Abstract
A simple, efficient, and mild procedure for a solvent-free one-step synthesis of various 4,4′-diaminotriarylmethane derivatives in the presence of antimony trichloride as catalyst is described. Triarylmethane derivatives were prepared in good to excellent yields and characterized by elemental analysis, FTIR, 1H and 13C NMR spectroscopic techniques. The structural and vibrational analysis were investigated by performing theoretical calculations at the HF and DFT levels of theory by standard 6-31G*, 6-31G*/B3LYP, and B3LYP/cc-pVDZ methods and good agreement was obtained between experimental and theoretical results.
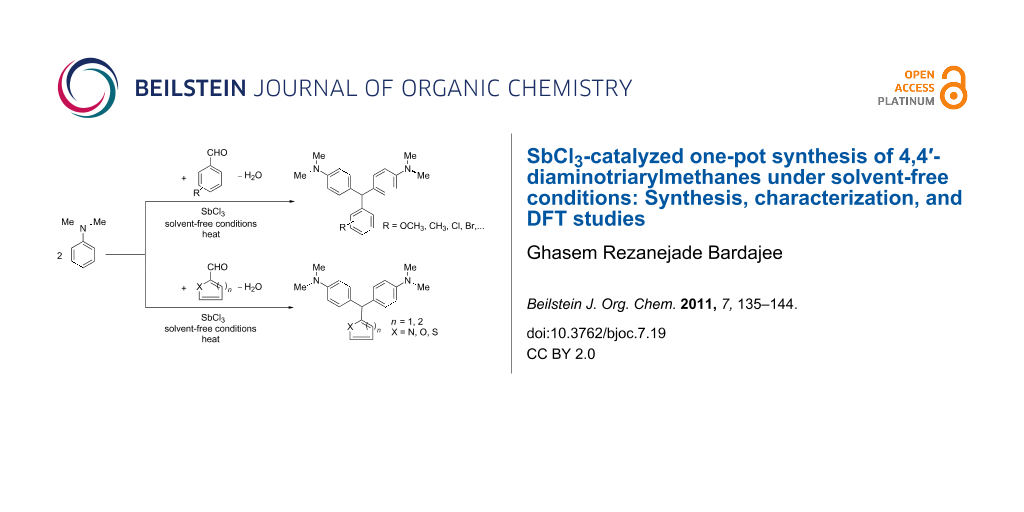
Graphical Abstract
Introduction
The leuco forms of triarylmethine dyes are compounds with numerous diverse industrial, biological, and analytical applications. They have a broad spectrum of technological applications. For instance, they have been used not only in textile industry to dye wool, nylon, silk, leather, cotton, and polyacrylonitrile fibers, but they also have applications for coloring of plastics, varnishes, waxes, and oils. They have been used in novel types of colorless copying papers, pressure and heat-sensitive materials, light-sensitive papers, ultrasonic recording papers, electrothermic heat-sensitive recording papers, inks, crayons, typewritten ribbons, and photoimaging systems [1-3]. These compounds have been employed as dye precursors in nanocomposite preparations [4], in photoresponsive polymers [5], and as the thermal iniferter (initiator–transfer–terminator agent) in pseudo-living radical polymerizations [6]. They are also used in low-dose dosimeters [7], in chemical radiochromic dosimeters [8] and as sensitizers for photoconductivity [9].
The leuco forms of triarylmethine dyes are extensively used in biological applications. They show phototoxicity toward tumor cells [10] and also demonstrate antifungal [11-13], antitubercular [14], anti-infective, and antimicrobial activity [15]. Additionally, they have been used for sterilization of trypanosome cruizi-infected blood [16], in biotechnology process control [17,18], in dye-assisted laser inactivation of enzymes [19], in wastewater treatment plants [20], and in the photochemotherapy of neoplastic diseases [21-23]. In analytical chemistry, they are used as indicators in calorimetric and titrimetric determinations [1], in detection of various heavy metals [24], and for the detection of iodide [25] and carboxylic acids [26].
Diaminotriphenylmethine (DTM) dyes are the most important group of triarylmethine dyes and were selected for the present study due to their brilliance, high pictorial strength, low cost, and wide variety of applications. This group includes a broad range of dyes such as Cresol Red, Bromocresol Green, Light Green SF Yellowish, Victoria Blue BO, Ethyl Green, Brilliant Green, Diaminotriphenylmethane, Fast Green FCF, Green S, Fuchsine Acid, Chlorophenol Red, Crystal Violet Lactone, Fuchsine, Pararosaniline, Water Blue, Thymolphthalein, Bromocresol Purple, and Aurin. These compounds are usually soluble in non-polar organic solvents and are insoluble in water. Because of the wide range of applications of DTMs, the development of new and more efficient synthetic methods for their preparation is of importance.
Various procedures for the preparation of triarylmethane compounds can be found in the literature. For instance, they can be prepared by the palladium-catalyzed arylation of aryl(azaaryl)methanes with aryl halides [27], cationic Pd(II)/bipyridine-catalyzed addition of arylboronic acids to arylaldehydes [28], and by Friedel–Crafts type catalytic alkylation of aromatic rings with aromatic aldehydes and their imines [29-34].
The reaction of arylaldehydes with N,N-dimethylaniline is one of the most efficient methods for the synthesis of DTMs. This reaction is usually carried out in the presence of Brønsted acids such as sulfuric acid, hydrochloric acid, methanesulfonic acid, or p-TSA, as well as Lewis acids such as zinc chloride, zeolites, montmorillonite K-10, and polymer-supported sulfonic acid (NKC-9) [35-40]. Microwave-assisted synthesis of DTMs in the presence of aniline hydrochloride has also been described [41]. Recently, bismuth(III) nitrate and zirconium(IV) dichloride oxide octahydrate have been successfully used for the preparation of DTMs in our group [42,43].
The reported procedures are associated with certain limitations, such as low yields, the use of corrosive acids and excess solvent, severe reaction conditions, long reaction times, costly reagents, as well as the inconvenience in handling the reagents. Considering these restrictions, the development of new and simple synthetic methods for the efficient preparation of DTMs is therefore an interesting challenge. In this contribution, we describe a new route for the preparation of DTM derivatives under solvent-free conditions by the use of SbCl3 as a versatile catalyst. In addition, structural and vibrational studies of leuco compounds by DFT methods were investigated for the first time.
Results and Discussion
Synthesis of DTM
Our investigations showed that N,N-dimethylaniline reacts smoothly with arylaldehydes and heterocyclic aldehydes in the presence of SbCl3 to produce the corresponding DTMs in good to excellent yields. At first we focused on the reaction of benzaldehyde and N,N-dimethylaniline as a model reaction under various reaction conditions (with solvent, solvent-free, and microwave-assisted). Various protic and aprotic solvents such as dimethyl sulfoxide, N,N-dimethylformamide, dichloromethane, ethanol, diethyl ether, and n-hexane were examined: The best results were obtained under solvent-free conditions without microwave irradiation. The general route for the synthesis of these compounds is shown in Scheme 1.
Scheme 1: General route for the synthesis of 4,4’-diaminotriarylmethane derivatives in the presence of SbCl3: (a) with arylaldehydes, (b) with heteroaryl aldehydes.
Scheme 1: General route for the synthesis of 4,4’-diaminotriarylmethane derivatives in the presence of SbCl3:...
In a typical procedure, the reaction of benzaldehyde (0.50 mmol, 1 equiv) and N,N-dimethylaniline (1.25 mmol, 2.5 equiv) in the presence of SbCl3 (30 mol %) under solvent-free conditions at 120 °C for 4 h afforded compound 1a in 80% yield (Table 1, entry 1). To determine the influence of different substituents on the generality of this reaction, a variety of aromatic aldehydes was examined (Table 1, entries 1–16). Furthermore, the number, nature, and position of these substituents affect the hue or color of the resulting dyes and the type of their applications.
Table 1: Synthesized diaminotriphenylmethanes (DTMs) 1a–1s [39-45].
| Entry | Product | Time (h) | Yield (%)a | Entry | Product | Time (h) | Yield (%)a |
|---|---|---|---|---|---|---|---|
| 1 |
|
4 | 80 | 11 |
|
2 | 61 |
| 2 |
|
2 | 84 | 12 |
|
2 | 76 |
| 3 |
|
3 | 81 | 13 |
|
3 | 58 |
| 4 |
|
2 | 88 | 14 |
|
3 | 54 |
| 5 |
|
3 | 86 | 15 |
|
4 | 67 |
| 6 |
|
3 | 83 | 16 |
|
4 | 36 |
| 7 |
|
3 | 82 | 17 |
|
4 | 74 |
| 8 |
|
2 | 78 | 18 |
|
3 | 65 |
| 9 |
|
4 | 65 | 19 |
|
3 | 62 |
| 10 |
|
4 | 57 | ||||
aIsolated yield.
The dominant mechanism for the reaction can be summarized as a tandem regioselective electrophilic aromatic substitution reaction of N,N-dimethylaniline and aldehydes, in which SbCl3 (as a Lewis acid catalyst) activates the carbonyl group of the aldehydes. If we accept this mechanism, one can expect a general influence of electron-donating and electron-withdrawing groups on the feasibility of the reaction. Electron-withdrawing groups such as halo and nitro substituents at the para-position of the arylaldehydes increase the electrophilic strength of the carbonyl group and subsequently increase the yield of the reaction (Table 1, entries 4–7). An electron-withdrawing nitro-substituent at the ortho- or meta- position of benzaldehyde also gave good product yields (Table 1, entries 2–3). The presence of a nitro group in the ortho-position of benzaldehyde increases the inductive effect of NO2 but at the same time decreases its resonance effect due to its steric effect. The resonance effect of a meta-nitro group is relatively low and leads to a slightly lower yield of product in comparison to ortho-nitrobenzaldehyde. Thus, in these two cases, the yields are lower than with the para-nitro substituted benzaldehyde. On the other hand, electron-rich groups such as para-methoxy decrease the product yield (Table 1, entries 10–11). Surprisingly, benzaldehydes with two or even three deactivating methoxy groups react with N,N-dimethylaniline to produce the corresponding products in moderately good yields (Table 1, entries 13–14) which highlights the application of SbCl3 as useful catalyst in this methodology. Furthermore, the application range of the reaction was expanded to fused aromatic rings such as 9H-fluorene and anthracene: 9H-Fluorene-2-carbaldehyde and anthracene-9-carbaldehyde react with N,N-dimethylaniline under the above reaction conditions to afford the desired products 1o and 1p in yields of 67% and 36% yields, respectively (Table 1, entries 15–16). In the next step, the application of this procedure was further expanded to heterocyclic aldehydes for the synthesis of diaryl heteroaryl methane compounds (Scheme 1). These heterocyclic derivatives have received increasing attention due to their biological activities such as antibacterial and antitumor properties [44,45].
Both, electron-rich and electron-poor heterocyclic aldehydes including furfural, thiophene-2- and pyridine-3-carbaldehydes react with N,N-dimethylaniline under the same optimized reaction conditions to afford the corresponding 4,4’-diaminodiaryl heteroaryl methane compounds in relatively good yields (Table 1, entries 17–19).
Computational details
To predict the molecular structure of the title compounds and to assign their vibrational spectra, we performed theoretical calculations with compound 11 as the model compound (Figure 1). All theoretical calculations were carried out with the GAMESS program [46].
Figure 1: Molecular structure of compound 11.
Figure 1: Molecular structure of compound 11.
The first stage for geometry optimization of this molecule was completed by the standard HF/6-31G* method. The resulted HF geometry was then employed in further DFT calculations and re-optimization was carried out by 6-31G*/B3LYP and B3LYP/cc-pVDZ methods. The optimized structures were then used in the vibrational frequency calculations. The vibrational frequencies for these species were calculated and scaled by 0.899, 0.960, and 0.970 for HF/6-31G*, B3LYP/6-31G*, and B3LYP/cc-pVDZ methods, respectively. No imaginary frequency modes were obtained for the optimized structure of compound 11, proving that a true minimum on the potential energy surface was found.
The molecular structure of leuco compounds
The optimized structural parameters of compound 11, calculated by HF and DFT levels with the HF/6-31G*, 6-31G*/B3LYP, and B3LYP/cc-pVDZ methods, are listed in Table 2. The optimized configuration is shown in Figure 2. Because of unavailability of X-ray crystal structures for these compounds, the optimized structure is compared with X-ray structures of similar compounds. This comparison shows good agreement between optimized and actual molecular structures. Here, we compare the bond lengths of the C–C, C–O, and C–N bonds of similar structures with the results of HF/6-31G*, 6-31G*/B3LYP, and B3LYP/cc-pVDZ calculations. The optimized C–C bond lengths in the N,N-dimethylbenzenamine rings of compound 11 are in the range of 138.0–140.0 pm for the 6-31G*, 139.1–141.3 pm for the 6-31G*/B3LYP, and 139.3–141.5 pm for the B3LYP/cc-pVDZ calculations, which are in good agreement with a similar molecular structure in which these bond lengths are in the range of 136.5–140.5 pm [47]. The optimized C–C bond lengths in the anisole ring of compound 11 fall in the range of 137.3–139.8 pm for the 6-31G*, 138.7–140.5 pm for the B3LYP/6-31G*, and 138.8–140.5 pm for the B3LYP/cc-pVDZ calculations, which shows a good agreement with a similar molecular structure where the bond lengths are between 137.0 and 139.8 pm [48].
Table 2: The optimized geometrical parameters for compound 11.
| Parameters |
HF/
6-31G* |
6-31G*/
B3LYP |
B3LYP/
cc-pVDZ |
|---|---|---|---|
| Bond length (pm) | |||
| C1–H1 | 110.201 | 110.336 | 110.319 |
| C1–C2 | 153.050 | 153.188 | 153.164 |
| C1–C3 | 152.841 | 153.016 | 153.005 |
| C1–C20 | 153.084 | 153.398 | 153.361 |
| C6–N15 | 141.685 | 141.353 | 139.428 |
| C11–N14 | 139.574 | 139.584 | 139.411 |
| C23–O26 | 135.024 | 136.774 | 136.779 |
| O26–C27 | 139.755 | 141.779 | 141.676 |
| Bond angles (°) | |||
| C2–C1–C20 | 112.699 | 112.568 | 112.54 |
| C3–C1–C20 | 113.353 | 113.395 | 113.444 |
| C6–N15–C18 | 116.859 | 117.694 | 118.941 |
| C6–N15–C19 | 115.066 | 117.357 | 118.907 |
| C11–N14–C16 | 118.162 | 118.850 | 119.011 |
| C11–N14–C17 | 118.057 | 118.796 | 119.017 |
| C23–O26–C27 | 119.551 | 118.066 | 118.089 |
| Dihedral angles (°) | |||
| C1–C2–C8–C7 | 179.221 | 179.4578 | 178.960 |
| C1–C2–C4–C5 | 178.147 | 178.777 | 178.759 |
| C1–C3–C9–C10 | 179.235 | 179.229 | 179.182 |
| C1–C3–C13–C12 | 179.053 | 179.387 | 179.424 |
| C1–C20–C21–C22 | 179.273 | 178.632 | 178.864 |
| C1–C20–C25–C24 | 179.475 | 178.831 | 179.053 |
| C22–C23-O26–C27 | 0.06182 | 0.02378 | 0.41239 |
| C24–C23–O26–C27 | 179.766 | 179.688 | 179.849 |
![[1860-5397-7-19-2]](/bjoc/content/figures/1860-5397-7-19-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: The molecular structure of compound 11 optimized by the B3LYP/cc-pVDZ method [47].
Figure 2: The molecular structure of compound 11 optimized by the B3LYP/cc-pVDZ method [47].
For the C23–O26 bond, the optimized bond lengths are 135.0 pm for the HF/6-31G* method, 136.7 pm for the B3LYP/6-31G* method, and 136.7 pm for the B3LYP/cc-pVDZ calculations which is in good agreement with a bond length of 137.0 pm found in similar compounds [48].
The bond lengths of C11–N14 and C6–N15 are 139.5 pm and 141.6 pm for the HF\6-31G*, 139.5 pm and 141.3 pm for the B3LYP/6-31G*, and 139.4 pm and 139.4 pm for the B3LYP/cc-pVDZ calculations. As one can see, these bond lengths are in good agreement with the X-ray results obtained for similar compounds where the bond lengths are 137.8 and 140.0 pm [49]. Although there are some differences between our results and those from X-ray structure analysis of similar compounds, the optimized structural parameters are very close to the X-ray values and are good enough for further vibrational calculations.
Assignments of vibrational frequencies
To our best knowledge, no vibrational data and assignments of the modes have been reported for leuco compounds. The experimental infrared spectrum and the calculated infrared spectra of 11 are shown in Figure 3.
Figure 3: Experimental infrared spectrum of compound 11 and infrared spectrum calculated with B3LYP and cc-pVDZ methods.
Figure 3: Experimental infrared spectrum of compound 11 and infrared spectrum calculated with B3LYP and cc-pV...
A comparison between the infrared spectra of this compound can be made by establishing a correlation between the observed and the theoretically calculated wavenumbers. Linear correlations between the experimental and calculated wave numbers have been found (Figure 4). The correlation coefficients indicate a good linearity between the calculated and experimental wavenumbers. These correlation coefficients are 0.998, 0.999, and 0.999 for HF/6-31G*, 6-31G*/B3LYP, and B3LYP/cc-pVDZ methods, respectively.
Figure 4: Linear correlation between the experimental and the theoretical frequencies (cm−1) obtained by HF/6-31G*, 6-31G*/B3LYP/cc-PVDZ methods.
Figure 4: Linear correlation between the experimental and the theoretical frequencies (cm−1) obtained by HF/6...
Based on this comparison between the calculated and experimental IR spectrum, assignments of the fundamental modes were carried out on the basis of the B3LYP/cc-pVDZ calculations.
The resulting vibrational wavenumbers for the optimized geometry and the proposed assignments are given in Table 3. The observed bands at = 3067, 3003, 2945, 2924, 2890, 2861, 2829, and 2803 cm−1 are assigned to the aromatic CH and aliphatic CH3 stretching modes, whereas the C–H stretching vibrations in aromatic rings occur above
= 3000 cm−1. The principal bands in the 1600–1000 cm−1 region are the C–O and C–N stretching vibrations modes as well as the bending vibrations of C–H bonds (Table 3).
Table 3: Comparison of the observed and the calculated vibrational wavenumbers (cm−1) of compound 11 (ν, stretching; δ, in-plane bending; π, out-of-plane bending; ω, wagging. Subscript: asym: asymmetric; sym: symmetric).
| EXP | HF | B3LYP | cc-pVDZ | Assignment | |
|---|---|---|---|---|---|
| 1 | 561.576 | 574.84 | 559.79 | 559.73 | π (rings) |
| 2 | 814.226 | 829.10 | 798.49 | 810.33 | π (ring) |
| 3 | 945.731 | 861.27 | 934.31 | 941.38 | νsym (C–N) |
| 4 | 1031.39 | 938.77 | 1037.28 | 1041.51 | ν (C–O) |
| 5 | 1058.06 | 1062.23 | 1051.95 | 1045.08 | π (C–H of N(CH3)2 |
| 6 | 1103.49 | 1094.70 | 1114.83 | 1099.25 | π (C–H of N(CH3)2 |
| 7 | 1174.91 | 1147.57 | 1128.25 | 1148.25 | δ (C–H of anisole ring) |
| 8 | 1200.32 | 1212.88 | 1192.91 | 1229.80 | δ (C–H) |
| 9 | 1246.14 | 1276.49 | 1241.33 | 1241.87 | ν (C–O) |
| 10 | 1299.39 | 1296.32 | 1293.91 | 1296.63 | δ (C–H of Ph rings) |
| 11 | 1346.98 | 1336.18 | 1325.74 | 1326.77 | ν (C–N) |
| 12 | 1455.78 | 1466.38 | 1476.46 | 1458.62 | π (C–H of N(CH3)2) |
| 13 | 1515.04 | 1523.14 | 1506.76 | 1504.60 | δ (C–H of Ph rings) |
| 14 | 1564.87 | 1598.21 | 1566.91 | 1577.69 | δ (C–H of anisole ring) |
| 15 | 1612.29 | 1637.60 | 1604.45 | 1616.06 | δ (C–H of anisole ring) |
| 16 | 2803.27 | 2832.43 | 2848.99 | 2872.77 | νsym (C–H of N(CH3)2) |
| 17 | 2829.10 | 2832.43 | 2860.60 | 2880.32 | νsym (C–H of N(CH3)2) |
| 18 | 2860.91 | 2851.48 | 2860.60 | 2889.46 | νsym (C–H of N(CH3)2) |
| 19 | 2889.90 | 2881.90 | 2884.14 | 2889.82 | νsym (C–H of N(CH3)2) |
| 20 | 2923.99 | 2926.87 | 2897.76 | 2904.77 | νsym (C–H of OCH3) |
| 21 | 2944.57 | 2935.79 | 2953.94 | 2969.29 | νasym (C–H of N(CH3)2) |
| 22 | 3002.6 | 2979.15 | 3026.16 | 3024.22 | νasym (C–H of N(CH3)2) |
| 23 | 3066.86 | 3067.33 | 3056.959 | 3079.631 | νasym (C–H of anisole ring) |
Some vibrational frequencies over 2900 cm−1 are not observed clearly in the experimental spectra whereas they are obtained from DFT calculations. For instance, the peaks at 2951, 2955 and 3042 cm−1 are for [C–H of N(CH3)2] vibrations in the theoretical spectra (there is also a peak in 2976 cm−1 for
[C–H of N(CH3)2] in the calculated infrared spectra). Other peaks at 2971, 3039 cm−1 (for
C–H of OCH3), 3130 and 3134 cm−1 (C–H symmetric stretching vibrations) are also not discernable in the experimental IR spectrum.
Conclusion
In summary, it has been demonstrated that SbCl3 is a mild and efficient catalyst for the one-pot reaction of N,N-dimethylaniline with a variety of aryl and heteroaryl aldehydes under solvent-free conditions to give substituted triarylmethanes. Using SbCl3 as catalyst, even electron-rich benzaldehydes such as 3,4,5-trimethoxybenzaldehyde gave the corresponding products in good yields. Operational simplicity, high yields, and the ability to prepare a wide range of products are the advantages of this protocol. Molecular geometry parameters and vibrational wavenumbers of the triarylmethanes have been obtained from theoretical calculations for the first time. The theoretical results show good agreement between theoretical and experimental data.
Experimental
General
Commercial grade aldehydes and N,N-dimethylaniline were purchased from Merck or Aldrich. The solvents were of analytical grade and were used as received. Silica gel (Merck, grade 9385, 230–400 mesh, 60 Å) for column chromatography was used as received. The course of the synthesis and the purity of the products were monitored by TLC on silica gel plates (Merck F60 254, 20110, 0.2 mm, ready-to-use), with ethyl acetate/n-hexane (1:4) as eluent. The eluent for PTLC was the same as the TLC eluent. Melting points were determined with a Gallenkamp melting point apparatus and a Kofler hotplate, and are uncorrected. 1H and 13C NMR spectra were recorded with either a Bruker AC300 or a 500 MHz spectrometer at ambient temperature. 1H NMR spectra are referenced to tetramethylsilane (0.00 ppm) and 13C NMR spectra are referenced to residual solvent peaks (for example, 77.23 ppm for CDCl3). Chemical shifts are given in ppm. Infrared absorption spectra were obtained using a Shimadzu 4300 FTIR spectrometer as thin films between potassium bromide plates. IR is reported as characteristic bands (cm−1) at their maximum intensity. Broad signals are denoted as br. Elemental analyses were carried out with a Heareus CHN-RAPID instrument.
Typical procedure for the synthesis of of 4,4'-diaminotriarylmethane derivatives
A vial equipped with a stirring bar was charged with the arylaldehyde (0.5 mmol, 1.0 equiv), N,N-dimethylaniline (1.25 mmol, 2.5 equiv), and SbCl3 (30 mol %) and the vial was capped. The resulting mixture was heated in an oil bath at 120 °C for an appropriate time (Table 1). The progress of the reaction was monitored by TLC. Then the reaction mixture was cooled to room temperature, diluted with dichloromethane and filtered. The filtrate was concentrated in vacuo and the residue purified by crystallization or column chromatography on silica gel (ethyl acetate/n-hexane). The spectral data and elemental analysis for selected products are listed below.
3,4-Dimethoxyphenyl-bis[4-(dimethylamino)phenyl]methane (1m)
Yield: 58%; Colorless oil. IR (KBr): = 3007, 2896, 1618, 1515, 1455, 1340, 1246, 1175, 814 cm−1. 1H NMR (300 MHz, CDCl3, ppm): δ = 2.94 (s, 12H), 3,79 (s, 3H), 3.87 (s, 3H), 5.35 (s, 1H), 6.64 (dd, J = 1.5 Hz, 8.2 Hz, 1H), 6.71 (m, 5H), 6.79 (d, J = 8.2, 1H), 7.01 ppm (d, J = 8.8, 4H). 13C NMR (75 MHz, CDCl3, ppm): δ = 41.3, 55.0, 56.2, 56.3, 111.2, 113.1, 113.2, 121.7, 130.3, 133.8, 138.5, 147.6, 149.1, 149.3 ppm. Anal. Calcd. for C25H30N2O2 (390.5): C 76.89; H 7.74; N 7.17. Found: C 76.98; H 7.82; N 7.24.
3,4,5-Trimethoxyphenyl-bis[4-(dimethylamino)phenyl]methane (1n)
Yield: 54%; Colorless oil. IR (KBr): = 3004, 2891, 1612, 1541, 1348, 1290, 1183, 1031, 946, 817 cm−1. 1H NMR (300 MHz, CDCl3, ppm): δ = 2.97 (s, 12H), 3,75 (s, 6H), 3.84 (s, 3H), 5.33 (s, 1H), 6.35 (s, 2H), 6.78 (d, J = 8.6, 4H), 7.02 ppm (d, J = 8.6, 4H). 13C NMR (75 MHz, CDCl3, ppm): δ = 30.9, 55.3, 56.1, 60.8, 106.5, 130.0, 135.3, 137.5, 139.2, 148.4, 152.9, 153.6 ppm. Anal. Calcd. for C26H32N2O3 (420.5): C 74.26; H 7.67; N 6.66. Found: C 74.39; H 7.66; N 6.78.
(9H)-Fluoren-2-yl-bis[4-(dimethylamino)phenyl]methane (1o)
Yield: 67%; Colorless crystals; mp 184–185 °C. IR (KBr): = 3006, 2875, 1612, 1516, 1479, 1447, 1346 cm−1; 1H NMR (500 MHz, CDCl3, ppm): δ = 2.94 (s, 12H), 3.84 (s, 2H), 5.49 (s, 1H), 6.72 (d, J = 8.4 Hz, 4H), 7.06 (d, J = 8.4 Hz, 4H), 7.19 (d, J = 7.8 Hz, 1H), 7.27 (m, 1H), 7.36 (m, 2H), 7.52 (d, J = 7.5 Hz, 1H), 7.69 (d, J = 7.8 Hz, 1H), 7.76 ppm (d, J = 7.5 Hz, 1H). 13C NMR (125 MHz, CDCl3, ppm): δ = 37.4, 41.3, 55.6, 113.1, 119.9, 120.1, 125.4, 126.4, 126.7, 127.1, 128.6, 130.5, 133.6, 140, 142.2, 143.7, 143.8, 144.8, 149.3 ppm. Anal. Calcd. for C30H30N2 (418.5): C 86.08; H 7.22; N 6.69. Found: C 86.19; H 7.15; N 6.75.
Anthracen-9-yl-bis[4-(dimethylamino)phenyl]methane (1p)
Yield: 36%; Colorless crystals; mp 68–69 °C. IR (KBr): = 3011, 2835, 1612, 1521, 1449, 1346 cm−1; 1H NMR (500 MHz, CDCl3, ppm): δ = 2.85 (s, 6H), 2.96 (s, 6H), 5.13 (s, 1H), 6.61 (m, 4H), 6.98 (m, 2H), 7.18 (m, 3H), 7.28 (m, 4H), 7.35 (m, 2H), 7.55 (d, J = 7.6 Hz, 1H), 7.75 ppm (d, J = 7.6 Hz, 1H). 13C NMR (125 MHz, CDCl3, ppm): δ = 40.9, 52.2, 112.4, 123.9, 125.7, 126.9, 127.2, 127.5, 128.3, 128.5, 128.8, 129.1, 129.5, 130.2, 130.7, 135.3, 139.6, 141.8 ppm. Anal. Calcd. for C31H30N2 (430.5): C 86.47; H 7.02; N 6.51. Found: C 86.59; H 7.09; N 6.58.
Acknowledgements
The authors thank Jörg Saßmannshausen of the University of Strathclyde (UK) for theoretical calculations suggestions. We also thank Masahiko Suenaga of the Kyushu University (Japan) for the FACIO software [50]. This research project was supported by the Research Council of the Payame Noor University, Qazvin branch.
References
-
Muthyala, R.; Lan, X. The Chemistry of Leuco Triarylmethanes. In Chemistry and applications of leuco dyes; Muthyala, R., Ed.; Plenum Press: New York, 1997; pp 125 ff.
Return to citation in text: [1] [2] -
Zollinger, H. Color chemistry - Syntheses, Properties, and Applications of Organic Dyes and Pigments, 3rd ed.; Wiley-VCH: Weinheim, 2003; pp 101 ff.
Return to citation in text: [1] -
Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Part A27; Wiley-VCH: Weinheim, 2001.
Return to citation in text: [1] -
Chen, B. K.; Chiu, T. M.; Tsay, S. Y. J. Appl. Polym. Sci. 2004, 94, 382. doi:10.1002/app.20947
Return to citation in text: [1] -
Irie, M.; Kungwatchakun, D. Makromol. Chem., Rapid Commun. 1984, 5, 829. doi:10.1002/marc.1984.030051209
Return to citation in text: [1] -
Xu, Y. Q.; Lu, J. M.; Li, N. J.; Yan, F.; Xia, X. W.; Xu, Q. F. Eur. Polym. J. 2008, 44, 2404. doi:10.1016/j.eurpolymj.2008.05.007
Return to citation in text: [1] -
Mai, H. H.; Solomon, H. M.; Taguchi, M.; Kojima, T. Radiat. Phys. Chem. 2008, 77, 457. doi:10.1016/j.radphyschem.2007.06.012
Return to citation in text: [1] -
Farahani, M.; Liang, J. H.; McLaughlin, W. L. Appl. Radiat. Isot. 1990, 5, 41. doi:10.1016/0883-2889(90)90123-X
Return to citation in text: [1] -
Mitra, S. J. Polym. Sci., Polym. Symp. 2007, 74, 165. doi:10.1002/polc.5070740115
Return to citation in text: [1] -
Kandela, I. K.; Bartlett, J. A.; Indig, G. L. Photochem. Photobiol. Sci. 2002, 1, 309. doi:10.1039/b110572h
Return to citation in text: [1] -
Culp, S. J.; Beland, F. A. J. Am. Coll. Toxicol. 1996, 15, 219. doi:10.3109/10915819609008715
Return to citation in text: [1] -
Alderman, D. J. J. Fish. Dis. 1985, 8, 289. doi:10.1111/j.1365-2761.1985.tb00945.x
Return to citation in text: [1] -
Cho, B. P.; Yang, T.; Blankenship, L. R.; Moody, J. D.; Churchwell, M.; Beland, F. A.; Culp, S. J. Chem. Res. Toxicol. 2003, 16, 285. doi:10.1021/tx0256679
Return to citation in text: [1] -
Parai, M. K.; Panda, G.; Chaturvedi, V.; Manju, Y. K.; Sinha, S. Bioorg. Med. Chem. Lett. 2008, 18, 289. doi:10.1016/j.bmcl.2007.10.083
Return to citation in text: [1] -
Duxbury, D. F. Chem. Rev. 1993, 93, 381. doi:10.1021/cr00017a018
Return to citation in text: [1] -
Ramirez, L. E.; Lages-Silva, E.; Pianetti, G. M.; Rabelo, R. M. C.; Bordin, J. O.; Moraes-Souza, H. Transfusion 1995, 35, 226. doi:10.1046/j.1537-2995.1995.35395184279.x
Return to citation in text: [1] -
Dittrich, F.; Scholz, M. Verfahren zum quantitativen Spurennachweis von Wasserstoffperoxid. German Patent DD 235,115, April 23, 1986.
Chem. Abstr. 1986, 107, 112216j.
Return to citation in text: [1] -
Babb, B. E.; Daniel, D. S. Compositions and elements containing triarylmethane leuco dyes and methods using same. European Patent EP 162,685, Nov 27, 1985.
Chem. Abstr. 1985, 104, 105638h.
Return to citation in text: [1] -
Jay, D. G.; Keshishian, H. Nature 1990, 348, 548. doi:10.1038/348548a0
Return to citation in text: [1] -
Zepp, R. G.; Skurlatov, Y. I.; Ritmiller, L. F. Environ. Technol. Lett. 1988, 9, 287. doi:10.1080/09593338809384569
Return to citation in text: [1] -
Viola, A.; Hadjur, C.; Jeunet, A.; Julliard, M. J. Photochem. Photobiol., B 1996, 32, 49. doi:10.1016/1011-1344(95)07199-7
Return to citation in text: [1] -
Indig, G. L. Chem. Lett. 1997, 243. doi:10.1246/cl.1997.243
Return to citation in text: [1] -
Fiedorowicz, M.; Pituch-Noworolska, A.; Zembala, M. Photochem. Photobiol. 1997, 65, 855. doi:10.1111/j.1751-1097.1997.tb01934.x
Return to citation in text: [1] -
Smith, I. L. Analytical Applications of the Heavy Metal Induced Oxidation of the Leuco Bases of Triphenylmethane Dyes. Ph.D. Thesis, The University of Alabama, 1974.
Chem. Abstr. 1974, 83, 71097m.
Return to citation in text: [1] -
Pérez Ruiz, T.; Martínez Lozano, C.; Hernández Lozano, M. An. Univ. Murcia Cienc. 1984, 43, 251–268.
Chem. Abstr. 1984, 103, 639831.
Return to citation in text: [1] -
Thakore, P. V. Sci. Cult. 1989, 55, 105.
Return to citation in text: [1] -
Niwa, T.; Yorimitsu, H.; Oshima, K. Org. Lett. 2007, 9, 2373. doi:10.1021/ol0708119
Return to citation in text: [1] -
Lin, S.; Lu, X. J. Org. Chem. 2007, 72, 9757. doi:10.1021/jo071232k
Return to citation in text: [1] -
Podder, S.; Choudhury, J.; Roy, U. K.; Roy, S. J. Org. Chem. 2007, 72, 3100. doi:10.1021/jo062633n
Return to citation in text: [1] -
Nair, V.; Abhilash, K. G.; Vidya, N. Org. Lett. 2005, 7, 5857. doi:10.1021/ol052423h
Return to citation in text: [1] -
Esquivias, J.; Gómez Arrayas, R.; Carretero, J. C. Angew. Chem., Int. Ed. 2006, 118, 645. doi:10.1002/anie.200503305
Return to citation in text: [1] -
Li, Z.; Duan, Z.; Kang, J.; Wang, H.; Yu, L.; Wu, Y. Tetrahedron 2008, 64, 1924. doi:10.1016/j.tet.2007.11.080
Return to citation in text: [1] -
Kodomari, M.; Nagamatsu, M.; Akaike, M.; Aoyama, T. Tetrahedron Lett. 2008, 49, 2537. doi:10.1016/j.tetlet.2008.02.117
Return to citation in text: [1] -
Jaratjaroonphong, J.; Sathalalai, S.; Techasauvapak, P.; Reutrakul, V. Tetrahedron Lett. 2009, 50, 6012. doi:10.1016/j.tetlet.2009.08.036
Return to citation in text: [1] -
Muthyala, R.; Katritzky, A. R.; Lan, X. Dyes Pigm. 1994, 25, 303. doi:10.1016/0143-7208(94)87017-9
Return to citation in text: [1] -
Ritchie, C. D.; Sager, W. F.; Lewis, E. S. J. Am. Chem. Soc. 1962, 84, 2349. doi:10.1021/ja00871a016
Return to citation in text: [1] -
Alvaro, M.; Garcia, H.; Sanjuan, A.; Espla, M. Appl. Catal., A 1998, 175, 105. doi:10.1016/S0926-860X(98)00213-0
Return to citation in text: [1] -
Chalk, A. J.; Halpern, J.; Harkness, A. C. J. Am. Chem. Soc. 1959, 81, 5854. doi:10.1021/ja01531a004
Return to citation in text: [1] -
Zhang, Z. H.; Yang, F.; Li, T. S.; Fu, C. G. Synth. Commun. 1997, 27, 3823. doi:10.1080/00397919708007307
Return to citation in text: [1] [2] -
An, L. T.; Ding, F. Q.; Zou, J. P. Dyes Pigm. 2008, 77, 478. doi:10.1016/j.dyepig.2007.06.004
Return to citation in text: [1] [2] -
Guzman-Lucero, D.; Guzman, J.; Likhatchev, D.; Martinez-Palou, R. Tetrahedron Lett. 2005, 46, 1119. doi:10.1016/j.tetlet.2004.12.091
Return to citation in text: [1] [2] -
Bardajee, G. R.; Jafarpour, F. Cent. Eur. J. Chem. 2009, 7, 138. doi:10.2478/s11532-008-0100-x
Return to citation in text: [1] [2] -
Jafarpour, F.; Bardajee, G. R.; Pirelahi, H.; Oroojpour, V.; Dehnamaki, H.; Rahmdel, S. Chin. J. Chem. 2009, 27, 1415. doi:10.1002/cjoc.200990238
Return to citation in text: [1] [2] -
Drasar, B. S.; Hill, M. J. Human Intestinal Flora; Academic Press: New York, 1974.
Return to citation in text: [1] [2] -
Malpert, J. H.; Grinevich, O.; Strehmel, B.; Jarikov, V.; Mejiritski, A.; Neckers, D. C. Tetrahedron 2001, 57, 967. doi:10.1016/S0040-4020(00)01088-7
Return to citation in text: [1] [2] -
Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen, K. A.; Su, S. J.; Windus, T. L.; Dupuis, M.; Montgomery, J. A. J. Comput. Chem. 1993, 14, 1347. doi:10.1002/jcc.540141112
Return to citation in text: [1] -
Fujii, I.; Hirayama, N.; Aoyama, N.; Miike, A. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1995, 51, 2198. doi:10.1107/S0108270195006196
Return to citation in text: [1] [2] -
Patterson, I. L. J.; Glidewell, C.; Ferguson, G. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1998, 54, 1970. doi:10.1107/S0108270198009329
Return to citation in text: [1] [2] -
Bode, B. M.; Gordon, M. S. J. Mol. Graphics Modell. 1998, 16, 133. doi:10.1016/S1093-3263(99)00002-9
Return to citation in text: [1] -
Suenaga, M. J. Comput. Chem., Jpn. 2008, 7, 33.
Return to citation in text: [1]
| 44. | Drasar, B. S.; Hill, M. J. Human Intestinal Flora; Academic Press: New York, 1974. |
| 45. | Malpert, J. H.; Grinevich, O.; Strehmel, B.; Jarikov, V.; Mejiritski, A.; Neckers, D. C. Tetrahedron 2001, 57, 967. doi:10.1016/S0040-4020(00)01088-7 |
| 46. | Schmidt, M. W.; Baldridge, K. K.; Boatz, J. A.; Elbert, S. T.; Gordon, M. S.; Jensen, J. H.; Koseki, S.; Matsunaga, N.; Nguyen, K. A.; Su, S. J.; Windus, T. L.; Dupuis, M.; Montgomery, J. A. J. Comput. Chem. 1993, 14, 1347. doi:10.1002/jcc.540141112 |
| 47. | Fujii, I.; Hirayama, N.; Aoyama, N.; Miike, A. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1995, 51, 2198. doi:10.1107/S0108270195006196 |
| 1. | Muthyala, R.; Lan, X. The Chemistry of Leuco Triarylmethanes. In Chemistry and applications of leuco dyes; Muthyala, R., Ed.; Plenum Press: New York, 1997; pp 125 ff. |
| 2. | Zollinger, H. Color chemistry - Syntheses, Properties, and Applications of Organic Dyes and Pigments, 3rd ed.; Wiley-VCH: Weinheim, 2003; pp 101 ff. |
| 3. | Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Part A27; Wiley-VCH: Weinheim, 2001. |
| 7. | Mai, H. H.; Solomon, H. M.; Taguchi, M.; Kojima, T. Radiat. Phys. Chem. 2008, 77, 457. doi:10.1016/j.radphyschem.2007.06.012 |
| 20. | Zepp, R. G.; Skurlatov, Y. I.; Ritmiller, L. F. Environ. Technol. Lett. 1988, 9, 287. doi:10.1080/09593338809384569 |
| 6. | Xu, Y. Q.; Lu, J. M.; Li, N. J.; Yan, F.; Xia, X. W.; Xu, Q. F. Eur. Polym. J. 2008, 44, 2404. doi:10.1016/j.eurpolymj.2008.05.007 |
| 21. | Viola, A.; Hadjur, C.; Jeunet, A.; Julliard, M. J. Photochem. Photobiol., B 1996, 32, 49. doi:10.1016/1011-1344(95)07199-7 |
| 22. | Indig, G. L. Chem. Lett. 1997, 243. doi:10.1246/cl.1997.243 |
| 23. | Fiedorowicz, M.; Pituch-Noworolska, A.; Zembala, M. Photochem. Photobiol. 1997, 65, 855. doi:10.1111/j.1751-1097.1997.tb01934.x |
| 5. | Irie, M.; Kungwatchakun, D. Makromol. Chem., Rapid Commun. 1984, 5, 829. doi:10.1002/marc.1984.030051209 |
| 17. |
Dittrich, F.; Scholz, M. Verfahren zum quantitativen Spurennachweis von Wasserstoffperoxid. German Patent DD 235,115, April 23, 1986.
Chem. Abstr. 1986, 107, 112216j. |
| 18. |
Babb, B. E.; Daniel, D. S. Compositions and elements containing triarylmethane leuco dyes and methods using same. European Patent EP 162,685, Nov 27, 1985.
Chem. Abstr. 1985, 104, 105638h. |
| 4. | Chen, B. K.; Chiu, T. M.; Tsay, S. Y. J. Appl. Polym. Sci. 2004, 94, 382. doi:10.1002/app.20947 |
| 11. | Culp, S. J.; Beland, F. A. J. Am. Coll. Toxicol. 1996, 15, 219. doi:10.3109/10915819609008715 |
| 12. | Alderman, D. J. J. Fish. Dis. 1985, 8, 289. doi:10.1111/j.1365-2761.1985.tb00945.x |
| 13. | Cho, B. P.; Yang, T.; Blankenship, L. R.; Moody, J. D.; Churchwell, M.; Beland, F. A.; Culp, S. J. Chem. Res. Toxicol. 2003, 16, 285. doi:10.1021/tx0256679 |
| 48. | Patterson, I. L. J.; Glidewell, C.; Ferguson, G. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1998, 54, 1970. doi:10.1107/S0108270198009329 |
| 10. | Kandela, I. K.; Bartlett, J. A.; Indig, G. L. Photochem. Photobiol. Sci. 2002, 1, 309. doi:10.1039/b110572h |
| 16. | Ramirez, L. E.; Lages-Silva, E.; Pianetti, G. M.; Rabelo, R. M. C.; Bordin, J. O.; Moraes-Souza, H. Transfusion 1995, 35, 226. doi:10.1046/j.1537-2995.1995.35395184279.x |
| 49. | Bode, B. M.; Gordon, M. S. J. Mol. Graphics Modell. 1998, 16, 133. doi:10.1016/S1093-3263(99)00002-9 |
| 9. | Mitra, S. J. Polym. Sci., Polym. Symp. 2007, 74, 165. doi:10.1002/polc.5070740115 |
| 48. | Patterson, I. L. J.; Glidewell, C.; Ferguson, G. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1998, 54, 1970. doi:10.1107/S0108270198009329 |
| 8. | Farahani, M.; Liang, J. H.; McLaughlin, W. L. Appl. Radiat. Isot. 1990, 5, 41. doi:10.1016/0883-2889(90)90123-X |
| 14. | Parai, M. K.; Panda, G.; Chaturvedi, V.; Manju, Y. K.; Sinha, S. Bioorg. Med. Chem. Lett. 2008, 18, 289. doi:10.1016/j.bmcl.2007.10.083 |
| 47. | Fujii, I.; Hirayama, N.; Aoyama, N.; Miike, A. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1995, 51, 2198. doi:10.1107/S0108270195006196 |
| 25. |
Pérez Ruiz, T.; Martínez Lozano, C.; Hernández Lozano, M. An. Univ. Murcia Cienc. 1984, 43, 251–268.
Chem. Abstr. 1984, 103, 639831. |
| 1. | Muthyala, R.; Lan, X. The Chemistry of Leuco Triarylmethanes. In Chemistry and applications of leuco dyes; Muthyala, R., Ed.; Plenum Press: New York, 1997; pp 125 ff. |
| 24. |
Smith, I. L. Analytical Applications of the Heavy Metal Induced Oxidation of the Leuco Bases of Triphenylmethane Dyes. Ph.D. Thesis, The University of Alabama, 1974.
Chem. Abstr. 1974, 83, 71097m. |
| 42. | Bardajee, G. R.; Jafarpour, F. Cent. Eur. J. Chem. 2009, 7, 138. doi:10.2478/s11532-008-0100-x |
| 43. | Jafarpour, F.; Bardajee, G. R.; Pirelahi, H.; Oroojpour, V.; Dehnamaki, H.; Rahmdel, S. Chin. J. Chem. 2009, 27, 1415. doi:10.1002/cjoc.200990238 |
| 39. | Zhang, Z. H.; Yang, F.; Li, T. S.; Fu, C. G. Synth. Commun. 1997, 27, 3823. doi:10.1080/00397919708007307 |
| 40. | An, L. T.; Ding, F. Q.; Zou, J. P. Dyes Pigm. 2008, 77, 478. doi:10.1016/j.dyepig.2007.06.004 |
| 41. | Guzman-Lucero, D.; Guzman, J.; Likhatchev, D.; Martinez-Palou, R. Tetrahedron Lett. 2005, 46, 1119. doi:10.1016/j.tetlet.2004.12.091 |
| 42. | Bardajee, G. R.; Jafarpour, F. Cent. Eur. J. Chem. 2009, 7, 138. doi:10.2478/s11532-008-0100-x |
| 43. | Jafarpour, F.; Bardajee, G. R.; Pirelahi, H.; Oroojpour, V.; Dehnamaki, H.; Rahmdel, S. Chin. J. Chem. 2009, 27, 1415. doi:10.1002/cjoc.200990238 |
| 44. | Drasar, B. S.; Hill, M. J. Human Intestinal Flora; Academic Press: New York, 1974. |
| 45. | Malpert, J. H.; Grinevich, O.; Strehmel, B.; Jarikov, V.; Mejiritski, A.; Neckers, D. C. Tetrahedron 2001, 57, 967. doi:10.1016/S0040-4020(00)01088-7 |
| 35. | Muthyala, R.; Katritzky, A. R.; Lan, X. Dyes Pigm. 1994, 25, 303. doi:10.1016/0143-7208(94)87017-9 |
| 36. | Ritchie, C. D.; Sager, W. F.; Lewis, E. S. J. Am. Chem. Soc. 1962, 84, 2349. doi:10.1021/ja00871a016 |
| 37. | Alvaro, M.; Garcia, H.; Sanjuan, A.; Espla, M. Appl. Catal., A 1998, 175, 105. doi:10.1016/S0926-860X(98)00213-0 |
| 38. | Chalk, A. J.; Halpern, J.; Harkness, A. C. J. Am. Chem. Soc. 1959, 81, 5854. doi:10.1021/ja01531a004 |
| 39. | Zhang, Z. H.; Yang, F.; Li, T. S.; Fu, C. G. Synth. Commun. 1997, 27, 3823. doi:10.1080/00397919708007307 |
| 40. | An, L. T.; Ding, F. Q.; Zou, J. P. Dyes Pigm. 2008, 77, 478. doi:10.1016/j.dyepig.2007.06.004 |
| 41. | Guzman-Lucero, D.; Guzman, J.; Likhatchev, D.; Martinez-Palou, R. Tetrahedron Lett. 2005, 46, 1119. doi:10.1016/j.tetlet.2004.12.091 |
| 29. | Podder, S.; Choudhury, J.; Roy, U. K.; Roy, S. J. Org. Chem. 2007, 72, 3100. doi:10.1021/jo062633n |
| 30. | Nair, V.; Abhilash, K. G.; Vidya, N. Org. Lett. 2005, 7, 5857. doi:10.1021/ol052423h |
| 31. | Esquivias, J.; Gómez Arrayas, R.; Carretero, J. C. Angew. Chem., Int. Ed. 2006, 118, 645. doi:10.1002/anie.200503305 |
| 32. | Li, Z.; Duan, Z.; Kang, J.; Wang, H.; Yu, L.; Wu, Y. Tetrahedron 2008, 64, 1924. doi:10.1016/j.tet.2007.11.080 |
| 33. | Kodomari, M.; Nagamatsu, M.; Akaike, M.; Aoyama, T. Tetrahedron Lett. 2008, 49, 2537. doi:10.1016/j.tetlet.2008.02.117 |
| 34. | Jaratjaroonphong, J.; Sathalalai, S.; Techasauvapak, P.; Reutrakul, V. Tetrahedron Lett. 2009, 50, 6012. doi:10.1016/j.tetlet.2009.08.036 |
| 27. | Niwa, T.; Yorimitsu, H.; Oshima, K. Org. Lett. 2007, 9, 2373. doi:10.1021/ol0708119 |
© 2011 Bardajee; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)