Abstract
A one-pot synthesis of 4-nitro-3-phenylisoxazole has been carried out by treatment of cinnamyl alcohol dissolved in acetic acid with sodium nitrite; in addition, 4-phenyl-3-furoxanmethanol was obtained in 40% yield.
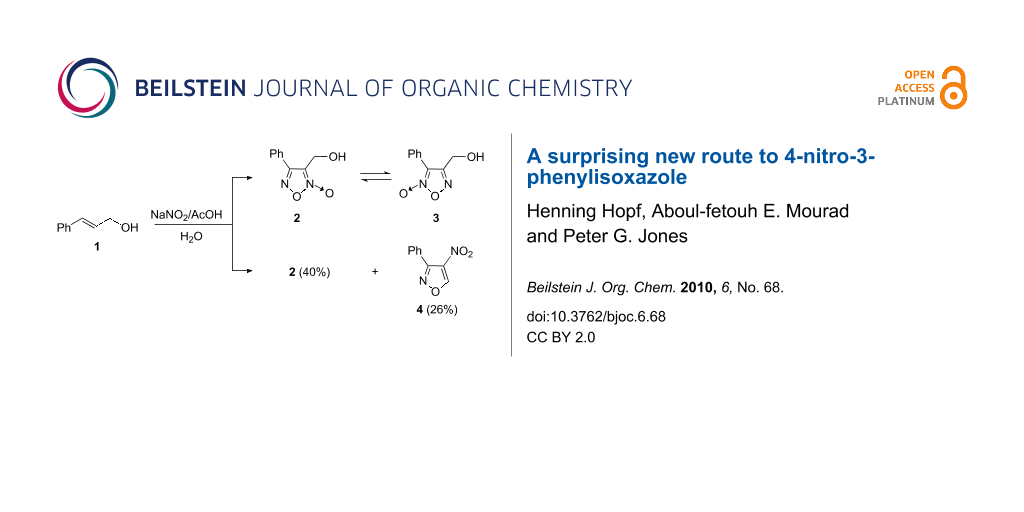
Graphical Abstract
Introduction
The isoxazole ring system, which can be easily obtained by [3 + 2] cycloaddition of nitric oxides to alkynes, is of interest since it forms a part of various biodynamic agents. Isoxazole derivatives that act as antithrombotic, hypolipidemic, nootropic, antiviral, antiobesity, and CNS modulation agents have been described [1].
On the other hand, derivatives having liquid crystalline properties have received a great deal of attention as they have a wide variety of applications, especially in flat-panel displays [2], light emitting diodes [3-5], anisotropic networks [6,7], and semiconductor materials [8]. Incorporation of the isoxazole moiety into such materials can result in significant changes in the corresponding mesogenic phases, since isoxazoles display classical nematic and smetic mesophases associated with their rod-like core structure [9].
The pioneering work on furoxans as nitric oxide donors by Gasco et al. has stimulated a large number of further studies. One of these reports deals with a synthetic route and structural characterization of two isomeric phenylfuroxans 2 and 3 [10]. According to the original report, 4-phenyl-3-furoxanmethanol (2) was obtained as the sole product in 40% yield, by treatment of cinnamyl alcohol (1) dissolved in acetic acid with concentrated aqueous sodium nitrite at 70 °C (Scheme 1).
Scheme 1: Preparation of 2 and 4 by treatment of cinnamyl alcohol (1).
Scheme 1: Preparation of 2 and 4 by treatment of cinnamyl alcohol (1).
Results and Discussion
Application of this approach to the synthesis of 2 and purification of the reaction product, as reported, by column chromatography with petroleum ether/ethyl acetate (4:1) as eluant resulted, in our hands, not only in the isolation of the desired reaction product 2 (40%), but surprisingly, also of compound 4 (26% yield). Compound 4 (Rf = 0.65) was eluted before compound 2 (Rf = 0.41). The structure of compound 4 was elucidated on the basis of its spectral (see Experimental section) and crystallographic data. The crystal structure of compound 4 is shown in Figure 1. Bond lengths and angles (e.g. C3–N2 1.3110(15), N2–O1 1.4283(13), O1–C5 1.2202(15) Å) may be considered normal. The five-membered ring is planar within a mean deviation of 0.002 Å, and subtends interplanar angles of 6.5° with the nitro and (in the same sense) 58.4° with the phenyl substituent. Molecules are connected to form broad ribbons in the (101) plane and parallel to the short y axis (Figure 2) by “weak” hydrogen bonds H12···O2 2.56 and H5···O3 2.39 Å.
![[1860-5397-6-68-1]](/bjoc/content/figures/1860-5397-6-68-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: The crystal structure of compound 4. Ellipsoids correspond to 50% probability levels.
Figure 1: The crystal structure of compound 4. Ellipsoids correspond to 50% probability levels.
![[1860-5397-6-68-2]](/bjoc/content/figures/1860-5397-6-68-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Packing diagram of compound 4 viewed perpendicular to (101). Hydrogen bonds are indicated by thick dashed bonds.
Figure 2: Packing diagram of compound 4 viewed perpendicular to (101). Hydrogen bonds are indicated by thick ...
A suggested mechanism for the formation of 4 is outlined in Scheme 2.
Scheme 2: Suggested mechanism for the formation of 4.
Scheme 2: Suggested mechanism for the formation of 4.
We propose that the nitro compound 4 is generated via the intermediate 6. This, in turn, could be generated by dehydration of the precursor 5, an addition product of HNO2 to the substrate 1 by a formal hetero Alder-ene reaction. Isoxazole 6 could then undergo electrophilic substitution via 7 to afford the nitroso compound 8, which in a final step would be oxidized to 4 under the reaction conditions.
Previously reported approaches to 4 include the reaction of aromatic α,β-unsaturated aldehydes with dinitrogen trioxide [11,12], the nitration of 3-phenyl-isoxazole [13,14], and the hydrolysis of methyl 4-nitro-3-phenyl-5-carboxylate, obtained by reaction of α-nitroacetophenone oxime and methoxalyl chloride [15].
In conclusion, a very simple route to 4-nitro-3-phenylisoxazole (4), as well as the furoxan derivative 2, which constitutes a nitric oxide (NO) donor site in NO donor systems [16], is disclosed.
Experimental
Melting points were measured on Büchi 350 apparatus and are uncorrected. IR spectra were measured with Bruker Tensor 27 instrument. 1H and 13C NMR spectra were recorded in CDCl3 with a Bruker DRX-400 instrument operating at 400 MHz and 100 MHz, respectively. Silica gel 60 (Merck, 70–230 mesh) was used for column chromatography. Macherey-Nagel polygram SilG/UV 254 was used for TLC. Petroleum ether refers to the fraction of bp 40–60 °C. The mass spectrum was taken on a Finnigan MAT 4515 spectrometer at 70 eV.
Preparation of 4-phenyl-3-furoxanmethanol (2) and 4-nitro-3-phenylisoxazole (4): To a stirred solution of cinnamyl alcohol (1, 4.08 g, 30 mmol) in glacial acetic acid (6 mL), was added saturated aqueous sodium nitrite (90 mmol) solution dropwise so that the temperature did not exceed 70 °C. Stirring was continued at room temperature for 1 h. The reaction mixture was diluted with water, extracted with diethyl ether, washed with brine, dried with anhydrous sodium sulfate and concentrated in vacuo. The residue was purified by column chromatography using petroleum ether/ethyl acetate (4:1) as eluent to give 4-phenyl-3-furoxanmethanol (2.29 g, 40%) and 4-nitro-3-phenylisoxazole (1.49 g, 26%), respectively.
4-Phenyl-3-furoxanmethanol (2): mp 67 °C (benzene/petroleum ether); lit. mp 66–67 °C [10]; IR: ν = 3127, 1569, 1523, 1449, 1404, 1372, 1195, 1130, 883, 767, 693 cm−1; 1H NMR: δ = 7.85–7.78 (m, 2H, Ar-H), 7.61–7.58 (m, 3H, Ar-H), 4.75 (s, 2H, CH2), 2.75 (br. s, 1H, OH); MS (EI): m/z = 191 [M]+ (42), 174 (7), 145 (12), 129 (16), 103 (100), 93 (8), 76 (38).
4-Nitro-3-phenylisoxazole (4): mp 113–114 °C (ethyl acetate); lit. mp 114.5 °C [17]; 1H NMR: δ = 9.36 (s, 1H, H-5), 7.70–7.67 (m, 2H, Ar-H), 7.59–7.49 (m, 3H, Ar-H); 13C NMR: δ = 160.6 (C-5), 156.5 (C-3), 133.9 (C-4), 131.1, 129.5, 128.6, 124.7 (Ph).
X-Ray structure determination of 4: Crystal data: C9H6N2O3, Mr = 190.16, monoclinic, P21/c, T = −173 °C, a = 12.1621(7), b = 5.6566(3), c = 12.0633(8) Å, β = 101.638(6)°, V = 812.85 Å3, Z = 4, F(000) = 392, λ(Mo Kα) = 0.71073 Å, μ = 0.12 mm−1, Dx = 1.554 g cm−3. Data collection: A colourless prism ca. 0.3 × 0.15 × 0.1 mm was mounted in inert oil on a glass fibre and transferred to the cold gas stream of an Oxford Diffraction Xcalibur E diffractometer. A total of 13,352 reflections were recorded to 2Θ 57.2°, of which 2100 were independent (Rint 0.037). Structure refinement: The structure was refined using SHELXL-97 [18]. Hydrogen atoms were included using a riding model. The final R2 (all reflections) was 0.084 for all intensities and 127 parameters, with R1 (I>2σ(I)) 0.035; S 0.94, max. Δρ 0.25 e Å−3.
X-ray crystallographic data (excluding structure factors) were deposited under the number CCDC-763982 and can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
References
-
Barta, S.; Srinivasan, T.; Rastogi, S. K.; Kundu, B.; Parta, A.; Bhaduri, A. P.; Dixit, M. Bioorg. Med. Chem. Lett. 2002, 12, 1905. doi:10.1016/S0960-894X(02)00333-5
and references cited therein.
Return to citation in text: [1] -
Demus, D.; Goodby, J. W.; Gray, G. W.; Spiess, H. W.; Vill, L. Low Molecular Weight Liquid Crystals I. Handbook of Liquid Crystals; Wiley-VCH: Weinheim, 1998; Vol. 2a. doi:10.1002/9783527619276
Return to citation in text: [1] -
Vlachos, P.; Mansoor, B.; Alfred, M. P.; O`Neil, M.; Kelly, S. M. Chem. Commun. 2005, 2921. doi:10.1039/b501875g
Return to citation in text: [1] -
Sonar, P.; Singh, S. P.; Sudhakar, S.; Dodabalapur, A.; Sellinger, A. Chem. Mater. 2008, 20, 3184. doi:10.1021/cm800139q
Return to citation in text: [1] -
Cristiano, R.; Ely, F.; Gallardo, H. Liq. Cryst. 2005, 32, 15. doi:10.1080/02678290412331329279
Return to citation in text: [1] -
Hsu, C.-S.; Chen, H.-L. J. Polym. Sci., Part A: Polym. Chem. 1999, 37, 3929. doi:10.1002/(SICI)1099-0518(19991101)37:21<3929::AID-POLA8>3.0.CO;2-9
Return to citation in text: [1] -
Cassano, R.; Dabrowski, R.; Dziaduszek, J.; Picci, C.; Chidichimo, G.; De Filpo, G.; Muzzalupo, R.; Puoci, F. Tetrahedron Lett. 2007, 48, 1447. doi:10.1016/j.tetlet.2006.12.094
Return to citation in text: [1] -
Melucci, M.; Favaretto, L.; Bettini, C.; Gazzano, M.; Camaioni, N.; Fukazawa, P.; Ostoja, P.; Monari, M.; Barbarella, G. Chem.–Eur. J. 2007, 13, 10046. doi:10.1002/chem.200701368
Return to citation in text: [1] -
Haino, T.; Tanaka, M.; Ideta, K.; Kubo, K.; Mori, A.; Maccagnani, Y. Tetrahedron Lett. 2004, 45, 2277. doi:10.1016/j.tetlet.2004.01.116
and references cited therein.
Return to citation in text: [1] -
Gasco, A. M.; Fruttero, R.; Sorba, G.; Gasco, A. Liebigs Ann. Chem. 1991, 1211. doi:10.1002/jlac.1991199101207
Return to citation in text: [1] [2] -
Quilico, A. Isoxazoles. In Five- and Six-Membered Compounds with Nitrogen and Oxygen (Excluding Oxazoles); Wiley, R. H.; Quilico, A.; Speroni, G.; Behr, L. C.; McKee, R. L., Eds.; Chemistry of Heterocyclic Compounds: A Series Of Monographs, Vol. 17; John Wiley & Sons, 1962; pp 5–94. doi:10.1002/9780470186787.ch1
Return to citation in text: [1] -
Wieland, H. Justus Liebigs Ann. Chem. 1903, 328, 154. doi:10.1002/jlac.19033280206
Return to citation in text: [1] -
Langella, M. R.; Finzi, P. V. Chim. Ind. (Milan) 1965, 47, 996.
Return to citation in text: [1] -
Sokolov, S. D.; Yudintseva, I. M.; Petrovskii, P. V.; Kaliuzhnaya, V. G. J. Org. Chem. USSR 1971, 7, 2051.
Return to citation in text: [1] -
Nesi, R.; Giomi, D.; Papaleo, S.; Turchi, S. J. Org. Chem. 1992, 57, 3713. doi:10.1021/jo00039a037
Return to citation in text: [1] -
Hwang, K.-J.; Jo, I.; Shin, Y. A.; Yoo, S.; Lee, J. H. Tetrahedron Lett. 1995, 36, 3337. doi:10.1016/0040-4039(95)00535-K
Return to citation in text: [1] -
Nesi, R.; Giomi, D.; Turchi, S.; Tedeschi, P. Gazz. Chim. Ital. 1993, 123, 633.
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. A 2008, 64, 112. doi:10.1107/S0108767307043930
Return to citation in text: [1]
| 1. |
Barta, S.; Srinivasan, T.; Rastogi, S. K.; Kundu, B.; Parta, A.; Bhaduri, A. P.; Dixit, M. Bioorg. Med. Chem. Lett. 2002, 12, 1905. doi:10.1016/S0960-894X(02)00333-5
and references cited therein. |
| 8. | Melucci, M.; Favaretto, L.; Bettini, C.; Gazzano, M.; Camaioni, N.; Fukazawa, P.; Ostoja, P.; Monari, M.; Barbarella, G. Chem.–Eur. J. 2007, 13, 10046. doi:10.1002/chem.200701368 |
| 6. | Hsu, C.-S.; Chen, H.-L. J. Polym. Sci., Part A: Polym. Chem. 1999, 37, 3929. doi:10.1002/(SICI)1099-0518(19991101)37:21<3929::AID-POLA8>3.0.CO;2-9 |
| 7. | Cassano, R.; Dabrowski, R.; Dziaduszek, J.; Picci, C.; Chidichimo, G.; De Filpo, G.; Muzzalupo, R.; Puoci, F. Tetrahedron Lett. 2007, 48, 1447. doi:10.1016/j.tetlet.2006.12.094 |
| 3. | Vlachos, P.; Mansoor, B.; Alfred, M. P.; O`Neil, M.; Kelly, S. M. Chem. Commun. 2005, 2921. doi:10.1039/b501875g |
| 4. | Sonar, P.; Singh, S. P.; Sudhakar, S.; Dodabalapur, A.; Sellinger, A. Chem. Mater. 2008, 20, 3184. doi:10.1021/cm800139q |
| 5. | Cristiano, R.; Ely, F.; Gallardo, H. Liq. Cryst. 2005, 32, 15. doi:10.1080/02678290412331329279 |
| 17. | Nesi, R.; Giomi, D.; Turchi, S.; Tedeschi, P. Gazz. Chim. Ital. 1993, 123, 633. |
| 2. | Demus, D.; Goodby, J. W.; Gray, G. W.; Spiess, H. W.; Vill, L. Low Molecular Weight Liquid Crystals I. Handbook of Liquid Crystals; Wiley-VCH: Weinheim, 1998; Vol. 2a. doi:10.1002/9783527619276 |
| 18. | Sheldrick, G. M. Acta Crystallogr., Sect. A 2008, 64, 112. doi:10.1107/S0108767307043930 |
| 13. | Langella, M. R.; Finzi, P. V. Chim. Ind. (Milan) 1965, 47, 996. |
| 14. | Sokolov, S. D.; Yudintseva, I. M.; Petrovskii, P. V.; Kaliuzhnaya, V. G. J. Org. Chem. USSR 1971, 7, 2051. |
| 16. | Hwang, K.-J.; Jo, I.; Shin, Y. A.; Yoo, S.; Lee, J. H. Tetrahedron Lett. 1995, 36, 3337. doi:10.1016/0040-4039(95)00535-K |
| 11. | Quilico, A. Isoxazoles. In Five- and Six-Membered Compounds with Nitrogen and Oxygen (Excluding Oxazoles); Wiley, R. H.; Quilico, A.; Speroni, G.; Behr, L. C.; McKee, R. L., Eds.; Chemistry of Heterocyclic Compounds: A Series Of Monographs, Vol. 17; John Wiley & Sons, 1962; pp 5–94. doi:10.1002/9780470186787.ch1 |
| 12. | Wieland, H. Justus Liebigs Ann. Chem. 1903, 328, 154. doi:10.1002/jlac.19033280206 |
| 10. | Gasco, A. M.; Fruttero, R.; Sorba, G.; Gasco, A. Liebigs Ann. Chem. 1991, 1211. doi:10.1002/jlac.1991199101207 |
| 10. | Gasco, A. M.; Fruttero, R.; Sorba, G.; Gasco, A. Liebigs Ann. Chem. 1991, 1211. doi:10.1002/jlac.1991199101207 |
| 9. |
Haino, T.; Tanaka, M.; Ideta, K.; Kubo, K.; Mori, A.; Maccagnani, Y. Tetrahedron Lett. 2004, 45, 2277. doi:10.1016/j.tetlet.2004.01.116
and references cited therein. |
| 15. | Nesi, R.; Giomi, D.; Papaleo, S.; Turchi, S. J. Org. Chem. 1992, 57, 3713. doi:10.1021/jo00039a037 |
© 2010 Hopf et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)