine-2,4-diamine intermediate 8 (route a) or the nucleophilic attack of the primary amine in 7 to the same imine carbon produces the corresponding N2-(tetrazole-5-yl)-6-aryl-1,3,5-triazine-2,4-diamines 4 (route b). Alternatively, intermediate 8 could be obtained by a condensation of 5-aminotetrazole (3) with aromatic aldehydes 2 followed by the addition of cyanoguanidine 5 to the formed Schiff’s base (route c). Product 4 was the sole isolable product as under reflux in pyridine as base, compound 8 well undergoes a Dimroth rearrangement forming the more thermodynamically stable product 4 [33]. We established route c, as the formation of the Schiff base is more favorable due to the high nucleophilicity of the exocyclic amino function attached to the electron-rich tetrazole ring [45]. In support of this assumption we stopped the reaction after 4 minutes of heating under microwave irradiation and inspected the prior formation of 9 via comparison with an authentic sample synthesized by a conventional method.
Conclusion
The synthesis of biologically relevant 6-aryl/heteroaryl-N2-(5H-tetrazole-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamines was achieved under controlled microwave heating via a simple one-pot, three-component reaction of cyanamide 1, aldehydes 2, and 5-amino-1,2,3,4-tetrazole (3) in excellent yields. The process proved to be an efficient synthetic route displaying high atom economy, short reaction times, and a simple work-up procedure. This protocol appeared to be general with a diversity of amines and aldehydes.
Experimental
All chemicals were purchased from Aldrich or Merck Companies. The 1H NMR (600 MHz) and 13C NMR (150 MHz) were run in a Bruker DPX instrument (δ ppm). Mass spectra were measured by using VG Autospec Q MS 30 and MS 9 (AEI) spectrometer, with EI (70 eV) mode. Melting points were recorded in a Gallenkamp melting point apparatus and are uncorrected. X-ray crystallographic structure determinations were performed by using Rigaku Rapid II and Bruker X8 Prospector single crystal X-ray diffractometers. The X-ray crystal structure data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk CCDC1961565 for compound 4i. All reactions were monitored by TLC with 1:1 ethyl acetate/petroleum ether as eluent and were carried out until starting materials were completely consumed. After 7 min microwave irradiation was stopped and the reaction mixture was analyzed by TLC; after further irradiation of 5 min the reaction was complete (total reaction time 12 min).
General procedure for the synthesis of N2,6-disubstituted dihydro-1,3,5-triazine-2,4-diamine derivatives
A solution of 1 (2 mmol), 2 (1 mmol), and 3 (1 mmol) in pyridine (10 mL) was heated under reflux in a Milestone Microwave Labstation at 120 °C for 12 min. The solvent was removed under reduced pressure and the solid product was isolated by filtration and recrystallized from DMF.
6-(4-Chlorophenyl)-N2-(5H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4a). Colorless crystals; mp 320–322 °C; yield 0.268 g, 92%; Rf 0.55 (1:1 ethyl acetate/petroleum ether); 1H NMR (600 MHz, DMSO-d6) δ 6.17 (s, 1H), 7.32 (s, 1H), 7.46 (d, J = 8.4 Hz, 2H), 7.53 (d, J = 9.2 Hz, 2H), 8.58 (s, 1H), 8.73 (brs, 1H), 10.82 (s, 1H), 11.22 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 61.52, 127.84, 128.94, 133.70, 139.75, 154.45, 157.61, 158.0; anal. calcd for C10H10ClN9: C, 41.17; H, 3.46; Cl, 12.15; N, 43.22; found: C, 41.22; H, 3.42; Cl, 12.11; N, 43.24; EIMS (m/z): 290.1 [M − 1]+.
6-(4-Methoxyphenyl)-N2-(5H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4b). Colorless crystals; mp 306–308 °C; yield 0.255 g, 89%; 1H NMR (600 MHz, DMSO-d6) δ 3.74 (s, 3H), 6.07 (s, 1H), 7.01 (d, J = 8.4, Hz, 2H), 7.38 (br s, 1H), 7.39 (d, J = 9 Hz, 2H), 8.29 (s, 1H), 8.60 (s, 1H), 10.69 (s, 1H), 11.15 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 55.26, 62.01, 114.29, 127.51, 132.45, 154.57, 157.79, 158.14, 159.92; anal. calcd for C11H13N9O: C, 45.99; H, 4.56; N, 43.88; found: C, 45.89; H, 4.52; N, 43.90; EIMS (m/z): 286.1 [M − 1]+.
6-Phenyl-N2-(5H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4c). Colorless crystals; mp 317–319 °C; yield 0.277 g, 93%; 1H NMR (600 MHz, DMSO-d6) δ 6.15 (s, 1H), 7.22 (s, 1H), 7.40–7.46 (m, 5H), 8.50 (br s, 1H), 8.71 (s, 1H), 10.77 (s, 1H), 11.20 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 62.2, 125.9, 128.9, 129.1, 154.5, 157.7, 158.0; anal. calcd for C10H11N9: C, 46.69; H,4.31; N, 49.0; found: C, 46.76; H, 4.41; N, 48.85; EIMS (m/z): 256.1 [M − 1]+.
6-(Benzo[d][1,3]dioxol-5-yl)-N2-(5H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4d). Colorless crystals; mp 304–306 °C; yield 0.265 g, 88%; 1H NMR (600 MHz, DMSO-d6) δ, 6.02 (s, 1H), 6.04 (d, 2H), 6.90–7.12 (m, 4H), 8.02 (brs, 1H), 8.55 (s, 1H), 10.68 (s, 1H), 11.10 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 56.0, 62.0, 101.4, 106.3, 108.29, 119.63, 134.38, 147.76, 147.93, 154.49, 157.72, 158.15; anal. calcd for C11H11N9O2: C, 43.85; H, 3.68; N, 41.84; found: C, 43.76; H, 3.59, N, 41.79.
6-(2-Methoxyphenyl)-N2-(5H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4e). Colorless crystals; mp 310–312 °C; yield 0.247 g, 88%; 1H NMR (600 MHz, DMSO-d6) δ 3.89 (s, 3H), 6.27 (s, 1H), 7.02 (t, J = 8.4 Hz, 1H), 7.12 (d, J = 8.4 Hz, 1H), 7.20, 7.21 (dd, J = 8.4, 1.8 Hz, 1H), 7.38–7.41 (m, 1H), 8.04 (brs, 1H), 8.36 (s, 1H), 10.87 (s, 1H), 11.07 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 55.85, 58.74, 111.65, 120.42, 125.28, 127.77, 130.50, 154.61, 156.3, 157.97, 158.2; anal. calcd for C11H13N9O: C, 45.99; H, 4.56; N, 43.88; found: C, 46.10; H, 4.69; N, 43.81; EIMS (m/z): 286.1 [M − 1]+.
6-(4-Methylphenyl)-N2-(5H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4f). Colorless crystals; mp 314−316 °C; yield 0.235 g, 87%; 1H NMR (600 MHz, DMSO-d6) δ 2.50 (s, 3H), 6.09 (s, 1H), 7.19 (s, 1H), 7.26 (d, J = 8.4 Hz, 2H), 7.34 (d, J = 8.4 Hz, 2H), 8.47 (brs, 1H), 8.66 (s, 1H), 10.7 (s, 1H), 11.18 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 20.73, 62.08, 125.90, 129.41, 137.64, 138.69, 154.53, 157.76, 158.07; anal. calcd for C11H13N9: C, 48.70; H, 4.83; N, 46.47; found: C, 48.75; H, 4.70; N, 46.56.
6-(2-Chlorophenyl)-N2-(5H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4g). Colorless crystals; mp 324–326 °C; yield 0.267 g, 92%; 1H NMR (600 MHz, DMSO-d6) δ 6.44 (s, 1H), 7.22 (s, 1H), 7.42–7.44 (m, 1H), 7.45–7.48 (m, 2H), 7.56–7.59 (m, 1H), 8.55 (brs, 1H), 8.65 (s, 1H), 10.94 (s, 1H), 11.29 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 60.55, 127.39, 127.98, 130.30, 131.05, 131.42, 137.07, 154.52, 157.71, 158.04, 162.31; anal. calcd for C10H10ClN9: C, 41.17; H, 3.46; Cl, 12.15; N, 43.22; found: C, 41.30; H, 3.34; Cl, 12.30; N, 43.38.
6-(3-Nitrophenyl)-N2-(5H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4h). Colorless crystals; mp 298–300 °C; yield 0.281 g, 93%; 1H NMR (600 MHz, DMSO-d6) δ 6.35 (s, 1H), 7.52 (s, 1H), 7.76 (t, J = 7.2 Hz, 1H), 7.88 (s, 1H), 8.26 (d, J = 7.8 Hz, 1H), 8.34 (s, 1H), 8.88 (brs, 2H), 10.95 (s, 1H), 11.26 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 61.28, 120.92, 123.91, 130.73, 132.27, 143.13, 147.95, 154.42, 157.62, 157.94; anal. calcd for C10H10N10O2: C, 39.74; H, 3.33; N, 46.34; found: C, 39.68; H, 3.47; N, 46.52.
6-(Furan-2-yl)-N2-(5H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4i). Colorless crystals; mp 208–210 °C; yield 0.224 g, 91%; 1H NMR (600 MHz, DMSO-d6) δ 6.24 (s, 1H), 6.46 (d, J = 16.8 Hz, 2H), 7.29 (brs, 1H), 7.70 (s, 1H), 8.52 (brs, 1H), 8.69 (s, 1H), 10.76 (s, 1H), 11.21 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 56.48, 107.88, 110.61, 143.88, 152.35, 154.52, 157.68, 157.97; anal. calcd for C8H9N9O: C, 38.87; H, 3.67; N, 50.99; found: C, 38.68; H, 3.76; N, 51.06; EIMS (m/z): 246.1 [M − 1]+.
6-(4-N,N-Dimethylaminophenyl)-N2-(5H-tetrazol-5-yl)-5,6-dihydro-1,3,5-triazine-2,4-diamine (4j). Colorless crystals; mp 278–280 °C; yield 0.264 g, 88%; 1H NMR (600 MHz, DMSO-d6) δ 2.91 (s, 6H), 5.96 (s, 1H), 6.59 (d, J = 8.4 Hz, 2H), 7.01 (s, 1H), 7.20 (d, J = 8.4 Hz, 2H), 7.78 (brs, 1H), 8.46 (s, 1H), 10.59 (s, 1H), 11.03 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 39.97, 40.03, 62.36, 111.42, 112.15, 127.01, 127.17, 128.32, 151.04, 154.64, 157.90, 158.24; anal. calcd for C12H16N10: C, 47.99; H, 5.37; N, 46.64; found: C, 48.10; H, 5.43; N, 46.52.
Supporting Information
| Supporting Information File 1: NMR and mass spectra. | ||
| Format: PDF | Size: 2.8 MB | Download |
Funding
K. U. Sadek is grateful to the Alexander von Humboldt Foundation for donation of a Milestone START Microwave Labstation. Saleh Al-Mousawi and Moustafa Sherief Moustafa are grateful to the Kuwait Foundation for the Advancement of Science, project number PR1714SC02 for supporting this work. Analytical facilities provided by Kuwait University GFS projects No. GS 01/03 and GS 03/08 are greatly appreciated.
References
-
Nishigaki, S.; Yoneda, F.; Matsumoto, H.; Morinaga, K. J. Med. Chem. 1969, 12, 39–42. doi:10.1021/jm00301a010
Return to citation in text: [1] -
Goda, F.; Tantawy, A.; Abouzeid, L.; Badr, S.; Selim, K. Saudi Pharm. J. 2008, 16, 103–111.
Return to citation in text: [1] -
Sączewski, F.; Bułakowska, A.; Bednarski, P.; Grunert, R. Eur. J. Med. Chem. 2006, 41, 219–225. doi:10.1016/j.ejmech.2005.10.013
Return to citation in text: [1] -
Chen, X.; Zhan, P.; Liu, X.; Cheng, Z.; Meng, C.; Shao, S.; Pannecouque, C.; Clercq, E. D.; Liu, X. Bioorg. Med. Chem. 2012, 20, 3856–3864. doi:10.1016/j.bmc.2012.04.030
Return to citation in text: [1] -
Landi, G.; Linciano, P.; Borsari, C.; Bertolacini, C. P.; Moraes, C. B.; Cordeiro-da-Silva, A.; Gul, S.; Witt, G.; Kuzikov, M.; Costi, M. P.; Pozzi, C.; Mangani, S. ACS Infect. Dis. 2019, 5, 1105–1114. doi:10.1021/acsinfecdis.8b00358
Return to citation in text: [1] -
Poirier, M.; Awale, M.; Roelli, M. A.; Giuffredi, G. T.; Ruddigkeit, L.; Evensen, L.; Stooss, A.; Calarco, S.; Lorens, J. B.; Charles, R.-P.; Reymond, J.-L. ChemMedChem 2019, 14, 224–236. doi:10.1002/cmdc.201800554
Return to citation in text: [1] -
Lourens, A. C. U.; Gravestock, D.; van Zyl, R. L.; Hoppe, H. C.; Kolesnikova, N.; Taweechai, S.; Yuthavong, Y.; Kamchonwongpaisan, S.; Rousseau, A. L. Org. Biomol. Chem. 2016, 14, 7899–7911. doi:10.1039/c6ob01350c
Return to citation in text: [1] -
Ma, X.; Tan, S.-T.; Khoo, C.-L.; Sim, H.-M.; Chan, L.-W.; Chui, W.-K. Bioorg. Med. Chem. Lett. 2011, 21, 5428–5431. doi:10.1016/j.bmcl.2011.06.125
Return to citation in text: [1] [2] -
Hanessian, S.; Sgarbi, P. W. M. Bioorg. Med. Chem. Lett. 2000, 10, 433–437. doi:10.1016/s0960-894x(00)00021-4
Return to citation in text: [1] -
Hajduk, P. J.; Dinges, J.; Schkeryantz, J. M.; Janowick, D.; Kaminski, M.; Tufano, M.; Augeri, D. J.; Petros, A.; Nienaber, V.; Zhong, P.; Hammond, R.; Coen, M.; Beutel, B.; Katz, L.; Fesik, S. W. J. Med. Chem. 1999, 42, 3852–3859. doi:10.1021/jm990293a
Return to citation in text: [1] -
Kosáry, J.; Kasztreiner, E.; Rablóczky, G.; Kürthy, M. Eur. J. Med. Chem. 1989, 24, 97–99. doi:10.1016/0223-5234(89)90171-2
Return to citation in text: [1] [2] -
Burger, A. Isosterism and bioisosterism in drug design. In Progress in Drug Research; Jucker, E., Ed.; Birkhäuser: Basel, Switzerland, 2011; Vol. 37, pp 287–371. doi:10.1007/978-3-0348-7139-6_7
Return to citation in text: [1] -
Ostrovskii, V. A.; Trifonov, R. E.; Popova, E. A. Russ. Chem. Bull. 2012, 61, 768–780. doi:10.1007/s11172-012-0108-4
Return to citation in text: [1] -
Malik, M. A.; Al-Thabaiti, S. A.; Malik, M. A. Int. J. Mol. Sci. 2012, 13, 10880–10898. doi:10.3390/ijms130910880
Return to citation in text: [1] -
Muralikrishna, S.; Raveendrareddy, P.; Ravindranath, L. K.; Harikrishna, S.; Pathange, J. Der Pharm. Chem. 2013, 5 (6), 87–93.
Return to citation in text: [1] -
Ostrovskii, V. A.; Koren, A. O. Heterocycles 2000, 53, 1421–1448. doi:10.3987/rev-00-530
Return to citation in text: [1] -
Mohite, P. B.; Bhaskar, V. H. Int. J. PharmTech Res. 2011, 3, 1557–1566.
Return to citation in text: [1] -
Adamec, J.; Waisser, K.; Kuneš, J.; Kaustová, J. Arch. Pharm. (Weinheim, Ger.) 2005, 338, 385–389. doi:10.1002/ardp.200400967
Return to citation in text: [1] -
Bachar, S. C.; Lahiri, S. C. Pharmazie 2004, 59, 435–438.
Return to citation in text: [1] -
Decker, M. Design of Hybrid Molecules for Drug Development; Elsevier: New York, NY, USA, 2017.
Return to citation in text: [1] -
Shaveta; Mishra, S.; Singh, P. Eur. J. Med. Chem. 2016, 124, 500–536. doi:10.1016/j.ejmech.2016.08.039
Return to citation in text: [1] -
Solomon, V. R.; Hu, C.; Lee, H. Bioorg. Med. Chem. 2009, 17, 7585–7592. doi:10.1016/j.bmc.2009.08.068
Return to citation in text: [1] -
Kelly, P. M.; Keely, N. O.; Bright, S. A.; Yassin, B.; Ana, G.; Fayne, D.; Zisterer, D. M.; Meegan, M. J. Molecules 2017, 22, 1440. doi:10.3390/molecules22091440
Return to citation in text: [1] -
Pingaew, R.; Saekee, A.; Mandi, P.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Eur. J. Med. Chem. 2014, 85, 65–76. doi:10.1016/j.ejmech.2014.07.087
Return to citation in text: [1] -
Pitts, W. J.; Guo, J.; Dhar, T. G. M.; Shen, Z.; Gu, H. H.; Watterson, S. H.; Bednarz, M. S.; Chen, B.-C.; Barrish, J. C.; Bassolino, D.; Cheney, D.; Fleener, C. A.; Rouleau, K. A.; Hollenbaugh, D. L.; Iwanowicz, E. J. Bioorg. Med. Chem. Lett. 2002, 12, 2137–2140. doi:10.1016/s0960-894x(02)00351-7
Return to citation in text: [1] -
Hirt, R.; Nidecker, H.; Berchtold, R.; Schönholzer, G. Helv. Chim. Acta 1950, 33, 1365–1369. doi:10.1002/hlca.19500330536
Return to citation in text: [1] -
List, M.; Puchinger, H.; Gabriel, H.; Monkowius, U.; Schwarzinger, C. J. Org. Chem. 2016, 81, 4066–4075. doi:10.1021/acs.joc.6b00355
Return to citation in text: [1] -
Alkalay, D.; Volk, J.; Bartlett, M. F. J. Pharm. Sci. 1976, 65, 525–529. doi:10.1002/jps.2600650412
Return to citation in text: [1] -
Yuki, Y.; Sakurai, S.; Kakurai, T.; Noguchi, T. Bull. Chem. Soc. Jpn. 1970, 43, 2130–2134. doi:10.1246/bcsj.43.2130
Return to citation in text: [1] -
Shapiro, S. L.; Isaacs, E. S.; Parrino, V. A.; Freedman, L. J. Org. Chem. 1961, 26, 68–74. doi:10.1021/jo01060a015
Return to citation in text: [1] -
Irikura, T.; Abe, Y.; Okamura, K.; Higo, K.; Maeda, A.; Morinaga, F.; Shirai, G.; Hatae, S. J. Med. Chem. 1970, 13, 1081–1089. doi:10.1021/jm00300a015
Return to citation in text: [1] -
Liu, C.; Lin, J.; Leftheris, K. Tetrahedron Lett. 2007, 48, 435–437. doi:10.1016/j.tetlet.2006.11.069
Return to citation in text: [1] -
Ma, X.; Poon, T.-Y.; Wong, P. T. H.; Chui, W.-K. Bioorg. Med. Chem. Lett. 2009, 19, 5644–5647. doi:10.1016/j.bmcl.2009.08.052
Return to citation in text: [1] [2] -
Carpenter, R. D.; Lam, K. S.; Kurth, M. J. J. Org. Chem. 2007, 72, 284–287. doi:10.1021/jo0618066
Return to citation in text: [1] -
Caddick, S. Tetrahedron 1995, 51, 10403–10432. doi:10.1016/0040-4020(95)00662-r
Return to citation in text: [1] -
Hameed, A. A.; Ahmed, E. K.; Fattah, A. A. A.; Andrade, C. K. Z.; Sadek, K. U. Res. Chem. Intermed. 2017, 43, 5523–5533. doi:10.1007/s11164-017-2944-1
Return to citation in text: [1] -
Sadek, K. U.; Mekheimer, R. A.; Mohamed, T. M.; Moustafa, M. S.; Elnagdi, M. H. Beilstein J. Org. Chem. 2012, 8, 18–24. doi:10.3762/bjoc.8.3
Return to citation in text: [1] -
Hayes, B. L. Microwave Synthesis: Chemistry at the Speed of Light; CEM Publishing: NC, USA, 2002.
Return to citation in text: [1] -
Kaval, N.; Ermolat'ev, D.; Appukkuttan, P.; Dehaen, W.; Kappe, C. O.; Van der Eycken, E. J. Comb. Chem. 2005, 7, 490–502. doi:10.1021/cc0498377
Return to citation in text: [1] -
Nüchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Green Chem. 2004, 6, 128–141. doi:10.1039/b310502d
Return to citation in text: [1] -
Abdel Hameed, A. M.; Moustafa, M. S.; Al-Mousawi, S. M.; Awed, R. R.; Sadek, K. U. Green Process. Synth. 2017, 6, 371–375. doi:10.1515/gps-2017-0019
Return to citation in text: [1] -
Mekheimer, R. A.; Hameed, A. A.; Sadek, K. U. Green Chem. 2008, 10, 592–593. doi:10.1039/b715126h
Return to citation in text: [1] -
Dyab, A. K. F.; Sadek, K. U. RSC Adv. 2018, 8, 23241–23251. doi:10.1039/c8ra04195d
Return to citation in text: [1] -
Abdel Hamid, A.; Abd-Elmonem, M.; Hayallah, A. M.; Abo Elsoud, F. A.; Sadek, K. U. ChemistrySelect 2017, 2, 10689–10693. doi:10.1002/slct.201702011
Return to citation in text: [1] -
Ostrovskii, V. A.; Koldobskii, G. I.; Trifonov, R. E. Tetrazoles. Comprehensive Heterocyclic Chemistry III; Elsevier: Amsterdam, Netherlands, 2008; Vol. 6, pp 257–423. doi:10.1016/b978-008044992-0.00517-4
Return to citation in text: [1]
| 1. | Nishigaki, S.; Yoneda, F.; Matsumoto, H.; Morinaga, K. J. Med. Chem. 1969, 12, 39–42. doi:10.1021/jm00301a010 |
| 5. | Landi, G.; Linciano, P.; Borsari, C.; Bertolacini, C. P.; Moraes, C. B.; Cordeiro-da-Silva, A.; Gul, S.; Witt, G.; Kuzikov, M.; Costi, M. P.; Pozzi, C.; Mangani, S. ACS Infect. Dis. 2019, 5, 1105–1114. doi:10.1021/acsinfecdis.8b00358 |
| 18. | Adamec, J.; Waisser, K.; Kuneš, J.; Kaustová, J. Arch. Pharm. (Weinheim, Ger.) 2005, 338, 385–389. doi:10.1002/ardp.200400967 |
| 4. | Chen, X.; Zhan, P.; Liu, X.; Cheng, Z.; Meng, C.; Shao, S.; Pannecouque, C.; Clercq, E. D.; Liu, X. Bioorg. Med. Chem. 2012, 20, 3856–3864. doi:10.1016/j.bmc.2012.04.030 |
| 3. | Sączewski, F.; Bułakowska, A.; Bednarski, P.; Grunert, R. Eur. J. Med. Chem. 2006, 41, 219–225. doi:10.1016/j.ejmech.2005.10.013 |
| 16. | Ostrovskii, V. A.; Koren, A. O. Heterocycles 2000, 53, 1421–1448. doi:10.3987/rev-00-530 |
| 2. | Goda, F.; Tantawy, A.; Abouzeid, L.; Badr, S.; Selim, K. Saudi Pharm. J. 2008, 16, 103–111. |
| 8. | Ma, X.; Tan, S.-T.; Khoo, C.-L.; Sim, H.-M.; Chan, L.-W.; Chui, W.-K. Bioorg. Med. Chem. Lett. 2011, 21, 5428–5431. doi:10.1016/j.bmcl.2011.06.125 |
| 9. | Hanessian, S.; Sgarbi, P. W. M. Bioorg. Med. Chem. Lett. 2000, 10, 433–437. doi:10.1016/s0960-894x(00)00021-4 |
| 10. | Hajduk, P. J.; Dinges, J.; Schkeryantz, J. M.; Janowick, D.; Kaminski, M.; Tufano, M.; Augeri, D. J.; Petros, A.; Nienaber, V.; Zhong, P.; Hammond, R.; Coen, M.; Beutel, B.; Katz, L.; Fesik, S. W. J. Med. Chem. 1999, 42, 3852–3859. doi:10.1021/jm990293a |
| 11. | Kosáry, J.; Kasztreiner, E.; Rablóczky, G.; Kürthy, M. Eur. J. Med. Chem. 1989, 24, 97–99. doi:10.1016/0223-5234(89)90171-2 |
| 14. | Malik, M. A.; Al-Thabaiti, S. A.; Malik, M. A. Int. J. Mol. Sci. 2012, 13, 10880–10898. doi:10.3390/ijms130910880 |
| 8. | Ma, X.; Tan, S.-T.; Khoo, C.-L.; Sim, H.-M.; Chan, L.-W.; Chui, W.-K. Bioorg. Med. Chem. Lett. 2011, 21, 5428–5431. doi:10.1016/j.bmcl.2011.06.125 |
| 15. | Muralikrishna, S.; Raveendrareddy, P.; Ravindranath, L. K.; Harikrishna, S.; Pathange, J. Der Pharm. Chem. 2013, 5 (6), 87–93. |
| 7. | Lourens, A. C. U.; Gravestock, D.; van Zyl, R. L.; Hoppe, H. C.; Kolesnikova, N.; Taweechai, S.; Yuthavong, Y.; Kamchonwongpaisan, S.; Rousseau, A. L. Org. Biomol. Chem. 2016, 14, 7899–7911. doi:10.1039/c6ob01350c |
| 6. | Poirier, M.; Awale, M.; Roelli, M. A.; Giuffredi, G. T.; Ruddigkeit, L.; Evensen, L.; Stooss, A.; Calarco, S.; Lorens, J. B.; Charles, R.-P.; Reymond, J.-L. ChemMedChem 2019, 14, 224–236. doi:10.1002/cmdc.201800554 |
| 12. | Burger, A. Isosterism and bioisosterism in drug design. In Progress in Drug Research; Jucker, E., Ed.; Birkhäuser: Basel, Switzerland, 2011; Vol. 37, pp 287–371. doi:10.1007/978-3-0348-7139-6_7 |
| 13. | Ostrovskii, V. A.; Trifonov, R. E.; Popova, E. A. Russ. Chem. Bull. 2012, 61, 768–780. doi:10.1007/s11172-012-0108-4 |
| 27. | List, M.; Puchinger, H.; Gabriel, H.; Monkowius, U.; Schwarzinger, C. J. Org. Chem. 2016, 81, 4066–4075. doi:10.1021/acs.joc.6b00355 |
| 20. | Decker, M. Design of Hybrid Molecules for Drug Development; Elsevier: New York, NY, USA, 2017. |
| 21. | Shaveta; Mishra, S.; Singh, P. Eur. J. Med. Chem. 2016, 124, 500–536. doi:10.1016/j.ejmech.2016.08.039 |
| 22. | Solomon, V. R.; Hu, C.; Lee, H. Bioorg. Med. Chem. 2009, 17, 7585–7592. doi:10.1016/j.bmc.2009.08.068 |
| 23. | Kelly, P. M.; Keely, N. O.; Bright, S. A.; Yassin, B.; Ana, G.; Fayne, D.; Zisterer, D. M.; Meegan, M. J. Molecules 2017, 22, 1440. doi:10.3390/molecules22091440 |
| 24. | Pingaew, R.; Saekee, A.; Mandi, P.; Nantasenamat, C.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Eur. J. Med. Chem. 2014, 85, 65–76. doi:10.1016/j.ejmech.2014.07.087 |
| 25. | Pitts, W. J.; Guo, J.; Dhar, T. G. M.; Shen, Z.; Gu, H. H.; Watterson, S. H.; Bednarz, M. S.; Chen, B.-C.; Barrish, J. C.; Bassolino, D.; Cheney, D.; Fleener, C. A.; Rouleau, K. A.; Hollenbaugh, D. L.; Iwanowicz, E. J. Bioorg. Med. Chem. Lett. 2002, 12, 2137–2140. doi:10.1016/s0960-894x(02)00351-7 |
| 26. | Hirt, R.; Nidecker, H.; Berchtold, R.; Schönholzer, G. Helv. Chim. Acta 1950, 33, 1365–1369. doi:10.1002/hlca.19500330536 |
| 45. | Ostrovskii, V. A.; Koldobskii, G. I.; Trifonov, R. E. Tetrazoles. Comprehensive Heterocyclic Chemistry III; Elsevier: Amsterdam, Netherlands, 2008; Vol. 6, pp 257–423. doi:10.1016/b978-008044992-0.00517-4 |
| 41. | Abdel Hameed, A. M.; Moustafa, M. S.; Al-Mousawi, S. M.; Awed, R. R.; Sadek, K. U. Green Process. Synth. 2017, 6, 371–375. doi:10.1515/gps-2017-0019 |
| 42. | Mekheimer, R. A.; Hameed, A. A.; Sadek, K. U. Green Chem. 2008, 10, 592–593. doi:10.1039/b715126h |
| 43. | Dyab, A. K. F.; Sadek, K. U. RSC Adv. 2018, 8, 23241–23251. doi:10.1039/c8ra04195d |
| 44. | Abdel Hamid, A.; Abd-Elmonem, M.; Hayallah, A. M.; Abo Elsoud, F. A.; Sadek, K. U. ChemistrySelect 2017, 2, 10689–10693. doi:10.1002/slct.201702011 |
| 33. | Ma, X.; Poon, T.-Y.; Wong, P. T. H.; Chui, W.-K. Bioorg. Med. Chem. Lett. 2009, 19, 5644–5647. doi:10.1016/j.bmcl.2009.08.052 |
| 33. | Ma, X.; Poon, T.-Y.; Wong, P. T. H.; Chui, W.-K. Bioorg. Med. Chem. Lett. 2009, 19, 5644–5647. doi:10.1016/j.bmcl.2009.08.052 |
| 34. | Carpenter, R. D.; Lam, K. S.; Kurth, M. J. J. Org. Chem. 2007, 72, 284–287. doi:10.1021/jo0618066 |
| 35. | Caddick, S. Tetrahedron 1995, 51, 10403–10432. doi:10.1016/0040-4020(95)00662-r |
| 36. | Hameed, A. A.; Ahmed, E. K.; Fattah, A. A. A.; Andrade, C. K. Z.; Sadek, K. U. Res. Chem. Intermed. 2017, 43, 5523–5533. doi:10.1007/s11164-017-2944-1 |
| 37. | Sadek, K. U.; Mekheimer, R. A.; Mohamed, T. M.; Moustafa, M. S.; Elnagdi, M. H. Beilstein J. Org. Chem. 2012, 8, 18–24. doi:10.3762/bjoc.8.3 |
| 38. | Hayes, B. L. Microwave Synthesis: Chemistry at the Speed of Light; CEM Publishing: NC, USA, 2002. |
| 39. | Kaval, N.; Ermolat'ev, D.; Appukkuttan, P.; Dehaen, W.; Kappe, C. O.; Van der Eycken, E. J. Comb. Chem. 2005, 7, 490–502. doi:10.1021/cc0498377 |
| 40. | Nüchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Green Chem. 2004, 6, 128–141. doi:10.1039/b310502d |
| 11. | Kosáry, J.; Kasztreiner, E.; Rablóczky, G.; Kürthy, M. Eur. J. Med. Chem. 1989, 24, 97–99. doi:10.1016/0223-5234(89)90171-2 |
| 28. | Alkalay, D.; Volk, J.; Bartlett, M. F. J. Pharm. Sci. 1976, 65, 525–529. doi:10.1002/jps.2600650412 |
| 29. | Yuki, Y.; Sakurai, S.; Kakurai, T.; Noguchi, T. Bull. Chem. Soc. Jpn. 1970, 43, 2130–2134. doi:10.1246/bcsj.43.2130 |
| 30. | Shapiro, S. L.; Isaacs, E. S.; Parrino, V. A.; Freedman, L. J. Org. Chem. 1961, 26, 68–74. doi:10.1021/jo01060a015 |
| 31. | Irikura, T.; Abe, Y.; Okamura, K.; Higo, K.; Maeda, A.; Morinaga, F.; Shirai, G.; Hatae, S. J. Med. Chem. 1970, 13, 1081–1089. doi:10.1021/jm00300a015 |
| 32. | Liu, C.; Lin, J.; Leftheris, K. Tetrahedron Lett. 2007, 48, 435–437. doi:10.1016/j.tetlet.2006.11.069 |
© 2020 Moustafa et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)





![[1860-5397-16-142-1]](/bjoc/content/figures/1860-5397-16-142-1.png?scale=2.0&max-width=1024&background=FFFFFF)