Abstract
Two new and five known oxazoles were identified from two different Pseudomonas strains in addition to the known pyrones pseudopyronine A and B. Labeling experiments confirmed their structures and gave initial evidence for a novel biosynthesis pathway of these natural oxazoles. In order to confirm their structure, they were synthesized, which also allowed tests of their bioactivity. Additionally, the bioactivities of the synthesis intermediates were also investigated revealing interesting biological activities for several compounds despite their overall simple structures.
Graphical Abstract
Findings
During our search for novel natural products from entomopathogenic bacteria, strain PB22.5 was isolated from a soil sample collected in Thailand by using the baiting technique for the isolation of entomopathogenic bacteria and/or entomopathogenic nematodes, as described previously [1-4]. HPLC–MS analysis of extracts from strain PB22.5 grown in LB showed several peaks (Supporting Information File 1, Figure S1) that have not been detected in other entomopathogenic bacteria (data not shown). Subsequent large scale cultivation and purification of the main components led to the isolation of five major compounds, which were subjected to structure elucidation by MS (Supporting Information File 1, Figure S2) and NMR analysis (Supporting Information File 1, Tables S1–S6). Whereas the structures 1 and 2 could be identified as pseudopyronines A (1, 267.4 m/z [M + H]+, C16H26O3) and B (2, 295.4 m/z [M + H]+, C18H30O3) [5-7], three other compounds were identified as the known oxazole derivatives labradorin 1 (3, 241.1 m/z [M + H]+, C15H16N2O), labradorin 2 (4, 255.2 m/z [M + H]+, C16H18N2O) and pimprinaphine (5, 275.1 m/z [M + H]+, C18H14N2O) (Figure 1) [8,9].
Figure 1: Structures of pseudopyronines A (1) and B (2) and natural oxazoles (3–8) as well as synthetic oxazole derivatives (9–11). All natural and synthetic oxazoles show the characteristic MS fragment 130 m/z [M + H]+. The intermediates of the oxazole syntheses (12–27) are also shown as they were also tested for their biological activity.
Figure 1: Structures of pseudopyronines A (1) and B (2) and natural oxazoles (3–8) as well as synthetic oxazo...
Detailed analysis of the HPLC–MS data showed WS-30581 A (6, 227.1 m/z [M + H]+, C14H14N2O) [10] as an additional oxazole derivative, but which was only produced in minute amounts. WS-30581 A (6) was identified by comparing the retention time and the MS fragmentation (Supporting Information File 1, Figure S2) of the product contained in the extract, with a synthesized compound. All oxazoles showed a characteristic fragment ion of 130 m/z [M + H]+ (Supporting Information File 1, Figure S2), and labeling experiments (Supporting Information File 1, Figure S3) enabled the elucidation of this ion as 3-methylidene-3H-indolium (Figure 1).
In order to determine the genus of the producing strain PB22.5, we sequenced its 16S-rRNA gene revealing it to be a Pseudomonas sp. with closest homology to P. putida (100% similarity: Supporting Information File 1, Figure S4) [11-14].
As judged on the basis of high-resolution MALDI–MS and LC–ESIMS/MS data the well-known entomopathogenic P. entomophila [15,16] also produces compounds 3–6 but not 1 and 2. Furthermore two novel oxazole derivatives named labradorin 3 (7, 268.4 m/z [M + H]+, C17H20N2O) and labradorin 4 (8, 283.2 m/z [M + H]+, C18H22N2O) were detected, but they could not be isolated due to their very low production.
Labeling experiments were performed in order to confirm the oxazole structures and to reveal their biosynthesis. Therefore, both strains were cultivated in fully labeled 13C or 15N media and 12C precursors were added (Figure 2).
Figure 2: MS data from strain PB22.5, which was cultivated in [U-13C] and [U-15N] medium background and LB medium as a control. Feeding experiments with 12C and 14N amino acids confirmed structures 3 and 5 and gave initial insights into the biosynthesis.
Figure 2: MS data from strain PB22.5, which was cultivated in [U-13C] and [U-15N] medium background and LB me...
Five carbons of leucine are incorporated in compound 3, while eight carbons of compound 5 originate from phenylalanine, which confirms these moieties to be amino-acid derived (Figure 3).
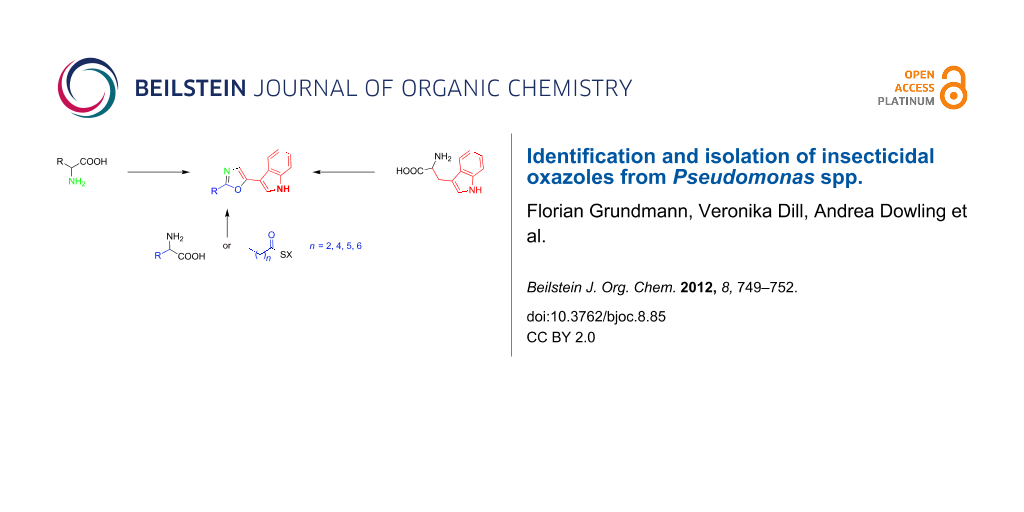
Figure 3: All incorporated biosynthetic precursors of the oxazoles are shown in color. The nitrogen shown in green is derived from transamination as part of the amino acid metabolism. SX = activated ester, which may be coenzyme A or enzyme (acyl carrier protein or polyketide synthase or nonribosomal peptide synthetase) bound.
Figure 3: All incorporated biosynthetic precursors of the oxazoles are shown in color. The nitrogen shown in ...
Ten carbons and one nitrogen of tryptophan were incorporated in all oxazoles, confirming the indole moiety to be derived from tryptophan. Surprisingly, no incorporation of carbon from tryptamine was observed, and the fact that nitrogen labeling also occurs when 14N-tryptamine, leucine or phenylalanine is fed indicates an efficient transaminase activity in both strains, as one would indeed expect for a bacterial strain living on proteinogenic substrates such as insects. Indole acetaldehyde was also fed in 13C-labeled medium, but, in this case as well, no incorporation was observed (data not shown).
Thus, indolepyruvate or an unknown degradation product thereof is proposed as a precursor for the indole moiety. Unfortunately, the production of 7 and 8 was insufficient for analysis in the labeled media, but the nature of the side chains was concluded from a fatty-acid analysis of the producing strain, which showed only straight-chain fatty acids (data not shown).
In order to confirm the structure of all oxazole derivatives, to compare their retention times, and to provide enough material for bioactivity tests, oxazoles 3–6 and 8 were synthesized. Briefly, the respective tryptamine derivatives were formed, which were then oxidized at the alpha-position with the help of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and cyclized to give the oxazoles by using phosphorylchloride as described [9]. Additionally, three nonnatural derivatives were synthesized to get a more diverse set of oxazole derivatives differing from the natural compounds by unusual (compound 9 and 10) or very long (compound 11) acyl chains.
Additionally, the synthetic intermediates were also tested as precursors in labeling experiments as described above, but again no incorporation could be observed. Thus, the biosynthesis via tryptamine amides, which are subsequently oxidized and cyclized to give the oxazoles, can be excluded and more experiments are needed to fully elucidate the biosynthesis of these simple heterocyclic compounds.
All synthesized compounds and intermediates thereof were tested for their biological activity, as a broad range of activities has already been described for these oxazoles, including anticonvulsant and antithrombotic activity as well as activity against human lung and pancreas cancer cell lines [9,10,17].
We tested the bioactivity against the human breast-cancer cell line MCF-7, using the XTT assay [18], and against insect hemocytes from the greater wax moth Galleria melonella according to the method described by Proschak et al. [19]. Three oxazole compounds show LD50 [µg ml−1] values against Galleria hemocytes of 30 (compound 3), 1.7 (compound 6), and 103 (compound 9) (Table S7). Compounds 3, 5, 6, and 9 are active in the MCF-7 assay with EC50 [µg ml−1] values of 58, 363, 26, and 34, respectively. Similar to previous results [19], several tryptamine amide derivatives (20–24, 26) showed cytotoxic activity against the MCF-7 cells with 24 being the most potent compound (1.02 µg ml−1). Interestingly, 26 also showed activity against Galleria hemocytes, although its oxazole derivative 10 did not, which may point to different targets for both compound classes.
The class of oxazole compounds, which were identified in this article, are not only prevalent in Pseudomonas but also in other bacteria such as Streptomyces [20] or Streptoverticillium [8,10], suggesting a biological relevance also in these bacteria. However, as concluded from the observed activity against insect cells, they could significantly add to the overall insecticidal activity of the investigated Pseudomonas strains.
Supporting Information
| Supporting Information File 1: General experimental procedures, isolation of the strain and taxonomic identification, cultivation and extraction, isolation, labeling experiments, synthesis, bioactivity results and compound characterization. | ||
| Format: PDF | Size: 1017.4 KB | Download |
Acknowledgements
Work in the Bode lab was supported by the LOEWE Schwerpunkt Insektenbiotechnologie, the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 223328, which also supported work in the ffrench-Constant and Chantratita labs, and the Deutsche Forschungsgemeinschaft (DFG).
References
-
Akhurst, R. J. J. Gen. Microbiol. 1980, 121, 303–309.
Return to citation in text: [1] -
Bedding, R. A.; Akhurst, R. J. Nematologica 1975, 21, 109–110.
Return to citation in text: [1] -
White, G. F. Science 1927, 66, 302–303. doi:10.1126/science.66.1709.302-a
Return to citation in text: [1] -
Woodring, L. J. H.; Kaya, K. H. Steinernematid and Heterorhabditid nematodes. A Handbook of biology and techniques; Southern Cooperative Series Bulletin, Arkansas Agricultural Experimental Station: Fayetteville Arkansas, USA, 1988.
Return to citation in text: [1] -
Chu, M.; Mierzwa, R.; Xu, L.; He, L.; Terracciano, J.; Patel, M.; Zhao, W.; Black, T. A.; Chan, T. M. J. Antibiot. 2002, 55, 215–218.
Return to citation in text: [1] -
Kong, F.; Singh, M. P.; Carter, G. T. J. Nat. Prod. 2005, 68, 920–923. doi:10.1021/np050038v
Return to citation in text: [1] -
Singh, M. P.; Kong, F. M.; Janso, J. E.; Arias, D. A.; Suarez, P. A.; Bernan, V. S.; Petersen, P. J.; Weiss, W. J.; Carter, G.; Greenstein, M. J. Antibiot. 2003, 56, 1033–1044.
Return to citation in text: [1] -
Koyama, Y.; Yokose, K.; Dolby, L. J. J. Agric. Biol. Chem. 1981, 45, 1285–1287.
Return to citation in text: [1] [2] -
Pettit, G. R.; Knight, J. C.; Herald, D. L.; Davenport, R.; Pettit, R. K.; Tucker, B. E.; Schmidt, J. M. J. Nat. Prod. 2002, 65, 1793–1797. doi:10.1021/np020173x
Return to citation in text: [1] [2] [3] -
Umehara, K.; Yoshida, K.; Okamoto, M.; Iwami, M.; Tanaka, H.; Kohsaka, M.; Imanaka, H. J. Antibiot. 1984, 37, 1153–1160.
Return to citation in text: [1] [2] [3] -
Kimura, M. J. Mol. Evol. 1980, 16, 111–120. doi:10.1007/BF01731581
Return to citation in text: [1] -
Saitou, N.; Nei, M. Mol. Biol. Evol. 1987, 4, 406–425.
Return to citation in text: [1] -
Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. Mol. Biol. Evol. 2007, 24, 1596–1599. doi:10.1093/molbev/msm092
Return to citation in text: [1] -
Thompson, J. D.; Higgins, D. G.; Gibson, T. J. Nucleic Acids Res. 1994, 22, 4673–4680. doi:10.1093/nar/22.22.4673
Return to citation in text: [1] -
Vodovar, N.; Vinals, M.; Liehl, P.; Basset, A.; Degrouard, J.; Spellman, P.; Boccard, F.; Lemaitre, B. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 11414–11419. doi:10.1073/pnas.0502240102
Return to citation in text: [1] -
Vodovar, N.; Vallenet, D.; Cruveiller, S.; Rouy, Z.; Barbe, V.; Acosta, C.; Cattolico, L.; Jubin, C.; Lajus, A.; Segurens, B.; Vacherie, B.; Wincker, P.; Weissenbach, J.; Lemaitre, B.; Médigue, C.; Boccard, F. Nat. Biotechnol. 2006, 24, 673–679. doi:10.1038/nbt1212
Return to citation in text: [1] -
Naik, S. R.; Harindran, J.; Varde, A. B. J. Biotechnol. 2001, 88, 1–10. doi:10.1016/S0168-1656(01)00244-9
Return to citation in text: [1] -
Scudiero, D. A.; Shoemaker, R. H.; Paull, K. D.; Monks, A.; Tierney, S.; Nofziger, T. H.; Currens, M. J.; Seniff, D.; Boyd, M. R. Cancer Res. 1988, 48, 4827–4833.
Return to citation in text: [1] -
Proschak, A.; Schultz, K.; Herrmann, J.; Dowling, A. J.; Brachmann, A. O.; ffrench-Constant, R.; Müller, R.; Bode, H. B. ChemBioChem 2011, 12, 2011–2015. doi:10.1002/cbic.201100223
Return to citation in text: [1] [2] -
Joshi, B. S.; Taylor, W. I.; Bhate, D. S.; Karmarkar, S. S. Tetrahedron 1963, 19, 1437–1439. doi:10.1016/S0040-4020(01)98569-2
Return to citation in text: [1]
| 1. | Akhurst, R. J. J. Gen. Microbiol. 1980, 121, 303–309. |
| 2. | Bedding, R. A.; Akhurst, R. J. Nematologica 1975, 21, 109–110. |
| 3. | White, G. F. Science 1927, 66, 302–303. doi:10.1126/science.66.1709.302-a |
| 4. | Woodring, L. J. H.; Kaya, K. H. Steinernematid and Heterorhabditid nematodes. A Handbook of biology and techniques; Southern Cooperative Series Bulletin, Arkansas Agricultural Experimental Station: Fayetteville Arkansas, USA, 1988. |
| 11. | Kimura, M. J. Mol. Evol. 1980, 16, 111–120. doi:10.1007/BF01731581 |
| 12. | Saitou, N.; Nei, M. Mol. Biol. Evol. 1987, 4, 406–425. |
| 13. | Tamura, K.; Dudley, J.; Nei, M.; Kumar, S. Mol. Biol. Evol. 2007, 24, 1596–1599. doi:10.1093/molbev/msm092 |
| 14. | Thompson, J. D.; Higgins, D. G.; Gibson, T. J. Nucleic Acids Res. 1994, 22, 4673–4680. doi:10.1093/nar/22.22.4673 |
| 10. | Umehara, K.; Yoshida, K.; Okamoto, M.; Iwami, M.; Tanaka, H.; Kohsaka, M.; Imanaka, H. J. Antibiot. 1984, 37, 1153–1160. |
| 8. | Koyama, Y.; Yokose, K.; Dolby, L. J. J. Agric. Biol. Chem. 1981, 45, 1285–1287. |
| 9. | Pettit, G. R.; Knight, J. C.; Herald, D. L.; Davenport, R.; Pettit, R. K.; Tucker, B. E.; Schmidt, J. M. J. Nat. Prod. 2002, 65, 1793–1797. doi:10.1021/np020173x |
| 8. | Koyama, Y.; Yokose, K.; Dolby, L. J. J. Agric. Biol. Chem. 1981, 45, 1285–1287. |
| 10. | Umehara, K.; Yoshida, K.; Okamoto, M.; Iwami, M.; Tanaka, H.; Kohsaka, M.; Imanaka, H. J. Antibiot. 1984, 37, 1153–1160. |
| 5. | Chu, M.; Mierzwa, R.; Xu, L.; He, L.; Terracciano, J.; Patel, M.; Zhao, W.; Black, T. A.; Chan, T. M. J. Antibiot. 2002, 55, 215–218. |
| 6. | Kong, F.; Singh, M. P.; Carter, G. T. J. Nat. Prod. 2005, 68, 920–923. doi:10.1021/np050038v |
| 7. | Singh, M. P.; Kong, F. M.; Janso, J. E.; Arias, D. A.; Suarez, P. A.; Bernan, V. S.; Petersen, P. J.; Weiss, W. J.; Carter, G.; Greenstein, M. J. Antibiot. 2003, 56, 1033–1044. |
| 18. | Scudiero, D. A.; Shoemaker, R. H.; Paull, K. D.; Monks, A.; Tierney, S.; Nofziger, T. H.; Currens, M. J.; Seniff, D.; Boyd, M. R. Cancer Res. 1988, 48, 4827–4833. |
| 19. | Proschak, A.; Schultz, K.; Herrmann, J.; Dowling, A. J.; Brachmann, A. O.; ffrench-Constant, R.; Müller, R.; Bode, H. B. ChemBioChem 2011, 12, 2011–2015. doi:10.1002/cbic.201100223 |
| 9. | Pettit, G. R.; Knight, J. C.; Herald, D. L.; Davenport, R.; Pettit, R. K.; Tucker, B. E.; Schmidt, J. M. J. Nat. Prod. 2002, 65, 1793–1797. doi:10.1021/np020173x |
| 10. | Umehara, K.; Yoshida, K.; Okamoto, M.; Iwami, M.; Tanaka, H.; Kohsaka, M.; Imanaka, H. J. Antibiot. 1984, 37, 1153–1160. |
| 17. | Naik, S. R.; Harindran, J.; Varde, A. B. J. Biotechnol. 2001, 88, 1–10. doi:10.1016/S0168-1656(01)00244-9 |
| 20. | Joshi, B. S.; Taylor, W. I.; Bhate, D. S.; Karmarkar, S. S. Tetrahedron 1963, 19, 1437–1439. doi:10.1016/S0040-4020(01)98569-2 |
| 9. | Pettit, G. R.; Knight, J. C.; Herald, D. L.; Davenport, R.; Pettit, R. K.; Tucker, B. E.; Schmidt, J. M. J. Nat. Prod. 2002, 65, 1793–1797. doi:10.1021/np020173x |
| 15. | Vodovar, N.; Vinals, M.; Liehl, P.; Basset, A.; Degrouard, J.; Spellman, P.; Boccard, F.; Lemaitre, B. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 11414–11419. doi:10.1073/pnas.0502240102 |
| 16. | Vodovar, N.; Vallenet, D.; Cruveiller, S.; Rouy, Z.; Barbe, V.; Acosta, C.; Cattolico, L.; Jubin, C.; Lajus, A.; Segurens, B.; Vacherie, B.; Wincker, P.; Weissenbach, J.; Lemaitre, B.; Médigue, C.; Boccard, F. Nat. Biotechnol. 2006, 24, 673–679. doi:10.1038/nbt1212 |
| 19. | Proschak, A.; Schultz, K.; Herrmann, J.; Dowling, A. J.; Brachmann, A. O.; ffrench-Constant, R.; Müller, R.; Bode, H. B. ChemBioChem 2011, 12, 2011–2015. doi:10.1002/cbic.201100223 |
© 2012 Grundmann et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)