Abstract
Novel N-alkyl-N-(phosphonoethyl) substituted mono-, bis- and tris(meth)acrylamides 3 were synthesized by two different three-step reactions and characterized by IR, 1H NMR and 13C NMR spectroscopy as well as refractive index and viscosity. The phosphonoethyl substituted (meth)acrylamide monomers show improved hydrolytic stability compared to carboxylic esters. The highest stability was found for the phosphonoethyl substituted acrylamide monomers. Acrylamides have a larger polymerization enthalpy ranging from −50 to −70 kJ·mol−1 per double bond compared to methacrylamides which show −8.57 to −25.1 kJ·mol−1 per double bond. Depending on their structure (meth)acrylamides 3 exhibit an adhesion to enamel and dentin up to 19.5 MPa. The monomer 3c shows the highest adhesion values to both substrates, namely 15.3 ± 3.4 MPa to enamel and 18.5 ± 2.3 MPa to dentin.
Graphical Abstract
Introduction
In the past decades dental adhesives have been employed for fixation of direct and indirect restorations. These adhesives were composed of three-part systems, consisting of an etch gel, a primer and a bonding material. Each of these adhesive parts was applied step-by-step. At first the etch gel was applied to enamel and dentin surface, then the gel was removed by washing with water. Thereafter, the primer and the bonding were applied successively and light polymerized. This multi-step procedure is relatively time consuming and bears the danger of various failures. In order to reduce the complexity of the adhesives during application and to make the adhesion procedure more safe, easy and robust several generations of adhesives were developed which combined the etch and prime function or the prime and bond function in one part together.
At present, self-etching, self-priming dental adhesives are composed of two-part systems due to low hydrolytic stability of conventional polymerizable acidic ester monomers. The low hydrolytic stability arises from the hydrolysis of acidic and adhesive (meth)acrylester monomers in water or water/solvent mixtures. Therefore, the known acidic and adhesive monomers must be stored water-free and mixed with the aqueous part just before application. If conventional (meth)acrylesters are used for one-part self-etching, self-priming dental adhesives they necessitate a storage under refrigeration to guarantee a shelf-life, comparable to two-part systems. That is the case for the most of the presently available adhesives of this type. Today the demand is to have a one-part self-etching adhesive which combines all three steps of adhesive procedure in one and which does not need to be refrigerated. Therefore, there is a need for novel hydrolytically stable monomers with and without acidic functions.
It is well known that methacrylamides exhibit in acidic aqueous solutions an improved hydrolytic stability compared to ester containing monomers [1]. In the past different approaches were made for hydrolytically stable acidic monomers. Recently, some (N-methylacrylamido)alkylphosphonic acids [2], a 4-(N-methylacrylamidomethyl)benzylphosphonic acid [2] and a bis(meth)acrylamide comprising one phosphoric acid moiety [3] have been prepared and were investigated for a dental adhesive. Furthermore, (meth)acrylamides with one [4-8] or two [7-9] phosphonic acid groups were suggested as hydrolytically stable acidic monomers.
The aim of the presented work was to synthesize novel (meth)acrylamides with phosphonic acid moieties and to investigate the influence of monomers bearing different aliphatic side chains and a different number of polymerizable and acidic groups on the adhesion of enamel and dentin.
Results and Discussion
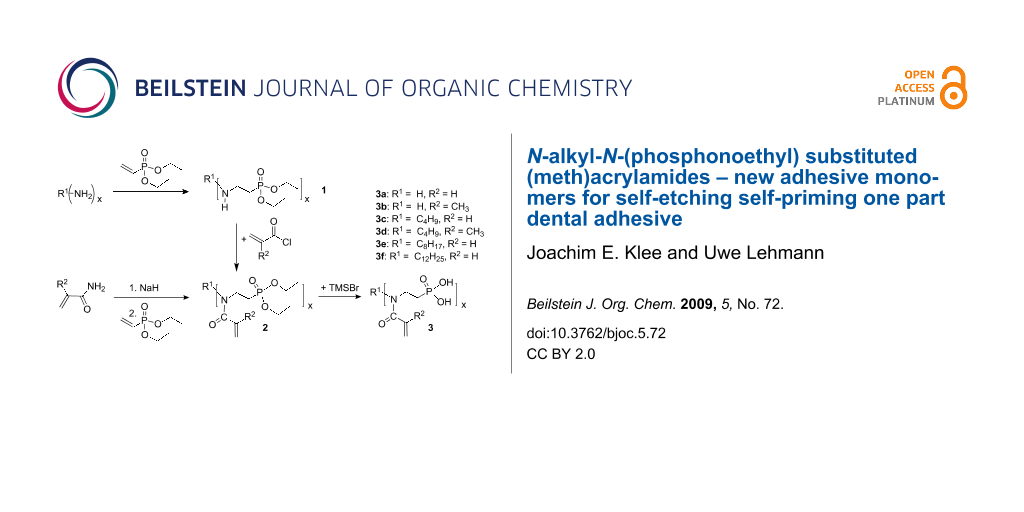
(Meth)acrylamides 3c–3f, bis(meth)acrylamides 3g and 3h and tris(meth)acrylamides 3i and 3j were prepared in a three step reaction via addition of vinylphosphonic acid to amines, a Schotten-Baumann acylation with (meth)acrylolyl chloride and subsequent methanolysis of the phosphonic acid ethyl ester with trimethylsilyl bromide (Scheme 1, Table 1). (Meth)acrylamides 3a and 3b were prepared by addition of vinylphosphonic acid ethyl ester to acrylamide and methacrylamide respectively and the same procedure for methanolysis of the phosphonic acid ethyl ester as that described above.
Scheme 1: Synthesis of novel N-alkyl-N-(ethyl phosphonate) (meth)acrylamides 3. For 2a, 2b, 3a, 3b: R1 = H, x = 1.
Scheme 1: Synthesis of novel N-alkyl-N-(ethyl phosphonate) (meth)acrylamides 3. For 2a, 2b, 3a, 3b: R1 = H, x...
Table 1: Formula, Mn-values and yields of N-alkyl-N-(phosphonoethyl) (meth)acrylamides 3.
| 3 | R1 | R2 | x | formula |
Mn
g mol−1 |
Yieldc
g (%) |
|---|---|---|---|---|---|---|
| a | H | H | 1 | C5H10O4NP | 179.11 | – |
| b | H | CH3 | 1 | C6H12O4NP | 193.14 | 2.0 (95.6) |
| c | C4H9 | H | 1 | C9H18O4NP | 235.22 | 32.2 (63.0) |
| d | C4H9 | CH3 | 1 | C10H20O4NP | 249.25 | 36.8 (75.2) |
| e | C8H17 | H | 1 | C13H26O4NP | 291.33 | 33.3 (100.0) |
| f | C12H25 | H | 1 | C17H34O4NP | 347.44 | 25.1 (100.0) |
| g |
|
H | 2 | C20H38O11N2P2 | 544.48 | 21.0 (100.0) |
| h |
|
CH3 | 2 | C22H42O11N2P2 | 572.53 | 16.9 (79.9) |
| i |
|
H | 3 | C35H64O12N3P | 750.89 | 75.9 (100.0) |
| j |
|
CH3 | 3 | C39H74O18N3P3 | 968.96 | 27.1 (95.8) |
amonophosphonic acid.
btriphosphonic acid.
cthe yields refer to methanolysis of 2 to 3.
The double bonds of 3 are detectable in the IR spectrum at 1533 cm−1 (3f) and in the 1H NMR spectrum at 5.3/5.5 ppm (3d). In the 13C NMR spectra signals of the sp2-hybridized C-atoms of the acrylamide double bonds appear at 128.9/129.1 (3c, 3e, 3f) and of the methacrylamides at 118.9/139.9 (3b), 115.7/141.2 ppm (3d), 121.7/140.2 ppm (3h). Absorptions of the P-OH moiety were found at 1078, 3390 and 3411 cm−1 (3c, 3d).
DSC investigation of 3
The polymerization behavior of N-alkyl-N-(phosphonoethyl)acrylamides and methacrylamides 3 was investigated by photopolymerization using a DSC7/DPA7 unit. The polymerization enthalpy of acrylamides 3 (Table 2) is ranging from −50 to −70 kJ·mol−1 per double bond. It is only slightly lower compared to the polymerization enthalpy of acrylamide which was measured with −75.4 [10] and −82.9 kJ·mol−1 [11] and to acrylic esters (−77.5 to −80.5 kJ·mol−1 [12,13]). The longer the alkyl substituent and the higher the steric hindrance, the lower is the polymerization enthalpy of N-alkyl-N-(phosphonoethyl)acrylamides in the order of 3c, 3e and 3f. The diacrylamide 3g shows a relatively low polymerization enthalpy of −21.5 kJ·mol−1 per double bond when polymerized photochemically or thermally with AIBN as initiator.
Table 2: Polymerization enthalpy of N-alkyl-N-(phosphonoethyl) substituted (meth)acrylamides 3 using a photo calorimeter DSC7/DPA7 (Perkin-Elmer); light intensity in the visible portion of the spectrum was 108 mW cm−2.
| 3 | a | b | c | d | e | f | g | h | i |
|---|---|---|---|---|---|---|---|---|---|
|
ΔRH
kJ·mol−1 |
−63.5 | −8.6a | −71.3 | −15.0a | −53.4 | −51.1 | −43.0a | −50.2 | −179.8 |
|
ΔRH
kJ·mol−1 per double bond |
−63.5 | −8.6 | −71.3 | −15.0 | −53.4 | −51.1 | −21.5 | −25.1 | −59.9 |
athermal polymerization initiated with AIBN.
The methacrylamides 3 exhibit a relatively strong delay of photopolymerization. The thermal initiated polymerization with AIBN leads to relatively low polymerization enthalpies ranging from −8.57 (3b) to −25.1 (3h) kJ·mol−1 per double bond only, which are significantly lower compared to methacrylic esters (−52.8 to −59.9 kJ·mol−1 [11-13]). Obviously, the steric hindrance of the methyl group and its electronic influence on the transition state limits the polymerization behavior. Already the polymerization enthalpy of methacrylamide is significantly lower (−35.2 kJ·mol−1 [12]) compared to methacrylic esters. Furthermore, it is well known, that N,N-disubstituted methacrylamides have relatively low reactivity in homopolymerization [14] due to the steric hindrance of the alkyl substituents.
Hydrolytic stability of monomers 3
It is well known that amides are more stable towards acidic hydrolysis than carboxylic ester compounds. On the other side it seems difficult to predict how stable the novel (meth)acrylamides are which contain, beside the (meth)acrylamide structures, acidic moieties within one molecule.
Hydrolytic stability was studied by detection of acrylic or methacrylic acid by HPLC using solutions of 1.5 mmol of 3c and 3d and solutions of 0.6 mmol 3g in 5 mL of a 1:1 mixture of distilled water and ethanol which were stored at 50 °C (Figure 1). Methacrylic acid phosphonomethyl ester (4) shows the highest degree of hydrolysis of approximately 80% after 42 d at 50 °C. The lowest degree of hydrolysis was found for acrylic amide monomers 3c and 3g, whereas the methacrylic amide monomer 3d exhibited a hydrolysis of 50% after 50 d at 50 °C. Obviously, the +I-effect of the methyl group in 3d leads to a transition state which facilitates the amide cleavage.
Figure 1: Hydrolysis of N-alkyl-N-(phosphonoethyl) substituted (meth)acrylamides 3 of Scheme 1 and of methacrylic acid phosphonomethyl ester (4) after storage in aqueous solution at 50 °C.
Figure 1: Hydrolysis of N-alkyl-N-(phosphonoethyl) substituted (meth)acrylamides 3 of Scheme 1 and of methacrylic aci...
Adhesion of phosphonic acids 3
One of the fundamental assumptions is that the adhesion of self-etching adhesives on enamel mainly depends on the acidity and consequently on the etch pattern that is formed. With the help of REM investigations the etch pattern of Conditioner 36, monomer 3g and Clearfil SE Primer are compared in Figure 2a–Figure 2c which illustrates that it seems possible to generate a deeper and more structured etch pattern than the one caused by the Clearfil SE Primer. However, the strong etch pattern of Conditioner 36 is not surpassed.
Figure 2: Etch pattern of enamel etched with a) Conditioner 36, magnification 11 × 103, b) acidic monomer 3g (c = 0.5 mol/l) in ethanol/water 1:1, magnification 11 × 103, c) Clearfil SE Primer, magnification 11 × 103.
Figure 2: Etch pattern of enamel etched with a) Conditioner 36, magnification 11 × 103, b) acidic monomer 3g (...
The adhesion of phosphonic acids 3c, 3e, 3f and 3g was investigated using an aqueous ethanol solution of N,N′-diethyl-1,3-bis(acrylamido)propane, phosphonic acid 3, and 3(4),8(9)-bis(acrylamidomethyl)tricyclo[5.2.1.02,6]decane (Figure 3) which was applied to enamel and dentin, followed by solvent evaporation by (air stream) and photopolymerization. Surprisingly, phosphonic acid 3c shows the highest adhesion to both enamel and dentin. Probably, 3c fulfils better than the other monomers some of the essential conditions for high adhesion to both tooth substrates such as the presence of hydrophilic/hydrophobic moieties of the adhesive molecule and a high polymerization tendency.
Especially with increasing chain length of the alkyl substituent the adhesion to enamel decreases strongly. The dentin adhesion seems to be independent of the alkyl substituent in the range of four to eight carbon atoms. Only longer aliphatic side chains of twelve carbon atoms limit the adhesion of dentin. These results are surprising in the light of the work of Kuraray [15] which showed a maximum of adhesion for relatively long C-10 alkyl spacers.
Figure 3: Shear bond strength (SBS) of phosphonic acids 3c, 3e, 3f and 3g in a formulation of an aqueous ethanol solution of N,N′-diethyl-1,3-bis(acrylamido)propane, phosphonic acid 3, and 3(4),8(9)-bis(acrylamidomethyl)tricyclo[5.2.1.02,6]decane.
Figure 3: Shear bond strength (SBS) of phosphonic acids 3c, 3e, 3f and 3g in a formulation of an aqueous etha...
In order to further improve the adhesion, polyfunctional monomers 3g–j were synthesized. It was expected that the adhesion of 3g, which contains two polymerizable moieties and two phosphonic acid groups, is superior compared to the monofunctional monomers. However, the adhesion of 3g is balanced on both enamel and dentin, but amounts to less than 10 MPa on each substrate only. Maybe the rather poor polymerization behavior and the relatively high molecular weight are responsible for the lower adhesion.
Conclusion
A series of novel N-alkyl-N-(phosphonoethyl) substituted mono-, bis- and tris(meth)acrylamides 3 was synthesized which shows improved hydrolytic stability compared to carboxylic esters. Their stability decreases in the order of acrylamide 3c ~ 3g > methacrylamide 3d > methacryl ester 4. Acrylamides show a higher polymerization enthalpy ranging from −50 to −70 kJ·mol−1 per double bond compared to methacrylamides which show only −8.57 to −25.1 kJ·mol−1 per double bond. The (meth)acrylamides 3 exhibit an adhesion to both enamel and dentin. The highest adhesion values to both tooth substrates were found using 3c with 15.3 ± 3.4 MPa to enamel and with 18.5 ± 2.3 MPa to dentin.
Experimental
Diethyl vinylphosphonate, acryloyl chloride, methacryloyl chloride, methacrylic acid, methylene chloride, NaOH, trimethylbromosilane, butylamine, 3,6-dioxaoctane-1,8-diamine, camphorquinone and 4-(N,N-dimethylamino)benzoic acid ethyl ester, diethyl (hydroxymethyl)phosphonate, 2,2′-azobisisobutyronitrile (AIBN) and 2,6-di-tert-butyl-4-methylphenol were purchased from Sigma-Aldrich and phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide from BASF Interorgana. N,N′-diethyl-1,3-(bisacrylamido)propane, 3(4),8(9)-bis(acrylamidomethyl)tricyclo[5.2.1.02,6]decane all of Dentsply. Spectrum TPH (Dentsply) is a dental composite comprising methacrylate basing resins and glass filler. Nupro (Dentsply) is a polishing paste. Conditioner 36 (Dentsply) mainly consist of phosphoric acid. Clearfil SE (Kuraray) is a water-free two-part self-etching adhesive containing 10-(methacryloyloxy)decyl dihydrogen phosphate.
The two (meth)acrylamido phosphonic acids 3a and 3b (Table 1) were synthesized according to the following general procedure.
1. (Meth)acrylamido phosphonic acid diethyl ester 2a and 2b: To a solution of acrylamide or methacrylamide in 30 mL CH2Cl2 were added a stoichiometric amount of NaH at 0 °C. After 1 h a stoichiometric amount of vinyl phosphonic acid diethyl ester was added to the reaction mixture.
2. (Meth)acrylamido phosphonic acids 3a and 3b: To the (meth)acrylamidoethylphosphonic acid diethyl ester dissolved in 10 mL methylene chloride was added trimethylbromosilane over a period of 20 min under stirring. Thereafter the reaction mixture was stirred for additional 3 h. The phosphonic acid silyl esters were hydrolyzed by adding methanol, extracted with CH2Cl2 and dried over MgSO4. Prior to removing the solvent 0.025 mol % 2,6-di-tert-butyl-4-methylphenol was added. The product was dried at 40 °C in vacuum.
The four monoalkyl (meth)acrylamido phosphonic acids 3c–f, the bis(meth)acrylamido phosphonic acids 3g–h and the tris (meth)acrylamido phosphonic acids 3i–j (Table 1) were synthesized according to the following general procedure.
1. Diethyl N-alkyl-2-aminoethylphosphonate: Diethyl vinylphosphonate was added to the alkyl amine and refluxed for 24 h at 65 °C. After this time signals of the vinyl group at 134.9 (CH2=) and 127.3/124.3 ppm (CH=) are completely missing from the 13C NMR spectrum.
2. N-Alkyl-N-[2-(diethylphosphono)ethyl]-(meth)acrylamide: In a 1 litre – 4-necked flask equipped with stirrer, thermometer and two 50 mL dropping funnels N-alkyl-2-aminoethylphosphonate was dissolved in 100 mL methylene chloride and cooled down to 0–5 °C. Acryloyl chloride or methacryloyl chloride dissolved in 130 mL methylene chloride and NaOH dissolved in 200 mL water was dropped simultaneously under stirring to this solution so that the temperature did not rise above 5 °C. Thereafter, the mixture was stirred for additional 2 h at room temperature. The reaction mixture was extracted twice with 150 mL 1 M HCl and 150 mL 1 M NaHCO3. Then the organic phase was washed with 150 mL deionized water, until the water had a pH-value of approximately 7. The separated organic phase was dried with Na2SO4 over night. The Na2SO4 was filtered off and extracted with methylene chloride. After addition of 0.025 mol % 2,6-di-tert-butyl-4-methylphenol relative to the (meth)acrylamide the methylene chloride was distilled off using a rotation evaporator until 8 mbar.
3. N-Alkyl-N-(2-phosphonoethyl)-(meth)acrylamide: In a 4-necked 1 litre flask equipped with a stirrer, a thermometer, refluxer with CaCl2-drying tube and 50 mL dropping funnels N-alkyl-N-[2-(diethylphosphono)ethyl]-(meth)acrylamide was dissolved in 100 mL of methylene chloride. Then trimethylbromosilane was added dropwise over a period of 20 min under stirring. Thereafter the reaction mixture was stirred for additional 2 h. By adding of 100 mL methanol the phosphonic acid silyl esters were hydrolyzed. Prior to removing the solvents 2,6-di-tert-butyl-4-methylphenol was added. The product was dried at 40 °C in vacuum.
2-(Acrylamido)ethylphosphonic acid (3a) was synthesized as described in [16].
2-(Methacrylamidoethyl)phosphonic acid (3b):
13C NMR (CH3OH-d4): δ = 166.5 (4), 139.9 (2), 118.9 (1), 34.7 (6), 28.2/26.9 (5), 18.8 (3) ppm.
N-Butyl-N-(2-phosphonoethyl)acrylamide (3c):
Soluble in CHCl3, CH3OH, C2H5OH, CHCl3/CH3OH, CH3OH/H2O, C2H5OH/H2O, acetone/water (1:1) and insoluble in acetone. mp = 129.7 °C. IR: 3411, 3390 (OH), 2973, 2929, 2885 (CH2/CH3), 1390 (CH2/CH3), 1078 cm−1 (OH). 1H NMR (CH3OH-d4): δ = 5.8 (P-OH), 5.7 (2), 5.3 (1), 3.9 (6), 4.45 (7), 2.7–2.3 (4, 5), 1.94 (8), 1.6 (9) ppm. 13C NMR (CH3OH-d4): δ = 168.4 (3), 129.1 (2), 128.9 (1), 43.8 (6), 32.6 (7), 30.8 (4), 27.9/25.8 (5), 21.2 (8), 14.2 (9) ppm. 31P NMR (CH3OH-d4): δ = 24.3/25.8 ppm.
N-Butyl-N-(2-phosphonoethyl)methacrylamide (3d):
Soluble in CHCl3, CH3OH, C2H5OH, CHCl3/CH3OH, CH3OH/H2O and C2H5OH/H2O. IR: 3411, 3390 (OH), 2973, 2929, 2885 (CH2/CH3), 1390 (CH2/CH3), 1078 cm−1 (OH). 1H NMR (CH3OH-d4): δ = 5.85 (P–OH), 5.5/5.3 (1), 3.9 (6), 3.65 (7), 2.2–2.4 (5), 1.84 (8), 1.6 (9), 1.2 (R1 = CH3) ppm. 13C NMR (CH3OH-d4): δ = 174.5 (3), 141.7 (2), 115.7 (1), 44.1 (6), 31.6 (4), 30.5 (7), 27.0/25.1 (5), 20.6 (8), 20.3 (R1=CH3), 13.8 (9) ppm.
N-Octyl-N-(2-phosphonoethyl)acrylamide (3e):
Soluble in CHCl3, CH3OH, C2H5OH, CHCl3/CH3OH, CH3OH/H2O, and C2H5OH/H2O. mp = 100.9 °C. IR: 3411, 3390 (OH), 2973, 2929, 2885 (CH2/CH3), 1390 (CH2/CH3), 1078 cm−1 (OH). 1H NMR (CH3OH-d4): δ = 6.6 (P–OH), 6.1 (2), 5.6 (1), 4.9 (6), 3.65 (7), 3.2–3.5 (4, 5, 8–11), 1.95 (12), 1.5 (13) ppm. 13C NMR (CDCl3): δ = 168.4 (3), 129.1 (2), 128.9 (1), 43.8 (6), 33.1 (7), 30.7 (4), 32.5 (11), 30.3 (9, 10), 28.7 /28.0 (8), 27.9/27.5 (5), 23.7 (12), 17.0 (13) ppm.
N-Dodecyl-N-(2-phosphonoethyl)acrylamide (3f):
Soluble in CHCl3, CH3OH, C2H5OH, CHCl3/CH3OH, CH3OH/H2O, C2H5OH/H2O (50/50) and insoluble in H2O. mp = 89.7 °C. IR: 2919, 2853 (CH2/CH3), 1641 (CO), 1533 (C=C), 1469, 1443, 1377 (CH2/CH3), 1183 (P–OH), 794 (C=C) cm−1. 1H NMR (CH3OH-d4): δ = 6.4 (P–OH), 6.1 (2), 5.6 (1), 4.8 (6), 3.7 (7), 3.3–3.6 (4, 5, 8–15), 1.9 (16), 1.5 (17) ppm. 13C NMR (CDCl3): δ = 168.4 (3), 129.1 (2), 128.9 (1), 43.8 (6), 33.1 (7), 32.5 (15), 30.7 (4), 30.3 (9–14), 28.7 /28.0 (8), 27.9/27.5 (5), 23.7 (16), 17.0 (17) ppm.
N,N′-Bis(2-phosphonoethyl)-N,N′-diacryloyl-4,7,10-trioxatridecane-1,13-diamine (3g):
Soluble in CHCl3, CHCl3/CH3OH, CH3OH, C2H5OH, CH3OH/H2O and C2H5OH/H2O (50/50).
= 1.5157 ± 0.0002, η23 °C = 436 ± 7 Pa·s
IR: 3373 (POH), 2933, 2877 (CH2/CH3), 1714, 1638 (CO), 1586 (C=C), 1460, 1437, 1370 (CH2/CH3), 1183 (P–OH), 794 (C=C) cm−1. 1H NMR (CH3OH-d4): δ = 7.4 (P–OH), 5.2 (2), 5.1 (1), 4.5 (8, 9), 3.6 (6), 3.2–3.3 (4, 5, 7) ppm. 13C NMR (CDCl3): δ = 164.7 (3), 140.2 (2), 121.7 (1), 70.6 (9), 69.4 (8), 48.2 (6), 32.5 (4) 31.9 (7), 29.5 (5) ppm.
N,N′-Bis(2-phosphonoethyl)-N,N′-bis(methacryloyl)-4,7,10-trioxatridecane-1,13-diamine (3h):
Soluble in CHCl3, CHCl3/CH3OH, CH3OH, C2H5OH, CH3OH/H2O and C2H5OH/H2O (50/50).
= 1.4900, η23 °C = 14.7 ± 0.3 Pa·s
IR: 3365 (POH), 2920, 2863 (CH2/CH3), 1722 (CO), 1584 (C=C), 1480, 1437, 1372 (CH2/CH3), 1109 (P–OH), 794 (C=C) cm−1. 1H NMR (CH3OH-d4): δ = 7.4 (P–OH), 5.1/5.2 (1), 4.5 (8, 9), 3.8 (6), 3.4–3.7 (4, 5, 7), 1.96 (10) ppm. 13C NMR (CDCl3): δ = 164.7 (3), 140.2 (2), 121.7 (1), 70.6 (9), 69.4 (8), 48.2 (6), 32.5 (4) 31.9 (7), 29.5 (5), 19.3 (10) ppm.
N-(2-Phosphonoethyl)-N,N′,N″-triacryloyl Jeffamine T403 (3i):
Soluble in CHCl3, CHCl3/CH3OH, CH3OH, C2H5OH, CH3OH/H2O and C2H5OH/H2O (50/50) and insoluble in acetone. The pH value of a solution in C2H5OH/H2O =50/50 is 1.75.
= 1.4924 ± 0.0003, η23 °C = 0.517 ± 0.018 Pa·s
IR: 3269 (POH), 2972/2933/2877 (CH2/CH3), 1734 (CO), 1652 (C=C), 795 (C=C), cm−1. 1H NMR (CH3OH-d4): δ = 6.8 (P–OH), 6.2/6.3 (2) 5.6/5.7 (1), 4.9 (8), 4.2 (9), 3.5–3.7 (4–6, 11), 1.4 (7), 1.2 (12) ppm. 13C NMR (CH3OH-d4): δ = 167.5 (3), 132.3 (2), 126.6 (1), 76.6 (8), 76.3 (9), 72.7 (6), 46.3 (5), 44.6 (4), 40.0 (10), 24.0 (11), 17.7 (7), 8.1 (12) ppm. 31P NMR (CH3OH-d4): δ = 29.5/29.9 ppm.
N,N′,N″-Tris(phosphonoethyl)-N,N′,N″-trismethacryloyl Jeffamine T403 (3j):
Soluble in CHCl3, CHCl3/CH3OH, CH3OH, C2H5OH, CH3OH/H2O and C2H5OH/H2O (50/50) and insoluble in acetone. The pH value of a solution in C2H5OH/H2O =50/50 is 1.75.
= 1.4927 ± 0.0004, η23 °C = 823 ± 1 Pa·s
IR: 3269 (POH), 2972/2933/2877 (CH2/CH3), 1734 (CO), 1652 (C=C), 795 (C=C), cm−1. 1H NMR (CH3OH-d4): δ = 6.1 (P–OH), 5.6 (1), 5.1 (8), 4.2 (9), 3.3–3.6 (4–6, 11), 1.9 (13), 1.4 (7), 1.1 (12) ppm. 13C NMR (CDCl3): δ = 174.9 (3), 140.7 (2), 115.7 (1), 121.7 (11), 70.6 (7), 69.4 (6), 62.3 (2), 48.2 (5), 32.5 (4), 25.1 (3), 19.3 (10), 16.4 (1) ppm. 31P NMR (CH3OH-d4): δ = 21.0/21.3 and 24.6/26.0 ppm.
Methacrylic acid phosphonomethyl ester (4):
This compound was synthesized by esterification of methacrylic acid with diethyl (hydroxymethyl)phosphonate and subsequent methanolysis of the phosphonic acid diethylester with trimethylbromosilane according to the procedure described for monomer 3b. Yield: 12.5 g (98.5% of th.), ΔRH = −39.0 kJ·mol−1. 13C NMR: δ = 166.8/166.6 (4), 134.3 (2), 127.4/125.2 (1), 58.7/56.0 (5), 17.6/17.4 (3) ppm.
The IR spectra were measured by using a FT-IR spectrometer (Nicolet 6700 FT-IR spectrometer (Thermo Scientific). The 1H NMR, 13C NMR and 31P NMR spectra were obtained by employing Bruker AC 250 MHz equipment. The viscosities were measured with the help of a Bohlin-Rheometer CS-50 at 23 °C.
The melting temperatures were measured by using a DSC 7 (Perkin-Elmer). The polymerization enthalpy was conducted in the isothermal mode at 37 °C, using the photo calorimeter DSC7/DPA7 (Perkin-Elmer). The light intensity in the visible portion of the spectrum was 108 mW·cm−2. Each DSC experiment included a short dark period (typically 6 s) and the subsequent illumination period. After the first run, an additional run was started using the polymerized material under the same experimental conditions. The subtraction of these runs from one another removed the effect of different baselines for the dark and the illumination periods. In the monomers were dissolved 0.3 mol % camphorquinone and 0.35 mol % 4-(N,N-dimethylamino)benzoic acid ethyl ester.
The hydrolytic stability was studied by detection of acrylic or methacrylic acid in HPLC using solutions of 1.5 mmol of 3c, 3d and 3e and solutions of 0.6 mmol 3g in 5 mL of a 1:1 mixture of distilled water and ethanol which were stored at 50 °C.
The adhesion on enamel and dentin of phosphonic acids 3 was tested using the following formulation: 0.2499 g N,N′-diethyl-1,3-bis(acrylamido)propane, 0.3000 g phosphonic acid 3, 0.4168 g 3(4),8(9)-bis(acrylamidomethyl)tricyclo[5.2.1.02,6]decane, 0.0071 g camphorquinone, 0.0083 g 4-(N,N-dimethylamino)benzoic acid ethyl ester and 0.0179 g bis bis(2,4,6-trimethylbenzoyl)-phenylphosphine oxide were dissolved in a solvent mixture composed of 0.5000 g ethanol and 0.5000 g water.
The following procedure was applied prior to adhesion measurement:
- At first teeth were abraded by 200 and 500 grit abrasive paper.
- Then the teeth were stored at 37 °C in water.
- Thereafter, teeth were treated with adhesive formulation for 20 s and the solvents were evaporated by air stream for 10 s.
- Now a light curing of adhesive layer occurred for 20 s.
- A polymerized Spectrum TPH body applied on the adhesive was cured on tooth three times for 20 s.
- At last the prepared teeth were stored in water at 37 °C for 2 h before measured.
REM investigation was done according to the following procedure: Tooth samples were polished with Nupro (Dentsply), etched with Conditioner 36 (Dentsply) or ethanol/water (1:1) solution of 3g (c = 0.5 mol l−1), for 30 s, washed with water for 10 s and with ethanol for another 10 s. Then the teeth were dried in an exsiccator under vacuum for 4 d.
References
-
Nishiyama, N.; Suzuki, K.; Yoshida, H.; Teshima, H.; Nemoto, K. Biomaterials 2004, 25, 965. doi:10.1016/S0142-9612(03)00616-1
Return to citation in text: [1] -
Catel, Y.; Degrange, M.; Le Pluart, L.; Madec, P.-J.; Pham, T.-N.; Picton, L. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 7074. doi:10.1002/pola.23013
Return to citation in text: [1] [2] -
Moszner, N.; Pavlinec, J.; Angermann, J. Macromol. Chem. Phys. 2007, 208, 529. doi:10.1002/macp.200600513
Return to citation in text: [1] -
Erdmann, C.; Ziegler, S.; Neffgen, S.; Bolln, C.; Mühlbauer, W.; Lück, R. Dental material containing phosphonic acids. WO 02/02057 A1, Jan 10, 2002.
Return to citation in text: [1] -
Klee, J. E.; Walz, U. One-part self-priming dental adhesive. WO 03/013444 A1, Feb 20, 2003.
Return to citation in text: [1] -
Ikemura, K.; Jyougetsu, Y.; Itou, S. Polymerisierbare Phosphonsäurederivate und Klebstoffzusammensetzungen enthaltend dieselben. Ger. Offen.. DE 10 2008 006 717 A1, Feb 19, 2009.
Return to citation in text: [1] -
Craciun, L.; Polishchuk, O.; Schriver, G. W.; Baisch, G.; Öhrlein, R. (Meth)acrylamide phosphorus monomer composition. WO 2007/006648 A1, Jan 18, 2007.
Return to citation in text: [1] [2] -
Kanzaki, Y. Phosphorus-acid-group-containing (meth)acrylamide, its polymer and use thereof, and their production methods. U.S. Patent 7,452,487 B2, Nov 18, 2008.
Return to citation in text: [1] [2] -
Abuelyaman, A. S.; Boardman, G. S.; Shukla, B. A.; Aasen, S. M.; Mitra, S. B.; Mikulla, M.; Cinader, D. K. Compositions including polymerizable bisphosphonic acids and methods. WO 2004/060327 A1, July 22, 2004.
Return to citation in text: [1] -
Kishore, K.; Santhanalakshmi, K. N. J. Polym. Sci., Polym. Chem. Ed. 1981, 19, 2367. doi:10.1002/pol.1981.170191001
Return to citation in text: [1] -
Dainton, F. S.; Ivin, K. J.; Walmsley, D. A. G. Trans. Faraday Soc. 1960, 56, 1784. doi:10.1039/tf9605601784
Return to citation in text: [1] [2] -
Joshi, R. M. J. Polym. Sci. 1962, 56, 313. doi:10.1002/pol.1962.1205616404
Return to citation in text: [1] [2] [3] -
Flammersheim, H.-J.; Klemm, E. Acta Polym. 1985, 36, 443. doi:10.1002/actp.1985.010360810
Return to citation in text: [1] [2] -
Otsu, T.; Inoue, M.; Yamada, B.; Mori, T. J. Polym. Sci., Polym. Lett. Ed. 1975, 13, 505. doi:10.1002/pol.1975.130130811
Return to citation in text: [1] -
Omura, I.; Yamauchi, J.; Nagase, Y.; Uemura, F. Adhesive composition. U.S. Patent 4,539,382, Sept 3, 1985.
Return to citation in text: [1] -
Neidlein, R.; Greulich, P. Helv. Chim. Acta 1992, 75, 2545. doi:10.1002/hlca.19920750809
Return to citation in text: [1]
| 1. | Nishiyama, N.; Suzuki, K.; Yoshida, H.; Teshima, H.; Nemoto, K. Biomaterials 2004, 25, 965. doi:10.1016/S0142-9612(03)00616-1 |
| 4. | Erdmann, C.; Ziegler, S.; Neffgen, S.; Bolln, C.; Mühlbauer, W.; Lück, R. Dental material containing phosphonic acids. WO 02/02057 A1, Jan 10, 2002. |
| 5. | Klee, J. E.; Walz, U. One-part self-priming dental adhesive. WO 03/013444 A1, Feb 20, 2003. |
| 6. | Ikemura, K.; Jyougetsu, Y.; Itou, S. Polymerisierbare Phosphonsäurederivate und Klebstoffzusammensetzungen enthaltend dieselben. Ger. Offen.. DE 10 2008 006 717 A1, Feb 19, 2009. |
| 7. | Craciun, L.; Polishchuk, O.; Schriver, G. W.; Baisch, G.; Öhrlein, R. (Meth)acrylamide phosphorus monomer composition. WO 2007/006648 A1, Jan 18, 2007. |
| 8. | Kanzaki, Y. Phosphorus-acid-group-containing (meth)acrylamide, its polymer and use thereof, and their production methods. U.S. Patent 7,452,487 B2, Nov 18, 2008. |
| 3. | Moszner, N.; Pavlinec, J.; Angermann, J. Macromol. Chem. Phys. 2007, 208, 529. doi:10.1002/macp.200600513 |
| 2. | Catel, Y.; Degrange, M.; Le Pluart, L.; Madec, P.-J.; Pham, T.-N.; Picton, L. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 7074. doi:10.1002/pola.23013 |
| 15. | Omura, I.; Yamauchi, J.; Nagase, Y.; Uemura, F. Adhesive composition. U.S. Patent 4,539,382, Sept 3, 1985. |
| 2. | Catel, Y.; Degrange, M.; Le Pluart, L.; Madec, P.-J.; Pham, T.-N.; Picton, L. J. Polym. Sci., Part A: Polym. Chem. 2008, 46, 7074. doi:10.1002/pola.23013 |
| 16. | Neidlein, R.; Greulich, P. Helv. Chim. Acta 1992, 75, 2545. doi:10.1002/hlca.19920750809 |
| 12. | Joshi, R. M. J. Polym. Sci. 1962, 56, 313. doi:10.1002/pol.1962.1205616404 |
| 13. | Flammersheim, H.-J.; Klemm, E. Acta Polym. 1985, 36, 443. doi:10.1002/actp.1985.010360810 |
| 11. | Dainton, F. S.; Ivin, K. J.; Walmsley, D. A. G. Trans. Faraday Soc. 1960, 56, 1784. doi:10.1039/tf9605601784 |
| 14. | Otsu, T.; Inoue, M.; Yamada, B.; Mori, T. J. Polym. Sci., Polym. Lett. Ed. 1975, 13, 505. doi:10.1002/pol.1975.130130811 |
| 10. | Kishore, K.; Santhanalakshmi, K. N. J. Polym. Sci., Polym. Chem. Ed. 1981, 19, 2367. doi:10.1002/pol.1981.170191001 |
| 7. | Craciun, L.; Polishchuk, O.; Schriver, G. W.; Baisch, G.; Öhrlein, R. (Meth)acrylamide phosphorus monomer composition. WO 2007/006648 A1, Jan 18, 2007. |
| 8. | Kanzaki, Y. Phosphorus-acid-group-containing (meth)acrylamide, its polymer and use thereof, and their production methods. U.S. Patent 7,452,487 B2, Nov 18, 2008. |
| 9. | Abuelyaman, A. S.; Boardman, G. S.; Shukla, B. A.; Aasen, S. M.; Mitra, S. B.; Mikulla, M.; Cinader, D. K. Compositions including polymerizable bisphosphonic acids and methods. WO 2004/060327 A1, July 22, 2004. |
| 11. | Dainton, F. S.; Ivin, K. J.; Walmsley, D. A. G. Trans. Faraday Soc. 1960, 56, 1784. doi:10.1039/tf9605601784 |
| 12. | Joshi, R. M. J. Polym. Sci. 1962, 56, 313. doi:10.1002/pol.1962.1205616404 |
| 13. | Flammersheim, H.-J.; Klemm, E. Acta Polym. 1985, 36, 443. doi:10.1002/actp.1985.010360810 |
© 2009 Klee and Lehmann; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)