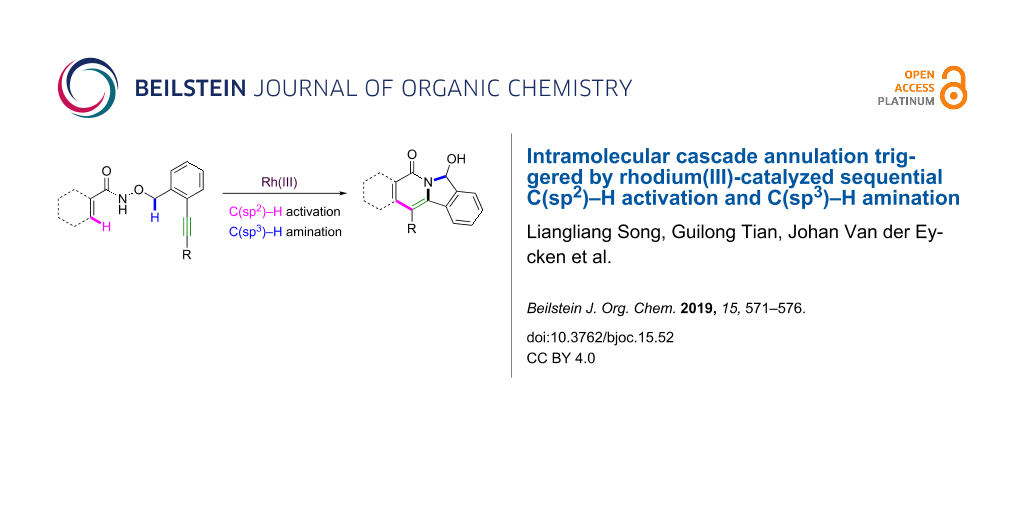
Abstract
A rhodium(III)-catalyzed intramolecular oxidative annulation of O-substituted N-hydroxyacrylamides for the construction of indolizinones via sequential C(sp2)–H activation and C(sp3)–H amination has been developed. This approach shows excellent functional-group tolerance. The synthesized scaffold forms the core of many natural products with pharmacological relevance.
Graphical Abstract
Introduction
Over the last decade, transition metal-catalyzed C(sp2)–H activation has emerged as an efficient strategy to access complex molecules [1-6]. Among the methodologies, RhIII-catalyzed oxidative annulation of a C(sp2)–H bond with 2π components (such as olefins, alkynes) stands out for the construction of carbo(hetero)cycles from easily available starting materials [7-10]. Compared to aromatic C(sp2)–H bonds, studies on activation of vinylic C(sp2)–H bonds have been less explored, due to an intrinsic inactivity, tended to undergo polymerization, prone to go through conjugate additions [11,12]. Moreover, the cyclometalation intermediates are unstable, and the β-substitution or α,β-disubstitution of acrylate sterically prevents the cyclometalation [13,14]. Despite these, several approaches have been developed to synthesize pyridones and highly substituted olefins using acrylamides. However, most of them are limited to one-step coupling or annulation and just a single ring is formed [15-22]. Therefore, it is necessary to explore a new cascade annulation of acrylamides to construct a polyfused-heteroarene skeleton in one operational step.
The tricyclic indolizinone scaffold is abundantly present in natural products, as, e.g., in the pharmacologically relevant mappicine [23,24], camptothecin [25,26], 10-hydroxycamptothecin and topotecan [27,28] (Figure 1). In 2012, Park and co-workers reported a RhIII-catalyzed intramolecular annulation of alkyne-tethered hydroxamic esters for the synthesis of isoquinolones and pyridines without using external oxidants (Scheme 1a) [22]. Recently, we reported an intramolecular annulation of benzamides to synthesize indolizinones through RhIII-catalyzed C(sp2)–H activation (Scheme 1b) [29-31]. Inspired by this work, we envisaged that tricyclic indolizinones could be built through rhodium(III)-catalyzed sequential C(sp2)–H activation and C(sp3)–H amination of O-substituted N-hydroxyacrylamides (Scheme 1c).
Figure 1: Selected examples of natural products with a tricyclic indolizinone scaffold.
Figure 1: Selected examples of natural products with a tricyclic indolizinone scaffold.
Scheme 1: Previous work and this approach.
Scheme 1: Previous work and this approach.
Results and Discussion
We selected N-hydroxyacrylamide 1a as our model substrate under standard conditions. In the presence of [RhCp*Cl2]2 (5 mol %) and CsOAc (2 equiv) in 1,4-dioxane (0.1 M) at 60 °C under air, the desired product 3a was obtained in 40% yield, together with 2a in 34% yield (Table 1, entry 1). Other solvents could not improve the yield of 3a (Table 1, entries 2 and 3). [Ru(p-cymene)Cl2]2 resulted in a very poor yield of 3a (Table 1, entry 4). Alternative rhodium catalyst [RhCp*(CH3CN)3](SbF6)2 gave 17% 2a and 35% 3a (Table 1, entry 5). Without CsOAc under [RhCp*(CH3CN)3](SbF6)2 catalysis, just 29% 2a was isolated (Table 1, entry 6). With CsOPiv instead of CsOAc, the products 3a and 2a were obtained in 32% and 21% yields, respectively (Table 1, entry 7). Without adding CsOAc, no products were formed (Table 1, entry 8). Also, when the reaction was treated under standard conditions for 0.5 h, the products 3a and 2a were isolated in 14% and 12% yields, respectively (Table 1, entry 9), which suggested that 3a was formed as soon as the reaction was performed.
Table 1: Optimization of the reaction conditions.a
|
|
||
| Entry | Changes to standard conditions | Yields (2a, 3a)b |
| 1 | none | (34%, 40%) |
| 2 | MeOH | (72%, 11%) |
| 3 | DCE | (49%, 30%) |
| 4 | [Ru(p-cymene)Cl2]2 | (52%, 10%) |
| 5 | [RhCp*(CH3CN)3](SbF6)2 | (17%, 35%) |
| 6c | [RhCp*(CH3CN)3](SbF6)2 | (29%, 0%) |
| 7 | CsOPiv instead of CsOAc | (21%, 32%) |
| 8 | without CsOAc | (0%, 0%) |
| 9 | 0.5 h instead of 8 h | (12%, 14%) |
aConditions: 1a (0.3 mmol), catalyst (0.015 mmol), CsOAc (0.6 mmol), solvent (3.0 mL). bIsolated yield. cWithout CsOAc.
Next, diverse substrates were explored to evaluate the scope of this approach under the optimal reaction conditions (Scheme 2). α-Methylacrylamide smoothly proceeded to give the corresponding indolizinone 3b in 41% yield. Acrylamide afforded the corresponding indolizinone 3c in 43% yield. Compared to α-substituted acrylamides, β-substituted acrylamides performed the reaction with lower yields under the same conditions (3d–f). It should be pointed out that α,β-disubstituted acrylamides were also suitable substrates for this transformation, and the corresponding indolizinones 3g–i were obtained in 39–45% yield. Substrates with different substituents on the alkyne, including 4-methylphenyl, 4-chlorophenyl, phenethyl and a TMS group, could deliver the corresponding indolizinones 3j–m in 47–52% yield. Interestingly, 2-ethynylquinoline as substrate worked well, yielding the corresponding indolizinone 3n in 32%, which has the same skeleton as mappicine.
Scheme 2: Reaction scope. Reaction conditions: 1 (0.3 mmol), [RhCp*Cl2]2 (0.015 mmol), CsOAc (0.6 mmol), 1,4-dioxane (3.0 mL), the ratio of isolated 3:2 was shown in parenthesis.
Scheme 2: Reaction scope. Reaction conditions: 1 (0.3 mmol), [RhCp*Cl2]2 (0.015 mmol), CsOAc (0.6 mmol), 1,4-...
To investigate the mechanism of this method, control experiments were carried out (Scheme 3). When 2a was performed under standard conditions, 3a could be obtained in 5% yield. Increasing the temperature to 80 °C or 100 °C has no dramatic effect on the yield of 3a. Other bases, like NaOAc or KOAc, could not improve the yield of 3a from 2a. On the contrary, 2a did not give 3a in the absence of CsOAc. These results indicate that 3a could be formed through two pathways, and the one from 2a is the minor pathway. The main pathway is directly from 1a.
Based on the above results, a plausible mechanism is proposed in Scheme 4 [31]. C(sp2)–H activation of acrylamide 1a, followed by subsequent intramolecular coordination of the alkyne gives intermediate B. Subsequent intramolecular migratory insertion affords intermediate C. Reductive elimination and subsequent oxidative addition give intermediate D. Then two pathways are involved in the following steps. In the main pathway (path a), intermediate D undergoes β-H elimination and tandem cyclization to give product 3a and Rh–H intermediate G, which could be oxidized by O2 to regenerate the catalyst. In the minor pathway (path b), intermediate D undergoes protonation by acetic acid to give product 2a, which undergoes deprotonation to form intermediate D again, then following the main pathway to give product 3a.
Conclusion
In summary, we have developed a rhodium(III)-catalyzed sequential C(sp2)–H activation and C(sp3)–H amination of O-substituted N-hydroxyacrylamides for the synthesis of indolizinones. This method shows excellent functional-group tolerance. The family of indolizinone products represents potential bioactive molecules for further studies.
Supporting Information
| Supporting Information File 1: Experimental details and characterization data. | ||
| Format: PDF | Size: 2.5 MB | Download |
References
-
Gensch, T.; Hopkinson, M. N.; Glorius, F.; Wencel-Delord, J. Chem. Soc. Rev. 2016, 45, 2900–2936. doi:10.1039/c6cs00075d
Return to citation in text: [1] -
Liu, W.; Ackermann, L. ACS Catal. 2016, 6, 3743–3752. doi:10.1021/acscatal.6b00993
Return to citation in text: [1] -
Su, B.; Cao, Z.-C.; Shi, Z.-J. Acc. Chem. Res. 2015, 48, 886–896. doi:10.1021/ar500345f
Return to citation in text: [1] -
Song, G.; Li, X. Acc. Chem. Res. 2015, 48, 1007–1020. doi:10.1021/acs.accounts.5b00077
Return to citation in text: [1] -
Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068. doi:10.1039/c1cs15082k
Return to citation in text: [1] -
Zhu, R.-Y.; Farmer, M. E.; Chen, Y.-Q.; Yu, J.-Q. Angew. Chem., Int. Ed. 2016, 55, 10578–10599. doi:10.1002/anie.201600791
Return to citation in text: [1] -
Yin, J.; Tan, M.; Wu, D.; Jiang, R.; Li, C.; You, J. Angew. Chem., Int. Ed. 2017, 56, 13094–13098. doi:10.1002/anie.201708127
Return to citation in text: [1] -
Wang, X.; Lerchen, A.; Gensch, T.; Knecht, T.; Daniliuc, C. G.; Glorius, F. Angew. Chem., Int. Ed. 2017, 56, 1381–1384. doi:10.1002/anie.201610117
Return to citation in text: [1] -
Hong, S. Y.; Jeong, J.; Chang, S. Angew. Chem., Int. Ed. 2017, 56, 2408–2412. doi:10.1002/anie.201612559
Return to citation in text: [1] -
Upadhyay, N. S.; Thorat, V. H.; Sato, R.; Annamalai, P.; Chuang, S.-C.; Cheng, C.-H. Green Chem. 2017, 19, 3219–3224. doi:10.1039/c7gc01221g
Return to citation in text: [1] -
Wang, K.; Hu, F.; Zhang, Y.; Wang, J. Sci. China: Chem. 2015, 58, 1252–1265. doi:10.1007/s11426-015-5362-5
Return to citation in text: [1] -
Shankar, M.; Guntreddi, T.; Ramesh, E.; Sahoo, A. K. Org. Lett. 2017, 19, 5665–5668. doi:10.1021/acs.orglett.7b02824
Return to citation in text: [1] -
Shang, X.; Liu, Z.-Q. Chem. Soc. Rev. 2013, 42, 3253–3260. doi:10.1039/c2cs35445d
Return to citation in text: [1] -
Colby, D. A.; Tsai, A. S.; Bergman, R. G.; Ellman, J. A. Acc. Chem. Res. 2012, 45, 814–825. doi:10.1021/ar200190g
Return to citation in text: [1] -
Hu, X.-H.; Yang, X.-F.; Loh, T.-P. Angew. Chem., Int. Ed. 2015, 54, 15535–15539. doi:10.1002/anie.201506437
Return to citation in text: [1] -
Zhao, Y.; Li, S.; Zheng, X.; Tang, J.; She, Z.; Gao, G.; You, J. Angew. Chem., Int. Ed. 2017, 56, 4286–4289. doi:10.1002/anie.201612147
Return to citation in text: [1] -
Meng, K.; Zhang, J.; Li, F.; Lin, Z.; Zhang, K.; Zhong, G. Org. Lett. 2017, 19, 2498–2501. doi:10.1021/acs.orglett.7b00695
Return to citation in text: [1] -
Yu, Y.; Huang, L.; Wu, W.; Jiang, H. Org. Lett. 2014, 16, 2146–2149. doi:10.1021/ol500611d
Return to citation in text: [1] -
Jiang, B.; Zhao, M.; Li, S.-S.; Xu, Y.-H.; Loh, T.-P. Angew. Chem., Int. Ed. 2018, 57, 555–559. doi:10.1002/anie.201710601
Return to citation in text: [1] -
Yu, C.; Li, F.; Zhang, J.; Zhong, G. Chem. Commun. 2017, 53, 533–536. doi:10.1039/c6cc07064g
Return to citation in text: [1] -
Liang, Q.-J.; Yang, C.; Meng, F.-F.; Jiang, B.; Xu, Y.-H.; Loh, T.-P. Angew. Chem., Int. Ed. 2017, 56, 5091–5095. doi:10.1002/anie.201700559
Return to citation in text: [1] -
Xu, X.; Liu, Y.; Park, C.-M. Angew. Chem., Int. Ed. 2012, 51, 9372–9376. doi:10.1002/anie.201204970
Return to citation in text: [1] [2] -
Zhang, Q.; Rivkin, A.; Curran, D. P. J. Am. Chem. Soc. 2002, 124, 5774–5781. doi:10.1021/ja025606x
Return to citation in text: [1] -
Henegar, K. E.; Baughman, T. A. J. Heterocycl. Chem. 2003, 40, 601–605. doi:10.1002/jhet.5570400407
Return to citation in text: [1] -
Xu, P.; Chen, D.-S.; Xi, J.; Yao, Z.-J. Chem. – Asian J. 2015, 10, 976–981. doi:10.1002/asia.201403190
Return to citation in text: [1] -
Yu, S.; Huang, Q.-Q.; Luo, Y.; Lu, W. J. Org. Chem. 2012, 77, 713–717. doi:10.1021/jo201974f
Return to citation in text: [1] -
Kingsbury, W. D.; Boehm, J. C.; Jakas, D. R.; Holden, K. G.; Hecht, S. M.; Gallagher, G.; Caranfa, M. J.; McCabe, F. L.; Faucette, L. F.; Johnson, R. K.; Hertzberg, R. P. J. Med. Chem. 1991, 34, 98–107. doi:10.1021/jm00105a017
Return to citation in text: [1] -
Li, K.; Ou, J.; Gao, S. Angew. Chem., Int. Ed. 2016, 55, 14778–14783. doi:10.1002/anie.201607832
Return to citation in text: [1] -
Song, L.; Tian, G.; He, Y.; Van der Eycken, E. V. Chem. Commun. 2017, 53, 12394–12397. doi:10.1039/c7cc06860c
Return to citation in text: [1] -
Song, L.; Tian, G.; Van der Eycken, E. V. Mol. Catal. 2018, 459, 129–134. doi:10.1016/j.mcat.2018.09.004
Return to citation in text: [1] -
Song, L.; Zhang, X.; Tian, G.; Robeyns, K.; Van Meervelt, L.; Harvey, J. N.; Van der Eycken, E. V. Mol. Catal. 2019, 463, 30–36. doi:10.1016/j.mcat.2018.11.016
Return to citation in text: [1] [2]
| 1. | Gensch, T.; Hopkinson, M. N.; Glorius, F.; Wencel-Delord, J. Chem. Soc. Rev. 2016, 45, 2900–2936. doi:10.1039/c6cs00075d |
| 2. | Liu, W.; Ackermann, L. ACS Catal. 2016, 6, 3743–3752. doi:10.1021/acscatal.6b00993 |
| 3. | Su, B.; Cao, Z.-C.; Shi, Z.-J. Acc. Chem. Res. 2015, 48, 886–896. doi:10.1021/ar500345f |
| 4. | Song, G.; Li, X. Acc. Chem. Res. 2015, 48, 1007–1020. doi:10.1021/acs.accounts.5b00077 |
| 5. | Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068. doi:10.1039/c1cs15082k |
| 6. | Zhu, R.-Y.; Farmer, M. E.; Chen, Y.-Q.; Yu, J.-Q. Angew. Chem., Int. Ed. 2016, 55, 10578–10599. doi:10.1002/anie.201600791 |
| 15. | Hu, X.-H.; Yang, X.-F.; Loh, T.-P. Angew. Chem., Int. Ed. 2015, 54, 15535–15539. doi:10.1002/anie.201506437 |
| 16. | Zhao, Y.; Li, S.; Zheng, X.; Tang, J.; She, Z.; Gao, G.; You, J. Angew. Chem., Int. Ed. 2017, 56, 4286–4289. doi:10.1002/anie.201612147 |
| 17. | Meng, K.; Zhang, J.; Li, F.; Lin, Z.; Zhang, K.; Zhong, G. Org. Lett. 2017, 19, 2498–2501. doi:10.1021/acs.orglett.7b00695 |
| 18. | Yu, Y.; Huang, L.; Wu, W.; Jiang, H. Org. Lett. 2014, 16, 2146–2149. doi:10.1021/ol500611d |
| 19. | Jiang, B.; Zhao, M.; Li, S.-S.; Xu, Y.-H.; Loh, T.-P. Angew. Chem., Int. Ed. 2018, 57, 555–559. doi:10.1002/anie.201710601 |
| 20. | Yu, C.; Li, F.; Zhang, J.; Zhong, G. Chem. Commun. 2017, 53, 533–536. doi:10.1039/c6cc07064g |
| 21. | Liang, Q.-J.; Yang, C.; Meng, F.-F.; Jiang, B.; Xu, Y.-H.; Loh, T.-P. Angew. Chem., Int. Ed. 2017, 56, 5091–5095. doi:10.1002/anie.201700559 |
| 22. | Xu, X.; Liu, Y.; Park, C.-M. Angew. Chem., Int. Ed. 2012, 51, 9372–9376. doi:10.1002/anie.201204970 |
| 13. | Shang, X.; Liu, Z.-Q. Chem. Soc. Rev. 2013, 42, 3253–3260. doi:10.1039/c2cs35445d |
| 14. | Colby, D. A.; Tsai, A. S.; Bergman, R. G.; Ellman, J. A. Acc. Chem. Res. 2012, 45, 814–825. doi:10.1021/ar200190g |
| 11. | Wang, K.; Hu, F.; Zhang, Y.; Wang, J. Sci. China: Chem. 2015, 58, 1252–1265. doi:10.1007/s11426-015-5362-5 |
| 12. | Shankar, M.; Guntreddi, T.; Ramesh, E.; Sahoo, A. K. Org. Lett. 2017, 19, 5665–5668. doi:10.1021/acs.orglett.7b02824 |
| 7. | Yin, J.; Tan, M.; Wu, D.; Jiang, R.; Li, C.; You, J. Angew. Chem., Int. Ed. 2017, 56, 13094–13098. doi:10.1002/anie.201708127 |
| 8. | Wang, X.; Lerchen, A.; Gensch, T.; Knecht, T.; Daniliuc, C. G.; Glorius, F. Angew. Chem., Int. Ed. 2017, 56, 1381–1384. doi:10.1002/anie.201610117 |
| 9. | Hong, S. Y.; Jeong, J.; Chang, S. Angew. Chem., Int. Ed. 2017, 56, 2408–2412. doi:10.1002/anie.201612559 |
| 10. | Upadhyay, N. S.; Thorat, V. H.; Sato, R.; Annamalai, P.; Chuang, S.-C.; Cheng, C.-H. Green Chem. 2017, 19, 3219–3224. doi:10.1039/c7gc01221g |
| 22. | Xu, X.; Liu, Y.; Park, C.-M. Angew. Chem., Int. Ed. 2012, 51, 9372–9376. doi:10.1002/anie.201204970 |
| 31. | Song, L.; Zhang, X.; Tian, G.; Robeyns, K.; Van Meervelt, L.; Harvey, J. N.; Van der Eycken, E. V. Mol. Catal. 2019, 463, 30–36. doi:10.1016/j.mcat.2018.11.016 |
| 27. | Kingsbury, W. D.; Boehm, J. C.; Jakas, D. R.; Holden, K. G.; Hecht, S. M.; Gallagher, G.; Caranfa, M. J.; McCabe, F. L.; Faucette, L. F.; Johnson, R. K.; Hertzberg, R. P. J. Med. Chem. 1991, 34, 98–107. doi:10.1021/jm00105a017 |
| 28. | Li, K.; Ou, J.; Gao, S. Angew. Chem., Int. Ed. 2016, 55, 14778–14783. doi:10.1002/anie.201607832 |
| 25. | Xu, P.; Chen, D.-S.; Xi, J.; Yao, Z.-J. Chem. – Asian J. 2015, 10, 976–981. doi:10.1002/asia.201403190 |
| 26. | Yu, S.; Huang, Q.-Q.; Luo, Y.; Lu, W. J. Org. Chem. 2012, 77, 713–717. doi:10.1021/jo201974f |
| 23. | Zhang, Q.; Rivkin, A.; Curran, D. P. J. Am. Chem. Soc. 2002, 124, 5774–5781. doi:10.1021/ja025606x |
| 24. | Henegar, K. E.; Baughman, T. A. J. Heterocycl. Chem. 2003, 40, 601–605. doi:10.1002/jhet.5570400407 |
| 29. | Song, L.; Tian, G.; He, Y.; Van der Eycken, E. V. Chem. Commun. 2017, 53, 12394–12397. doi:10.1039/c7cc06860c |
| 30. | Song, L.; Tian, G.; Van der Eycken, E. V. Mol. Catal. 2018, 459, 129–134. doi:10.1016/j.mcat.2018.09.004 |
| 31. | Song, L.; Zhang, X.; Tian, G.; Robeyns, K.; Van Meervelt, L.; Harvey, J. N.; Van der Eycken, E. V. Mol. Catal. 2019, 463, 30–36. doi:10.1016/j.mcat.2018.11.016 |
© 2019 Song et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)