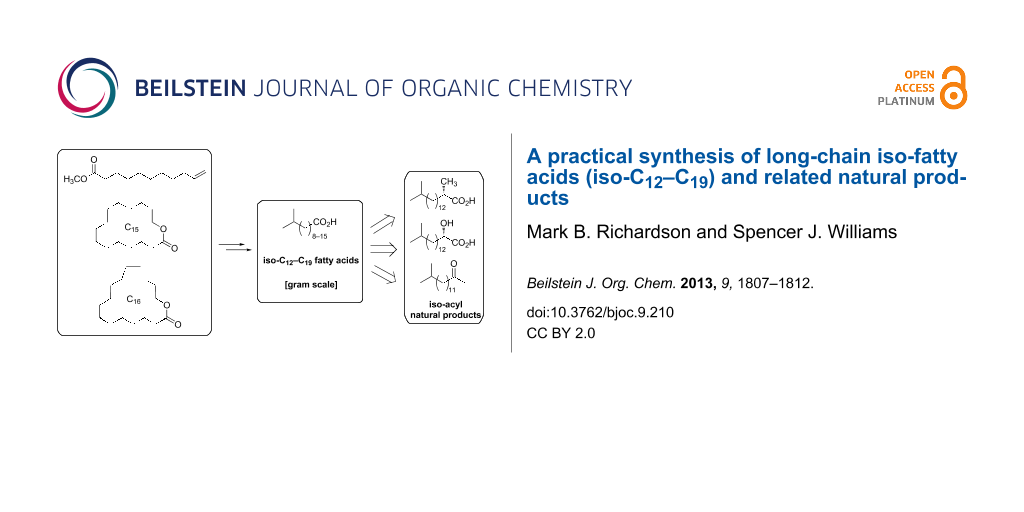
Abstract
A gram-scale synthesis of terminally-branched iso-fatty acids (iso-C12–C19) was developed commencing with methyl undec-10-enoate (methyl undecylenate) (for iso-C12–C14) or the C15 and C16 lactones pentadecanolide (for iso-C15–C17) and hexadecanolide (for iso-C18–C19). Central to the approaches outlined is the two-step construction of the terminal isopropyl group through addition of methylmagnesium bromide to the ester/lactones and selective reduction of the resulting tertiary alcohols. Thus, the C12, C17 and C18 iso-fatty acids were obtained in three steps from commercially-available starting materials, and the remaining C13–C16 and C19 iso-fatty acids were prepared by homologation or recursive dehomologations of these fatty acids or through intercepting appropriate intermediates. Highlighting the synthetic potential of the iso-fatty acids and various intermediates prepared herein, we describe the synthesis of the natural products (S)-2,15-dimethylpalmitic acid, (S)-2-hydroxy-15-methylpalmitic acid, and 2-oxo-14-methylpentadecane.
Graphical Abstract
Introduction
Long-chain iso-fatty acids occur in a broad range of organisms, and are especially abundant in bacteria where, through incorporation into phospholipids, they influence membrane fluidity [1]. Emerging evidence has revealed unexpected roles for certain iso-fatty acids; for example iso-C15 and iso-C17 fatty acids have been shown to be essential in the development of the model eukaryote Caenorhabditis elegans [2]. They are present as esters and amides in natural products including septacidin [3], teicoplanins [4], tunicaminyluracil-based antibiotics [5] (tunicamycins [6], corynetoxins [7], and streptovirudins [8]), the arylomycin glycopeptide antibiotics [9,10], maradolipids [11], plipastatin-type lipopeptides [12], Nod factors [13], glycosylglycerides [14,15], phosphoglycolipids [16], and various sphingolipids [17-19]. The terminal isopropyl group of the iso-fatty acids arises from valine and leucine, which through transamination and decarboxylation reactions yield isobutyryl-CoA and isovaleryl-CoA [20]. These starter units are elongated by fatty acid synthases to the final iso-fatty acids (even numbered for isobutyryl-CoA; odd-numbered for isovaleryl-CoA) through extension with malonyl-CoA [21,22]. Long-chain iso-fatty acids are important analytical reference compounds owing to the presence of these materials in tobacco [23], wool wax [24], butter fat [25], human sebaceous secretions [26] (adult skin [27], meibum [28], cerumen [29], and newborn vernix caseosa [30,31]), and a wide variety of microbiological samples [1].
Previous syntheses of iso-fatty acids have typically utilized extended, multi-step sequences. Two main approaches have been used: (1) two-component cross-couplings that include α-ketoester alkylation/decarboxylation [32,33], aldehyde–olefin photoaddition [34], acetylide alkylation (sp3–sp) [35,36], Wittig coupling [3,21,37-39], Kolbe electrosynthesis [35,40-42], organocadmium (sp2–sp3) [43-46], organomagnesium (sp2–sp3) [47], or organocopper (sp3–sp3) [48,49] cross-couplings; or (2) bidirectional extension of a central thiophene C4-fragment [50]. Two fundamentally different approaches worth special mention are the synthesis of the iso-C14 acid 3 by direct hydro-isopropylation of the terminal alkene of methyl undecylenate (available as a pyrolysis product of ricinoleic acid) using isopropyl chloroformate and ethyldichloroaluminium [51], and the synthesis of the iso-C17 acid 6 from methyl ustilate (15,16-dihydroxypalmitate) [52]. Despite the interest in natural products containing iso-fatty acids, these compounds are not readily acquired in multigram quantities due to the complexity of the synthetic routes or limited availability of starting materials. To overcome these problems, we report the scalable, gram-scale syntheses of eight common iso-C12–C19 acids 1–8 (Figure 1), from readily available starting materials. To illustrate the opportunities that our approach provides, we demonstrate the elaboration of the C17-iso-fatty acid 6 and an intermediate, 22, to several terminal-branched natural products that have not previously been synthesized.
Results and Discussion
Our approach to the iso-C12–C14 fatty acids 1–3 commenced from methyl undec-10-enoate (methyl undecylenate) 9. Reaction of 9 with methylmagnesium bromide afforded the tertiary alcohol 10 in 98% yield (Scheme 1). Selective reduction of the tertiary alcohol of 10 was achieved by ‘ionic hydrogenation’ with triethylsilane and BF3·Et2O [53], affording 11. Oxidative cleavage of 11 with KMnO4/Bu4NBr [54] afforded iso-C12 acid 1. Alternatively, anti-Markovnikov hydration of 11, using I2/NaBH4 then hydrogen peroxide [55], afforded the alcohol 12, and oxidation of 12 with KMnO4/Bu4NBr afforded iso-C13 acid 2. Alternatively, alcohol 12 could be intercepted and converted to the mesylate 13 using MsCl/Et3N [56] and thence the nitrile 14 (KCN in DMSO/THF). Finally, hydrolysis of the nitrile 14 with NaOH in H2O/EtOH afforded iso-C14 acid 3.
Scheme 1: Synthesis of iso-C12 1, iso-C13 2, and iso-C14 3 fatty acids from methyl undecylenate (9). Reagents and conditions: (a) MeMgBr, THF, 98%; (b) BF3·Et2O, Et3SiH, CH2Cl2, 99%; (c) KMnO4, Bu4NBr, AcOH, H2O, 88% for 1, 96% for 2; (d) i) I2, NaBH4, THF, ii) H2O2, 95%; (e) MsCl, Et3N, CH2Cl2, 98%; (f) KCN, DMSO, THF, 72%; (g) aq NaOH, EtOH, 96%.
Scheme 1: Synthesis of iso-C12 1, iso-C13 2, and iso-C14 3 fatty acids from methyl undecylenate (9). Reagents...
The iso-C15–C17 fatty acids 4–6 were prepared from the readily available C15 lactone pentadecanolide (exaltolide, 15) [57], a natural product that is produced industrially for use as a musk-odored perfumery fixative. Reaction of 15 with methylmagnesium bromide afforded the tertiary alcohol 16 in 98% yield (Scheme 2). Selective reduction of the tertiary alcohol of 16 was achieved using triethylsilane/BF3·Et2O [53], yielding 17. Finally, oxidation of 17 with KMnO4/Bu4NBr [54] afforded the iso-C17 acid 6. The iso-C15 acid 4 and iso-C16 acid 5 were prepared by recursive dehomologation through intercepting the alcohol 17. Preparation of the xanthate ester 18 (NaH, CS2, then MeI) [58] followed by Chugaev elimination afforded the terminal alkene 19. Oxidative cleavage of 19 using KMnO4/Bu4NBr [54] afforded iso-C16 acid 5. Reduction of 5 (BH3·Me2S) [59] afforded the alcohol 20 that when subjected to the same transformations as before, via the xanthate ester 21, delivered the terminal alkene 22. Finally, oxidative cleavage (KMnO4/Bu4NBr) [54] of 22 afforded iso-C15 acid 4.
Scheme 2: Synthesis of iso-C15 4, iso-C16 5, and iso-C17 6 fatty acids from pentadecanolide (15). Reagents and conditions: (a) MeMgBr, THF, 98%; (b) BF3·Et2O, Et3SiH, CH2Cl2, 96%; (c) KMnO4, Bu4NBr, AcOH, H2O, 95% for 6, 93% for 5; 86% for 4; (d) NaH, CS2 then MeI; (e) reflux, 89% for 19 over 2 steps from 17, 98% for 22 over 2 steps from 20; (f) BH3·SMe2, THF, 78%.
Scheme 2: Synthesis of iso-C15 4, iso-C16 5, and iso-C17 6 fatty acids from pentadecanolide (15). Reagents an...
The iso-C18 7 and iso-C19 8 fatty acids were synthesized through similar approaches from the related C16 lactone hexadecanolide 23 [60] (Scheme 3). Reaction of 23 with methylmagnesium bromide afforded 24; triethylsilane/BF3·Et2O [53] reduction gave 25; and KMnO4/Bu4NBr oxidation afforded iso-C18 acid 7 (Scheme 3). The iso-C19 acid 8 was readily prepared by a three-step homologation through intercepting the alcohol 25. Thus, mesylation of 25 (MsCl/Et3N [56]) afforded 26; substitution (KCN/DMSO) afforded the nitrile 27; and hydrolysis (NaOH in H2O/EtOH) of 27 afforded 8.
Scheme 3: Synthesis of iso-C18 7 and iso-C19 8 fatty acids from hexadecanolide 23. Reagents and conditions: (a) MeMgBr, THF, 97%; (b) BF3·Et2O, Et3SiH, CH2Cl2, 95%; (c) KMnO4, Bu4NBr, AcOH, H2O, 82%; (d) MsCl, Et3N, 96%; (e) KCN, DMSO, THF, 75%; (f) aq NaOH, EtOH, 86%.
Scheme 3: Synthesis of iso-C18 7 and iso-C19 8 fatty acids from hexadecanolide 23. Reagents and conditions: (...
The above routes enable the acquisition of (multi)gram quantities of the iso-C12–19 acids 1–8, and provide opportunities for their use as starting materials for the preparation of more complex fatty acids. To illustrate their potential we undertook the synthesis of several representative natural products (Scheme 4). 2,15-Dimethylpalmitic acid has been isolated from a microaerophilic subsurface microbial community [61], and is a component of human newborn vernix caseosa [31], although the absolute configuration of natural samples has not been determined. Conversion of iso-C17 acid 6 to the N-acyloxazolidinone 28 was achieved using pivalyl chloride/LiCl [62] and (S)-4-benzyloxazolidinone. Diastereoselective methylation [63] of the chelated Z-enolate, derived from deprotonation of 28, using NaHMDS, followed by addition of iodomethane, yielded 29 as a single diastereoisomer (as determined by 1H NMR) in 80% yield. Cleavage of the chiral auxiliary using LiOH/H2O2 (which occurs without racemization at the α-position) [64] afforded (S)-2,15-dimethylpalmitic acid (30) in 98% yield. 2-Hydroxy-15-methylpalmitic acid has been identified from a range of sources [1] including the myxobacterium Stigmatella aurantiaca [21,65], and the oral bacterium Veillonella parvula [66], although the absolute configuration has not been reported. Diastereoselective hydroxylation [67] of the chelated Z-enolate derived from 28 using the Davis oxaziridine [68] afforded the 2-hydroxy compound 31 as a single diastereoisomer (as determined by 1H NMR) in 71% yield. Esterification with MeOMgCl [69] (which has been shown not to cause epimerization at the α-position [67]) and saponification [70] afforded (S)-2-hydroxy-15-methylpalmitic acid (32). The ketone 33 was isolated from Xanthomonas campestris pv. vesicatoria 85-10 [71]. A direct one step synthesis of 33 was achieved in 51% yield by Wacker oxidation using Pd/O2 [72] of the alkene 22, intercepted from the synthesis of the iso-C15 acid 4.
Scheme 4: Synthesis of (A) 2-methyl- and 2-hydroxy-iso-fatty acids 30 and 32, and (B) the ketone 33. Reagents and conditions: (a) Et3N, PivCl, LiCl, DMAP, (S)-4-benzyloxazolidinone, 71%; (b) NaHMDS, MeI, THF, 80%; (c) LiOH, H2O2, THF, H2O, 98%; (d) NaHMDS, Davis oxaziridine, THF, 71%; (e) i) iPrMgCl, MeOH, 76%, ii) NaOH, MeOH, 83%; (f) O2, PdCl2, DMA, H2O, 51%.
Scheme 4: Synthesis of (A) 2-methyl- and 2-hydroxy-iso-fatty acids 30 and 32, and (B) the ketone 33. Reagents...
Conclusion
We have accomplished a highly practical synthesis of the homologous iso-fatty acids 1–8. The iso-C12 1, iso-C17 6 and iso-C18 7 acids were prepared from commercially-available starting materials through three-step sequences and produced more than 1 g of each of the three iso-fatty acids in just 2 days each. The remaining five fatty acids were each prepared on >1 g scale by homologation or dehomologation reactions, or through the elaboration of intermediates in the synthesis of 1. Underscoring the practicability of this approach, the iso-fatty acids or appropriate intermediates were used for the preparation of three natural products, enantiopure acids 30 and 32, and the ketone 33. The simplicity of our approach suggests that it will be of great utility in the preparation of iso-fatty acids for incorporation into more complex molecules.
References
-
Kaneda, T. Microbiol. Rev. 1991, 55, 288–302.
Return to citation in text: [1] [2] [3] -
Kniazeva, M.; Crawford, Q. T.; Seiber, M.; Wang, C. Y.; Han, M. PLoS Biol. 2004, 2, E257. doi:10.1371/journal.pbio.0020257
Return to citation in text: [1] -
Acton, E. M.; Ryan, K. J.; Luetzow, A. E. J. Med. Chem. 1977, 20, 1362–1371. doi:10.1021/jm00221a002
Return to citation in text: [1] [2] -
Borghi, A.; Antonini, P.; Zanol, M.; Ferrari, P.; Zerilli, L. F.; Lancini, G. C. J. Antibiot. 1989, 42, 361–366. doi:10.7164/antibiotics.42.361
Return to citation in text: [1] -
Cockrum, P. A.; Edgar, J. A. J. Chromatogr. 1983, 268, 245–254. doi:10.1016/S0021-9673(01)95411-1
Return to citation in text: [1] -
Ito, T.; Takatsuki, A.; Kawamura, K.; Sato, K.; Tamura, G. Agric. Biol. Chem. 1980, 44, 695–698. doi:10.1271/bbb1961.44.695
Return to citation in text: [1] -
Edgar, J. A.; Frahn, J. L.; Cockrum, P. A.; Anderton, N.; Jago, M. V.; Culvenor, C. C. J.; Jones, A. J.; Murray, K.; Shaw, K. J. J. Chem. Soc., Chem. Commun. 1982, 222–224. doi:10.1039/C39820000222
Return to citation in text: [1] -
Eckardt, K.; Wetzstein, H.; Thrum, H.; Ihn, W. J. Antibiot. 1980, 33, 908–910. doi:10.7164/antibiotics.33.908
Return to citation in text: [1] -
Kulanthaivel, P.; Kreuzman, A. J.; Strege, M. A.; Belvo, M. D.; Smitka, T. A.; Clemens, M.; Swartling, J. R.; Minton, K. L.; Zheng, F.; Angleton, E. L.; Mullen, D.; Jungheim, L. N.; Klimkowski, V. J.; Nicas, T. I.; Thompson, R. C.; Peng, S.-B. J. Biol. Chem. 2004, 279, 36250–36258. doi:10.1074/jbc.M405884200
Return to citation in text: [1] -
Liu, J.; Luo, C.; Smith, P. A.; Chin, J. K.; Page, M. G. P.; Paetzel, M.; Romesberg, F. E. J. Am. Chem. Soc. 2011, 133, 17869–17877. doi:10.1021/ja207318n
Return to citation in text: [1] -
Penkov, S.; Mende, F.; Zagoriy, V.; Erkut, C.; Martin, R.; Pässler, U.; Schuhmann, K.; Schwudke, D.; Gruner, M.; Mäntler, J.; Reichert-Müller, T.; Shevchenko, A.; Knölker, H.-J.; Kurzchalia, T. V. Angew. Chem., Int. Ed. 2010, 49, 9430–9435. doi:10.1002/anie.201004466
Return to citation in text: [1] -
Esumi, Y.; Suzuki, Y.; Itoh, Y.; Chijimatsu, M.; Uramoto, M.; Kimura, K.-i.; Nakayama, S.; Yoshihama, M.; Ichikawa, T.; Haramo, T.; Fujishige, J. J. Antibiot. 2003, 56, 716–720. doi:10.7164/antibiotics.56.716
Return to citation in text: [1] -
Poinsot, V.; Bélanger, E.; Laberge, S.; Yang, G.-P.; Antoun, H.; Cloutier, J.; Treilhou, M.; Dénarié, J.; Promé, J.-C.; Debellé, F. J. Bacteriol. 2001, 183, 3721–3728. doi:10.1128/JB.183.12.3721-3728.2001
Return to citation in text: [1] -
Hunter, S. W.; McNeil, M. R.; Brennan, P. J. J. Bacteriol. 1986, 168, 917–922.
Return to citation in text: [1] -
Orgambide, G. G.; Hollingsworth, R. I.; Dazzo, F. B. Carbohydr. Res. 1992, 233, 151–159. doi:10.1016/S0008-6215(00)90927-3
Return to citation in text: [1] -
Fujimoto, Y.; Mitsunobe, K.; Fujiwara, S.; Mori, M.; Hashimoto, M.; Suda, Y.; Kusumoto, S.; Fukase, K. Org. Biomol. Chem. 2013, 11, 5034–5041. doi:10.1039/c3ob40899j
Return to citation in text: [1] -
Minamino, M.; Sakaguchi, I.; Naka, T.; Ikeda, N.; Kato, Y.; Tomiyasu, I.; Yano, I.; Kobayashi, K. Microbiology 2003, 149, 2071–2081. doi:10.1099/mic.0.25922-0
Return to citation in text: [1] -
Nakayama, M. Seikatsu Eisei 1998, 42, 135–148.
Return to citation in text: [1] -
Yano, I.; Tomiyasu, I.; Yabuuchi, E. FEMS Microbiol. Lett. 1982, 15, 303–307. doi:10.1111/j.1574-6968.1982.tb00239.x
Return to citation in text: [1] -
Schulz, S.; Dickschat, J. S. Nat. Prod. Rep. 2007, 24, 814–842. doi:10.1039/b507392h
Return to citation in text: [1] -
Dickschat, J. S.; Bode, H. B.; Kroppenstedt, R. M.; Müller, R.; Schulz, S. Org. Biomol. Chem. 2005, 3, 2824–2831. doi:10.1039/b504889c
Return to citation in text: [1] [2] [3] -
Dickschat, J. S.; Bruns, H.; Riclea, R. Beilstein J. Org. Chem. 2011, 7, 1697–1712. doi:10.3762/bjoc.7.200
Return to citation in text: [1] -
Kolattukudy, P. E. Plant Physiol. 1968, 43, 375–383. doi:10.1104/pp.43.3.375
Return to citation in text: [1] -
Weitkamp, A. W. J. Am. Chem. Soc. 1945, 67, 447–454. doi:10.1021/ja01219a027
Return to citation in text: [1] -
Hansen, R. P.; Shorland, F. B. Biochem. J. 1951, 50, 207–210.
Return to citation in text: [1] -
James, A. T.; Wheatley, V. R. Biochem. J. 1956, 63, 269–273.
Return to citation in text: [1] -
Nicolaides, N.; Apon, J. M. B. Biomed. Mass Spectrom. 1977, 4, 337–347. doi:10.1002/bms.1200040604
Return to citation in text: [1] -
Harvey, D. J.; Tiffany, J. M.; Duerden, J. M.; Pandher, K. S.; Mengher, L. S. J. Chromatogr. 1987, 414, 253–263. doi:10.1016/0378-4347(87)80051-8
Return to citation in text: [1] -
Harvey, D. J. Biomed. Environ. Mass Spectrom. 1989, 18, 719–723. doi:10.1002/bms.1200180912
Return to citation in text: [1] -
Nicolaides, N. Lipids 1971, 6, 901–905. doi:10.1007/BF02531172
Return to citation in text: [1] -
Nicolaides, N.; Apon, J. M. Lipids 1976, 11, 781–790. doi:10.1007/BF02533404
Return to citation in text: [1] [2] -
Arosenius, K. E.; Stallberg, G.; Stenhagen, E.; Tagtstrom-Eketorp, B. Ark. Kemi, Mineral. Geol. 1948, 26A, 20.
Return to citation in text: [1] -
Weitzel, G.; Wojahn, J. Hoppe-Seyler's Z. Physiol. Chem. 1951, 287, 65–89. doi:10.1515/bchm2.1951.287.1-6.65
Return to citation in text: [1] -
Buu-Hoi, N. G. P. Recl. Trav. Chim. Pays-Bas 1953, 72, 84–87. doi:10.1002/recl.19530720110
Return to citation in text: [1] -
Hougen, F. W.; Ilse, D.; Sutton, D. A.; de Villiers, J. P. J. Chem. Soc. 1953, 98–102. doi:10.1039/JR9530000098
Return to citation in text: [1] [2] -
Silvius, J. R.; McElhaney, R. N. Chem. Phys. Lipids 1979, 24, 287–296. doi:10.1016/0009-3084(79)90034-3
Return to citation in text: [1] -
Bergel'son, L. D.; Vaver, V. A.; Bezzubov, A. A.; Shemyakin, M. M. Zh. Obshch. Khim. 1962, 32, 1807–1811.
Return to citation in text: [1] -
Carballeira, N.; Thompson, J. E.; Ayanoglu, E.; Djerassi, C. J. Org. Chem. 1986, 51, 2751–2756. doi:10.1021/jo00364a024
Return to citation in text: [1] -
Shioiri, T.; Terao, Y.; Irako, N.; Aoyama, T. Tetrahedron 1998, 54, 15701–15710. doi:10.1016/S0040-4020(98)00984-3
Return to citation in text: [1] -
Milburn, A. H.; Truter, E. V. J. Chem. Soc. 1954, 3344–3351. doi:10.1039/jr9540003344
Return to citation in text: [1] -
Norén, B.; Odham, G. Lipids 1973, 8, 573–583. doi:10.1007/BF02532714
Return to citation in text: [1] -
Streibl, M.; Jarolimek, P.; Wollrab, V. Collect. Czech. Chem. Commun. 1964, 29, 2522–2527. doi:10.1135/cccc19642522
Return to citation in text: [1] -
Akiya, S.; Nakazawa, Y. Yakugaku Zasshi 1956, 76, 1403–1405.
Return to citation in text: [1] -
Balzer, T.; Budzikiewicz, H. Z. Naturforsch., B: Chem. Sci. 1987, 42, 1367–1368.
Return to citation in text: [1] -
Cason, J. J. Am. Chem. Soc. 1942, 64, 1106–1110. doi:10.1021/ja01257a029
Return to citation in text: [1] -
Stein, J.; Budzikiewicz, H. Z. Naturforsch., B: Chem. Sci. 1987, 42, 1017–1020.
Return to citation in text: [1] -
Fordyce, C. R.; Johnson, J. R. J. Am. Chem. Soc. 1933, 55, 3368–3372. doi:10.1021/ja01335a054
Return to citation in text: [1] -
Edmunds, A. J. F.; Aluja, M.; Diaz-Fleischer, F.; Patrian, B.; Hagmann, L. Chimia 2010, 64, 37–42. doi:10.2533/chimia.2010.37
Return to citation in text: [1] -
Takikawa, H.; Nozawa, D.; Kayo, A.; Muto, S.-e.; Mori, K. J. Chem. Soc., Perkin Trans. 1 1999, 2467–2477. doi:10.1039/A904258J
Return to citation in text: [1] -
McGhie, J. F.; Ross, W. A.; Evans, D.; Tomlin, J. E. J. Chem. Soc. 1962, 350–355. doi:10.1039/JR9620000350
Return to citation in text: [1] -
Biermann, U.; Metzger, J. O. J. Am. Chem. Soc. 2004, 126, 10319–10330. doi:10.1021/ja048904y
Return to citation in text: [1] -
Crossley, A. T.; Craig, B. M. Can. J. Chem. 1955, 33, 1426–1432. doi:10.1139/v55-171
Return to citation in text: [1] -
Orfanopoulos, M.; Smonou, I. Synth. Commun. 1988, 18, 833–839. doi:10.1080/00397918808057852
Return to citation in text: [1] [2] [3] -
Herriott, A. W.; Picker, D. Tetrahedron Lett. 1974, 15, 1511–1514. doi:10.1016/S0040-4039(01)93123-5
Return to citation in text: [1] [2] [3] [4] -
Prasad, A. S. B.; Kanth, J. V. B.; Periasamy, M. Tetrahedron 1992, 48, 4623–4628. doi:10.1016/S0040-4020(01)81236-9
Return to citation in text: [1] -
Crossland, R. K.; Servis, K. L. J. Org. Chem. 1970, 35, 3195–3196. doi:10.1021/jo00834a087
Return to citation in text: [1] [2] -
Kerschbaum, M. Ber. Dtsch. Chem. Ges. 1927, 60, 902–909. doi:10.1002/cber.19270600411
Return to citation in text: [1] -
Barton, D. H. R.; McCombie, S. W. J. Chem. Soc., Perkin Trans. 1 1975, 1574–1585. doi:10.1039/P19750001574
Return to citation in text: [1] -
Adams, R. M.; Braun, L. M.; Braun, R. A.; Crissman, H. R.; Opprman, M. J. Org. Chem. 1971, 36, 2388–2389. doi:10.1021/jo00815a047
Return to citation in text: [1] -
Bougault, J. C. R. Acad. Sci. 1910, 150, 874–876.
Return to citation in text: [1] -
Hedrick, D.; Peacock, A.; Long, P.; White, D. Lipids 2008, 43, 843–851. doi:10.1007/s11745-008-3206-1
Return to citation in text: [1] -
Ho, G.-J.; Mathre, D. J. J. Org. Chem. 1995, 60, 2271–2273. doi:10.1021/jo00112a060
Return to citation in text: [1] -
Evans, D. A.; Ennis, M. D.; Mathre, D. J. J. Am. Chem. Soc. 1982, 104, 1737–1739. doi:10.1021/ja00370a050
Return to citation in text: [1] -
Evans, D. A.; Britton, T. C.; Ellman, J. A. Tetrahedron Lett. 1987, 28, 6141–6144. doi:10.1016/S0040-4039(00)61830-0
Return to citation in text: [1] -
Fautz, E.; Rosenfelder, G.; Grotjahn, L. J. Bacteriol. 1979, 140, 852–858.
Return to citation in text: [1] -
Leuckfeld, I.; Paster, B. J.; Kristoffersen, A. K.; Olsen, I. APMIS 2010, 118, 230–242. doi:10.1111/j.1600-0463.2009.02584.x
Return to citation in text: [1] -
Evans, D. A.; Morrissey, M. M.; Dorow, R. L. J. Am. Chem. Soc. 1985, 107, 4346–4348. doi:10.1021/ja00300a054
Return to citation in text: [1] [2] -
Davis, F. A.; Chattopadhyay, S.; Towson, J. C.; Lal, S.; Reddy, T. J. Org. Chem. 1988, 53, 2087–2089. doi:10.1021/jo00244a043
Return to citation in text: [1] -
Verma, R.; Ghosh, S. J. Chem. Soc., Perkin Trans. 1 1999, 265–270. doi:10.1039/A808840C
Return to citation in text: [1] -
Theodorou, V.; Skobridis, K.; Tzakos, A. G.; Ragoussis, V. Tetrahedron Lett. 2007, 48, 8230–8233. doi:10.1016/j.tetlet.2007.09.074
Return to citation in text: [1] -
Weise, T.; Kai, M.; Gummesson, A.; Troeger, A.; von Reuß, S.; Piepenborn, S.; Kosterka, F.; Sklorz, M.; Zimmermann, R.; Francke, W.; Piechulla, B. Beilstein J. Org. Chem. 2012, 8, 579–596. doi:10.3762/bjoc.8.65
Return to citation in text: [1] -
Mitsudome, T.; Umetani, T.; Nosaka, N.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Angew. Chem., Int. Ed. 2006, 45, 481–485. doi:10.1002/anie.200502886
Return to citation in text: [1]
| 34. | Buu-Hoi, N. G. P. Recl. Trav. Chim. Pays-Bas 1953, 72, 84–87. doi:10.1002/recl.19530720110 |
| 35. | Hougen, F. W.; Ilse, D.; Sutton, D. A.; de Villiers, J. P. J. Chem. Soc. 1953, 98–102. doi:10.1039/JR9530000098 |
| 36. | Silvius, J. R.; McElhaney, R. N. Chem. Phys. Lipids 1979, 24, 287–296. doi:10.1016/0009-3084(79)90034-3 |
| 3. | Acton, E. M.; Ryan, K. J.; Luetzow, A. E. J. Med. Chem. 1977, 20, 1362–1371. doi:10.1021/jm00221a002 |
| 21. | Dickschat, J. S.; Bode, H. B.; Kroppenstedt, R. M.; Müller, R.; Schulz, S. Org. Biomol. Chem. 2005, 3, 2824–2831. doi:10.1039/b504889c |
| 37. | Bergel'son, L. D.; Vaver, V. A.; Bezzubov, A. A.; Shemyakin, M. M. Zh. Obshch. Khim. 1962, 32, 1807–1811. |
| 38. | Carballeira, N.; Thompson, J. E.; Ayanoglu, E.; Djerassi, C. J. Org. Chem. 1986, 51, 2751–2756. doi:10.1021/jo00364a024 |
| 39. | Shioiri, T.; Terao, Y.; Irako, N.; Aoyama, T. Tetrahedron 1998, 54, 15701–15710. doi:10.1016/S0040-4020(98)00984-3 |
| 52. | Crossley, A. T.; Craig, B. M. Can. J. Chem. 1955, 33, 1426–1432. doi:10.1139/v55-171 |
| 53. | Orfanopoulos, M.; Smonou, I. Synth. Commun. 1988, 18, 833–839. doi:10.1080/00397918808057852 |
| 50. | McGhie, J. F.; Ross, W. A.; Evans, D.; Tomlin, J. E. J. Chem. Soc. 1962, 350–355. doi:10.1039/JR9620000350 |
| 51. | Biermann, U.; Metzger, J. O. J. Am. Chem. Soc. 2004, 126, 10319–10330. doi:10.1021/ja048904y |
| 47. | Fordyce, C. R.; Johnson, J. R. J. Am. Chem. Soc. 1933, 55, 3368–3372. doi:10.1021/ja01335a054 |
| 48. | Edmunds, A. J. F.; Aluja, M.; Diaz-Fleischer, F.; Patrian, B.; Hagmann, L. Chimia 2010, 64, 37–42. doi:10.2533/chimia.2010.37 |
| 49. | Takikawa, H.; Nozawa, D.; Kayo, A.; Muto, S.-e.; Mori, K. J. Chem. Soc., Perkin Trans. 1 1999, 2467–2477. doi:10.1039/A904258J |
| 35. | Hougen, F. W.; Ilse, D.; Sutton, D. A.; de Villiers, J. P. J. Chem. Soc. 1953, 98–102. doi:10.1039/JR9530000098 |
| 40. | Milburn, A. H.; Truter, E. V. J. Chem. Soc. 1954, 3344–3351. doi:10.1039/jr9540003344 |
| 41. | Norén, B.; Odham, G. Lipids 1973, 8, 573–583. doi:10.1007/BF02532714 |
| 42. | Streibl, M.; Jarolimek, P.; Wollrab, V. Collect. Czech. Chem. Commun. 1964, 29, 2522–2527. doi:10.1135/cccc19642522 |
| 43. | Akiya, S.; Nakazawa, Y. Yakugaku Zasshi 1956, 76, 1403–1405. |
| 44. | Balzer, T.; Budzikiewicz, H. Z. Naturforsch., B: Chem. Sci. 1987, 42, 1367–1368. |
| 45. | Cason, J. J. Am. Chem. Soc. 1942, 64, 1106–1110. doi:10.1021/ja01257a029 |
| 46. | Stein, J.; Budzikiewicz, H. Z. Naturforsch., B: Chem. Sci. 1987, 42, 1017–1020. |
| 54. | Herriott, A. W.; Picker, D. Tetrahedron Lett. 1974, 15, 1511–1514. doi:10.1016/S0040-4039(01)93123-5 |
| 55. | Prasad, A. S. B.; Kanth, J. V. B.; Periasamy, M. Tetrahedron 1992, 48, 4623–4628. doi:10.1016/S0040-4020(01)81236-9 |
| 56. | Crossland, R. K.; Servis, K. L. J. Org. Chem. 1970, 35, 3195–3196. doi:10.1021/jo00834a087 |
| 54. | Herriott, A. W.; Picker, D. Tetrahedron Lett. 1974, 15, 1511–1514. doi:10.1016/S0040-4039(01)93123-5 |
| 54. | Herriott, A. W.; Picker, D. Tetrahedron Lett. 1974, 15, 1511–1514. doi:10.1016/S0040-4039(01)93123-5 |
| 59. | Adams, R. M.; Braun, L. M.; Braun, R. A.; Crissman, H. R.; Opprman, M. J. Org. Chem. 1971, 36, 2388–2389. doi:10.1021/jo00815a047 |
| 54. | Herriott, A. W.; Picker, D. Tetrahedron Lett. 1974, 15, 1511–1514. doi:10.1016/S0040-4039(01)93123-5 |
| 58. | Barton, D. H. R.; McCombie, S. W. J. Chem. Soc., Perkin Trans. 1 1975, 1574–1585. doi:10.1039/P19750001574 |
| 57. | Kerschbaum, M. Ber. Dtsch. Chem. Ges. 1927, 60, 902–909. doi:10.1002/cber.19270600411 |
| 53. | Orfanopoulos, M.; Smonou, I. Synth. Commun. 1988, 18, 833–839. doi:10.1080/00397918808057852 |
| 56. | Crossland, R. K.; Servis, K. L. J. Org. Chem. 1970, 35, 3195–3196. doi:10.1021/jo00834a087 |
| 61. | Hedrick, D.; Peacock, A.; Long, P.; White, D. Lipids 2008, 43, 843–851. doi:10.1007/s11745-008-3206-1 |
| 53. | Orfanopoulos, M.; Smonou, I. Synth. Commun. 1988, 18, 833–839. doi:10.1080/00397918808057852 |
| 5. | Cockrum, P. A.; Edgar, J. A. J. Chromatogr. 1983, 268, 245–254. doi:10.1016/S0021-9673(01)95411-1 |
| 17. | Minamino, M.; Sakaguchi, I.; Naka, T.; Ikeda, N.; Kato, Y.; Tomiyasu, I.; Yano, I.; Kobayashi, K. Microbiology 2003, 149, 2071–2081. doi:10.1099/mic.0.25922-0 |
| 18. | Nakayama, M. Seikatsu Eisei 1998, 42, 135–148. |
| 19. | Yano, I.; Tomiyasu, I.; Yabuuchi, E. FEMS Microbiol. Lett. 1982, 15, 303–307. doi:10.1111/j.1574-6968.1982.tb00239.x |
| 66. | Leuckfeld, I.; Paster, B. J.; Kristoffersen, A. K.; Olsen, I. APMIS 2010, 118, 230–242. doi:10.1111/j.1600-0463.2009.02584.x |
| 4. | Borghi, A.; Antonini, P.; Zanol, M.; Ferrari, P.; Zerilli, L. F.; Lancini, G. C. J. Antibiot. 1989, 42, 361–366. doi:10.7164/antibiotics.42.361 |
| 20. | Schulz, S.; Dickschat, J. S. Nat. Prod. Rep. 2007, 24, 814–842. doi:10.1039/b507392h |
| 3. | Acton, E. M.; Ryan, K. J.; Luetzow, A. E. J. Med. Chem. 1977, 20, 1362–1371. doi:10.1021/jm00221a002 |
| 14. | Hunter, S. W.; McNeil, M. R.; Brennan, P. J. J. Bacteriol. 1986, 168, 917–922. |
| 15. | Orgambide, G. G.; Hollingsworth, R. I.; Dazzo, F. B. Carbohydr. Res. 1992, 233, 151–159. doi:10.1016/S0008-6215(00)90927-3 |
| 2. | Kniazeva, M.; Crawford, Q. T.; Seiber, M.; Wang, C. Y.; Han, M. PLoS Biol. 2004, 2, E257. doi:10.1371/journal.pbio.0020257 |
| 16. | Fujimoto, Y.; Mitsunobe, K.; Fujiwara, S.; Mori, M.; Hashimoto, M.; Suda, Y.; Kusumoto, S.; Fukase, K. Org. Biomol. Chem. 2013, 11, 5034–5041. doi:10.1039/c3ob40899j |
| 21. | Dickschat, J. S.; Bode, H. B.; Kroppenstedt, R. M.; Müller, R.; Schulz, S. Org. Biomol. Chem. 2005, 3, 2824–2831. doi:10.1039/b504889c |
| 65. | Fautz, E.; Rosenfelder, G.; Grotjahn, L. J. Bacteriol. 1979, 140, 852–858. |
| 9. | Kulanthaivel, P.; Kreuzman, A. J.; Strege, M. A.; Belvo, M. D.; Smitka, T. A.; Clemens, M.; Swartling, J. R.; Minton, K. L.; Zheng, F.; Angleton, E. L.; Mullen, D.; Jungheim, L. N.; Klimkowski, V. J.; Nicas, T. I.; Thompson, R. C.; Peng, S.-B. J. Biol. Chem. 2004, 279, 36250–36258. doi:10.1074/jbc.M405884200 |
| 10. | Liu, J.; Luo, C.; Smith, P. A.; Chin, J. K.; Page, M. G. P.; Paetzel, M.; Romesberg, F. E. J. Am. Chem. Soc. 2011, 133, 17869–17877. doi:10.1021/ja207318n |
| 12. | Esumi, Y.; Suzuki, Y.; Itoh, Y.; Chijimatsu, M.; Uramoto, M.; Kimura, K.-i.; Nakayama, S.; Yoshihama, M.; Ichikawa, T.; Haramo, T.; Fujishige, J. J. Antibiot. 2003, 56, 716–720. doi:10.7164/antibiotics.56.716 |
| 63. | Evans, D. A.; Ennis, M. D.; Mathre, D. J. J. Am. Chem. Soc. 1982, 104, 1737–1739. doi:10.1021/ja00370a050 |
| 8. | Eckardt, K.; Wetzstein, H.; Thrum, H.; Ihn, W. J. Antibiot. 1980, 33, 908–910. doi:10.7164/antibiotics.33.908 |
| 13. | Poinsot, V.; Bélanger, E.; Laberge, S.; Yang, G.-P.; Antoun, H.; Cloutier, J.; Treilhou, M.; Dénarié, J.; Promé, J.-C.; Debellé, F. J. Bacteriol. 2001, 183, 3721–3728. doi:10.1128/JB.183.12.3721-3728.2001 |
| 64. | Evans, D. A.; Britton, T. C.; Ellman, J. A. Tetrahedron Lett. 1987, 28, 6141–6144. doi:10.1016/S0040-4039(00)61830-0 |
| 7. | Edgar, J. A.; Frahn, J. L.; Cockrum, P. A.; Anderton, N.; Jago, M. V.; Culvenor, C. C. J.; Jones, A. J.; Murray, K.; Shaw, K. J. J. Chem. Soc., Chem. Commun. 1982, 222–224. doi:10.1039/C39820000222 |
| 6. | Ito, T.; Takatsuki, A.; Kawamura, K.; Sato, K.; Tamura, G. Agric. Biol. Chem. 1980, 44, 695–698. doi:10.1271/bbb1961.44.695 |
| 11. | Penkov, S.; Mende, F.; Zagoriy, V.; Erkut, C.; Martin, R.; Pässler, U.; Schuhmann, K.; Schwudke, D.; Gruner, M.; Mäntler, J.; Reichert-Müller, T.; Shevchenko, A.; Knölker, H.-J.; Kurzchalia, T. V. Angew. Chem., Int. Ed. 2010, 49, 9430–9435. doi:10.1002/anie.201004466 |
| 62. | Ho, G.-J.; Mathre, D. J. J. Org. Chem. 1995, 60, 2271–2273. doi:10.1021/jo00112a060 |
| 21. | Dickschat, J. S.; Bode, H. B.; Kroppenstedt, R. M.; Müller, R.; Schulz, S. Org. Biomol. Chem. 2005, 3, 2824–2831. doi:10.1039/b504889c |
| 22. | Dickschat, J. S.; Bruns, H.; Riclea, R. Beilstein J. Org. Chem. 2011, 7, 1697–1712. doi:10.3762/bjoc.7.200 |
| 69. | Verma, R.; Ghosh, S. J. Chem. Soc., Perkin Trans. 1 1999, 265–270. doi:10.1039/A808840C |
| 67. | Evans, D. A.; Morrissey, M. M.; Dorow, R. L. J. Am. Chem. Soc. 1985, 107, 4346–4348. doi:10.1021/ja00300a054 |
| 67. | Evans, D. A.; Morrissey, M. M.; Dorow, R. L. J. Am. Chem. Soc. 1985, 107, 4346–4348. doi:10.1021/ja00300a054 |
| 68. | Davis, F. A.; Chattopadhyay, S.; Towson, J. C.; Lal, S.; Reddy, T. J. Org. Chem. 1988, 53, 2087–2089. doi:10.1021/jo00244a043 |
| 32. | Arosenius, K. E.; Stallberg, G.; Stenhagen, E.; Tagtstrom-Eketorp, B. Ark. Kemi, Mineral. Geol. 1948, 26A, 20. |
| 33. | Weitzel, G.; Wojahn, J. Hoppe-Seyler's Z. Physiol. Chem. 1951, 287, 65–89. doi:10.1515/bchm2.1951.287.1-6.65 |
| 29. | Harvey, D. J. Biomed. Environ. Mass Spectrom. 1989, 18, 719–723. doi:10.1002/bms.1200180912 |
| 30. | Nicolaides, N. Lipids 1971, 6, 901–905. doi:10.1007/BF02531172 |
| 31. | Nicolaides, N.; Apon, J. M. Lipids 1976, 11, 781–790. doi:10.1007/BF02533404 |
| 27. | Nicolaides, N.; Apon, J. M. B. Biomed. Mass Spectrom. 1977, 4, 337–347. doi:10.1002/bms.1200040604 |
| 72. | Mitsudome, T.; Umetani, T.; Nosaka, N.; Mori, K.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Angew. Chem., Int. Ed. 2006, 45, 481–485. doi:10.1002/anie.200502886 |
| 28. | Harvey, D. J.; Tiffany, J. M.; Duerden, J. M.; Pandher, K. S.; Mengher, L. S. J. Chromatogr. 1987, 414, 253–263. doi:10.1016/0378-4347(87)80051-8 |
| 70. | Theodorou, V.; Skobridis, K.; Tzakos, A. G.; Ragoussis, V. Tetrahedron Lett. 2007, 48, 8230–8233. doi:10.1016/j.tetlet.2007.09.074 |
| 71. | Weise, T.; Kai, M.; Gummesson, A.; Troeger, A.; von Reuß, S.; Piepenborn, S.; Kosterka, F.; Sklorz, M.; Zimmermann, R.; Francke, W.; Piechulla, B. Beilstein J. Org. Chem. 2012, 8, 579–596. doi:10.3762/bjoc.8.65 |
© 2013 Richardson and Williams; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)