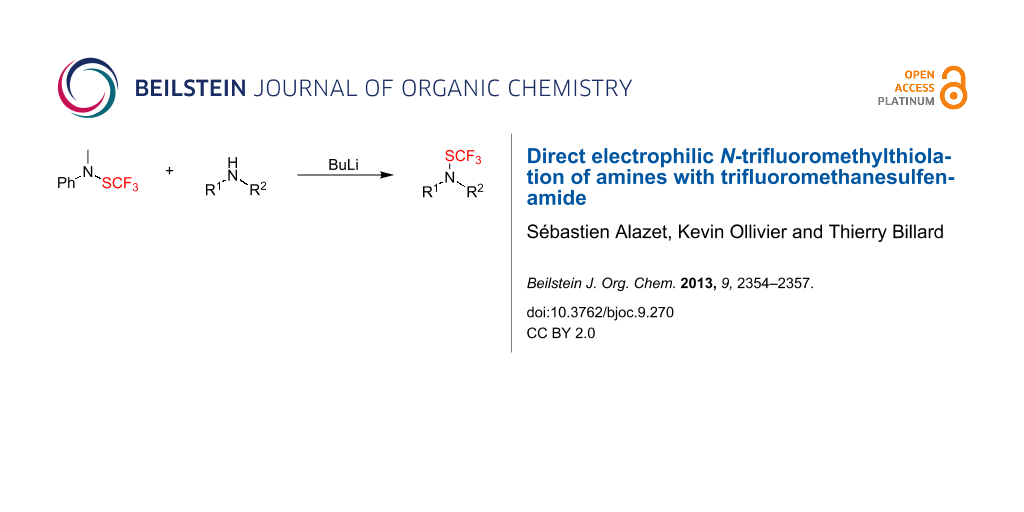
Abstract
The CF3SN moiety is a substituent with interesting properties. However, there is no easy synthetic access to molecules bearing this group. The trifluoromethanesulfenamide is a new reagent for the electrophilic trifluoromethylthiolation which reacts easily with amines to obtain trifluoromethylsulfanylamines with good yields.
Graphical Abstract
Introduction
In past decades, fluorinated molecules have found more and more applications in a variety of fields, especially in the design of new compounds for medicinal chemistry or agrochemistry [1-9]. More recently, new substituents have emerged which associate the trifluoromethyl group with heteroatoms such as CF3O or CF3S. Because of its high hydrophobicity (Hansch parameter πR = 1.44), the CF3S moiety is of particular interest [10]. Compounds with this group constitute important targets for applications in pharmaceuticals and agrochemicals [4,11-13].
The association of a CF3 group to more than one heteroatom is rarely described in literature. In particular, there are only a few investigations regarding the trifluoromethylsulfanylamine moiety (CF3SN). However, this group has found applications in agrochemical and medicinal chemistry [14-20]. From a physicochemical point of view, the CF3SN group possesses a Hansch’s hydrophobicity parameter πR = 1.50 [21]. This value, slightly superior to the Hansch’s hydrophobicity parameter of the CF3S group, could be of great interest in the development of biological active compounds. Yet, the common synthetic route to these compounds use the highly toxic and gaseous CF3SCl [16,22-35].
Results and Discussion
Several years ago, we have described an easy access to trifluoromethanesulfenamides [36], starting from DAST, Ruppert reagent, and primary amines [37]. However, even though this strategy gave good results with primary amines, secondary amines do not react under these conditions, thus limiting the access to a large panel of trifluoromethylsulfanylamines. The trifluoromethanesulfenamide 1a is an efficient reagent for the electrophilic trifluoromethylthiolation of carbon nucleophiles [38-44]. Therefore, this reagent should react with amines to perform transamination reactions with secondary amines leading to various trifluoromethylsulfanylamines 3. The reaction has been optimized with phenylpiperazine (2a) (Table 1).
Table 1: Reaction of phenylpiperazine (2a) with 1a under basic conditions.
|
|
||||
| Entry | Base | T (°C) | t | 3a (%)a |
|---|---|---|---|---|
| 1 | BuLi | −78 | 15 min | 65 |
| 2 | BuLi | 0 | 15 min | 86 |
| 3 | BuLi | 0 | 3 h | 84 |
| 4 | NaH | 0 | 15 min | 0 |
| 5 | Cs2CO3 | 80 | 2 h 30 | 0 |
aCrude yields determined by 19F NMR spectroscopy by using PhOCF3 as an internal standard.
After preliminary deprotonation of 2a with BuLi, the trifluoromethanesulfenamide 1a is added. The expected product 3a is obtained in 15 min with good yield. To improve the kinetic of the reaction, the deprotonation and the transamination should be performed at 0 °C (Table 1, entries 1 and 2). Longer reaction times do not increase the yield, the reaction seems to be finished in 15 min (Table 1, entries 2 and 3). As previously observed in other works, the use of other bases with sodium or cesium cations is not efficient since only Li is a Lewis acid strong enough to activate 1a [38,44]. These optimal conditions have been extended to selected amines 2 (Figure 1).
Figure 1: Transamination of 1a with amines. (Isolated yields, in parentheses crude yields determined by 19F NMR with PhOCF3 as an internal standard).
Figure 1: Transamination of 1a with amines. (Isolated yields, in parentheses crude yields determined by 19F N...
The reaction gives, in general, good yields with various secondary amines (3a–k). Because of their high volatility, some compounds (3h and 3i) have not been isolated. Imines can be also trifluoromethylthiolated in good yields (3m). Even if our first developed method is compatible with primary amines [37], they can also react under these new conditions, as illustrated with the aniline (3n).
Amino alcohols and bis-amines can also be trifluoromethylthiolated, with the most nucleophile atom as a target (Figure 2). In this case, 2.1 equiv of BuLi are required and reaction times are loner (20 h).
Figure 2: Reaction of 1a with bis-nucleophiles. (Isolated yields, in parentheses crude yields determined by 19F NMR with PhOCF3 as an internal standard).
Figure 2: Reaction of 1a with bis-nucleophiles. (Isolated yields, in parentheses crude yields determined by 19...
This new method was applied to synthesize a trifluoromethylthio analog (3l) of the well-known tricyclic antidepressant imipramine (Figure 3). Since the pentafluoroethyl analog 1b of reagent 1a has also been described previously, a pentafluoroethylthio analog of imipramine was synthesized (4l) (Figure 3). In the latter case, the obtained yield was lower, certainly due to the steric hindrance of the CF3CF2S moiety. The pharmacological properties of these new compounds are under investigation.
Figure 3: Synthesis of fluoroalkylthio analogs of imipramine. (Isolated yields, in parentheses crude yields determined by 19F NMR with PhOCF3 as an internal standard).
Figure 3: Synthesis of fluoroalkylthio analogs of imipramine. (Isolated yields, in parentheses crude yields d...
Conclusion
In conclusion, the trifluoromethanesulfenamide 1a is a very efficient reagent for the electrophilic trifluoromethylthiolation which can also react with amines to open a new access to trifluoromethylsulfanylamines. These compounds belong to a new class of products which may exhibit interesting properties for further applications – in particular in medicinal chemistry – owing to the characteristics of the CF3SN moiety.
Supporting Information
| Supporting Information File 1: Experimental procedure. | ||
| Format: PDF | Size: 434.0 KB | Download |
References
-
Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley: Chichester, 2009. doi:10.1002/9781444312096
Return to citation in text: [1] -
Dunitz, J. D. ChemBioChem 2004, 5, 614–621. doi:10.1002/cbic.200300801
Return to citation in text: [1] -
Biffinger, J. C.; Kim, H. W.; DiMagno, S. G. ChemBioChem 2004, 5, 622–627. doi:10.1002/cbic.200300910
Return to citation in text: [1] -
Becker, A. Inventory of Industrial Fluoro-biochemicals; Eyrolles: Paris, 1996.
Return to citation in text: [1] [2] -
Jeschke, P. ChemBioChem 2004, 5, 570–589. doi:10.1002/cbic.200300833
Return to citation in text: [1] -
Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881–1886. doi:10.1126/science.1131943
Return to citation in text: [1] -
Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c
Return to citation in text: [1] -
Hagmann, W. K. J. Med. Chem. 2008, 51, 4359–4369. doi:10.1021/jm800219f
Return to citation in text: [1] -
Bégué, J.-P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; John Wiley & Sons, Inc.: Hoboken, 2008. doi:10.1002/9780470281895
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
Return to citation in text: [1] -
Langlois, B. R.; Billard, T.; Large, S.; Roques, N. Potentially bioactive CF3S- and CF3S(O)-substituted compounds. Fluorinated Bioactive Compounds in the Agricultural & Medical Fields; Chemical & Polymer: Cheshire, 1999; 24/1–24/8.
Return to citation in text: [1] -
Szmuszkovicz, J.; Glenn, E. M.; Heinzelman, R. V.; Hester, J. B., Jr.; Youngdale, G. A. J. Med. Chem. 1966, 9, 527–536. doi:10.1021/jm00322a020
Return to citation in text: [1] -
Cherkofsky, S. C. Antiinflammatory 4,5-diaryl-2-(substituted-thio)pyrroles and their corresponding sulfoxides and sulfones. U.S. Patent US4267184A, May 12, 1981.
Return to citation in text: [1] -
Davies, P.; Doherty, J. B.; Finke, P. E.; Humes, J. L.; Leudke, E. S.; Maccoss, M.; Mumford, R. A.; Shah, S. K. Substituted azetidinones useful in the treatment of leukemia. Brit. Patent GB2266527A, March 11, 1993.
Return to citation in text: [1] -
Dorn, C. P.; Finke, P. E.; Maccoss, M.; Doherty, J. B.; Shah, S. K.; Hagmann, W. K. Preparation of substituted azetidinones as anti-inflammatory and antidegenerative agents. Eur. Pat. EP0481671A1, April 22, 1992.
Return to citation in text: [1] -
Firestone, R. A.; Barker, P. L.; Pisano, J. M.; Ashe, B. M.; Dahlgren, M. E. Tetrahedron 1990, 46, 2255–2262. doi:10.1016/S0040-4020(01)82006-8
Return to citation in text: [1] [2] -
Nakayama, M.; Yamada, M. Sulfonamide derivative-containing agricultural and horticultural compositions. WO Patent WO2002054867A1, July 18, 2002.
Return to citation in text: [1] -
Sahu, K. K.; Ravichandran, V.; Mourya, V. K.; Agrawal, R. K. Med. Chem. Res. 2007, 15, 418–430. doi:10.1007/s00044-006-0020-2
Return to citation in text: [1] -
Schallner, O.; Schwarz, H.-G.; Hoischen, D.; Linker, K.-H.; Drewes, M. W.; Dahmen, P.; Feucht, D.; Pontzen, R. Preparation of benzoxazinones and related compounds as herbicides. WO Patent WO2002006277A1, Jan 24, 2002.
Return to citation in text: [1] -
Tabuchi, T.; Yamamoto, T.; Nakayama, M. Preparation of aryl- or heterocyclylsulfonamide derivatives as agricultural and horticultural microbicides. WO Patent WO2000065913A1, Nov 1, 2000.
Return to citation in text: [1] -
Ferry, A. New reactivity of DAST and its analogs: Synthesis and applications of fluorinated sulfinamidines and sulfanylamines. Ph.D. Thesis, University of Lyon, Lyon, France, 2007.
Return to citation in text: [1] -
Borowski, H. E.; Haas, A. Chem. Ber. 1982, 115, 523–532. doi:10.1002/cber.19821150215
Return to citation in text: [1] -
Ceacareanu, D. M.; Gerstenberger, M. R. C.; Haas, A. Chem. Ber. 1983, 116, 3325–3331. doi:10.1002/cber.19831161008
Return to citation in text: [1] -
Geisel, M.; Mews, R. Chem. Ber. 1987, 120, 1675–1677. doi:10.1002/cber.19871201011
Return to citation in text: [1] -
Gerstenberger, M. R. C.; Haas, A.; Wille, R.; Yazdanbakhsch, M. Rev. Chim. Miner. 1986, 23, 485–496.
Return to citation in text: [1] -
Gupta, O. D.; Kamil, W. A.; Shreeve, J. M. Inorg. Chem. 1985, 24, 2126–2129. doi:10.1021/ic00208a004
Return to citation in text: [1] -
Haas, A.; Lieb, M. J. Heterocycl. Chem. 1986, 23, 1079–1084. doi:10.1002/jhet.5570230423
Return to citation in text: [1] -
Haas, A.; Lieb, M.; Schwederski, B. Rev. Roum. Chim. 1987, 32, 1219–1224.
Return to citation in text: [1] -
Kolasa, A.; Lieb, M. J. Fluorine Chem. 1995, 70, 45–47. doi:10.1016/0022-1139(94)03086-F
Return to citation in text: [1] -
Kost, D.; Egozy, H. J. Org. Chem. 1989, 54, 4909–4913. doi:10.1021/jo00281a038
Return to citation in text: [1] -
Munavalli, S.; Rohrbaugh, D. K.; Rossman, D. I.; Berg, F. J.; Wagner, G. W.; Durst, H. D. Synth. Commun. 2000, 30, 2847–2854. doi:10.1080/00397910008087435
Return to citation in text: [1] -
Munavalli, S.; Rohrbaugh, D. K.; Rossman, D. I.; Durst, H. D. Phosphorus, Sulfur Silicon Relat. Elem. 2002, 177, 1109–1116. doi:10.1080/10426500211704
Return to citation in text: [1] -
Munavalli, S.; Rohrbaugh, D. K.; Rossman, D. I.; Wagner, W. G.; Durst, H. D. Phosphorus, Sulfur Silicon Relat. Elem. 2003, 178, 107–113. doi:10.1080/10426500307779
Return to citation in text: [1] -
Peach, M. E. Can. J. Chem. 1967, 45, 429–432. doi:10.1139/v67-076
Return to citation in text: [1] -
Yagupol'skii, L. M.; Bezdudnyi, A. V.; Yagupol'skii, Yu. L. Russ. J. Org. Chem. 2006, 42, 1275–1279. doi:10.1134/S1070428006090041
Return to citation in text: [1] -
Various nomenclatures have been used for these compounds in previous publications, including our own.According to the IUPAC rules it appears that the true name must be trifluoromethanesulfenamide. Alternatively, trifluoromethylsulfanylamine could be used.
Return to citation in text: [1] -
Ferry, A.; Billard, T.; Langlois, B. R.; Bacqué, E. J. Org. Chem. 2008, 73, 9362–9365. doi:10.1021/jo8018544
Return to citation in text: [1] [2] -
Baert, F.; Colomb, J.; Billard, T. Angew. Chem. 2012, 124, 10528–10531. doi:10.1002/ange.201205156
Angew. Chem., Int. Ed. 2012, 51, 10382–10385. doi:10.1002/anie.201205156
Return to citation in text: [1] [2] -
Ferry, A.; Billard, T.; Bacqué, E.; Langlois, B. R. J. Fluorine Chem. 2012, 134, 160–163. doi:10.1016/j.jfluchem.2011.02.005
Return to citation in text: [1] -
Ferry, A.; Billard, T.; Langlois, B. R.; Bacqué, E. Angew. Chem. 2009, 121, 8703–8707. doi:10.1002/ange.200903387
Angew. Chem., Int. Ed. 2009, 48, 8551–8555. doi:10.1002/anie.200903387
Return to citation in text: [1] -
Liu, J.; Chu, L.; Qing, F.-L. Org. Lett. 2013, 15, 894–897. doi:10.1021/ol400032g
Return to citation in text: [1] -
Tlili, A.; Billard, T. Angew. Chem. 2013, 125, 6952–6954. doi:10.1002/ange.201301438
Angew. Chem., Int. Ed. 2013, 52, 6818–6819. doi:10.1002/anie.201301438
Return to citation in text: [1] -
Yang, Y.; Jiang, X.; Qing, F.-L. J. Org. Chem. 2012, 77, 7538–7547. doi:10.1021/jo3013385
Return to citation in text: [1] -
Alazet, S.; Zimmer, L.; Billard, T. Angew. Chem. 2013, 125, 11014–11017. doi:10.1002/ange.201305179
Angew. Chem., Int. Ed. 2013, 52, 10814–10817. doi:10.1002/anie.201305179
Return to citation in text: [1] [2]
| 1. | Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley: Chichester, 2009. doi:10.1002/9781444312096 |
| 2. | Dunitz, J. D. ChemBioChem 2004, 5, 614–621. doi:10.1002/cbic.200300801 |
| 3. | Biffinger, J. C.; Kim, H. W.; DiMagno, S. G. ChemBioChem 2004, 5, 622–627. doi:10.1002/cbic.200300910 |
| 4. | Becker, A. Inventory of Industrial Fluoro-biochemicals; Eyrolles: Paris, 1996. |
| 5. | Jeschke, P. ChemBioChem 2004, 5, 570–589. doi:10.1002/cbic.200300833 |
| 6. | Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881–1886. doi:10.1126/science.1131943 |
| 7. | Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 320–330. doi:10.1039/b610213c |
| 8. | Hagmann, W. K. J. Med. Chem. 2008, 51, 4359–4369. doi:10.1021/jm800219f |
| 9. | Bégué, J.-P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; John Wiley & Sons, Inc.: Hoboken, 2008. doi:10.1002/9780470281895 |
| 21. | Ferry, A. New reactivity of DAST and its analogs: Synthesis and applications of fluorinated sulfinamidines and sulfanylamines. Ph.D. Thesis, University of Lyon, Lyon, France, 2007. |
| 14. | Davies, P.; Doherty, J. B.; Finke, P. E.; Humes, J. L.; Leudke, E. S.; Maccoss, M.; Mumford, R. A.; Shah, S. K. Substituted azetidinones useful in the treatment of leukemia. Brit. Patent GB2266527A, March 11, 1993. |
| 15. | Dorn, C. P.; Finke, P. E.; Maccoss, M.; Doherty, J. B.; Shah, S. K.; Hagmann, W. K. Preparation of substituted azetidinones as anti-inflammatory and antidegenerative agents. Eur. Pat. EP0481671A1, April 22, 1992. |
| 16. | Firestone, R. A.; Barker, P. L.; Pisano, J. M.; Ashe, B. M.; Dahlgren, M. E. Tetrahedron 1990, 46, 2255–2262. doi:10.1016/S0040-4020(01)82006-8 |
| 17. | Nakayama, M.; Yamada, M. Sulfonamide derivative-containing agricultural and horticultural compositions. WO Patent WO2002054867A1, July 18, 2002. |
| 18. | Sahu, K. K.; Ravichandran, V.; Mourya, V. K.; Agrawal, R. K. Med. Chem. Res. 2007, 15, 418–430. doi:10.1007/s00044-006-0020-2 |
| 19. | Schallner, O.; Schwarz, H.-G.; Hoischen, D.; Linker, K.-H.; Drewes, M. W.; Dahmen, P.; Feucht, D.; Pontzen, R. Preparation of benzoxazinones and related compounds as herbicides. WO Patent WO2002006277A1, Jan 24, 2002. |
| 20. | Tabuchi, T.; Yamamoto, T.; Nakayama, M. Preparation of aryl- or heterocyclylsulfonamide derivatives as agricultural and horticultural microbicides. WO Patent WO2000065913A1, Nov 1, 2000. |
| 4. | Becker, A. Inventory of Industrial Fluoro-biochemicals; Eyrolles: Paris, 1996. |
| 11. | Langlois, B. R.; Billard, T.; Large, S.; Roques, N. Potentially bioactive CF3S- and CF3S(O)-substituted compounds. Fluorinated Bioactive Compounds in the Agricultural & Medical Fields; Chemical & Polymer: Cheshire, 1999; 24/1–24/8. |
| 12. | Szmuszkovicz, J.; Glenn, E. M.; Heinzelman, R. V.; Hester, J. B., Jr.; Youngdale, G. A. J. Med. Chem. 1966, 9, 527–536. doi:10.1021/jm00322a020 |
| 13. | Cherkofsky, S. C. Antiinflammatory 4,5-diaryl-2-(substituted-thio)pyrroles and their corresponding sulfoxides and sulfones. U.S. Patent US4267184A, May 12, 1981. |
| 10. | Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004 |
| 38. |
Baert, F.; Colomb, J.; Billard, T. Angew. Chem. 2012, 124, 10528–10531. doi:10.1002/ange.201205156
Angew. Chem., Int. Ed. 2012, 51, 10382–10385. doi:10.1002/anie.201205156 |
| 39. | Ferry, A.; Billard, T.; Bacqué, E.; Langlois, B. R. J. Fluorine Chem. 2012, 134, 160–163. doi:10.1016/j.jfluchem.2011.02.005 |
| 40. |
Ferry, A.; Billard, T.; Langlois, B. R.; Bacqué, E. Angew. Chem. 2009, 121, 8703–8707. doi:10.1002/ange.200903387
Angew. Chem., Int. Ed. 2009, 48, 8551–8555. doi:10.1002/anie.200903387 |
| 41. | Liu, J.; Chu, L.; Qing, F.-L. Org. Lett. 2013, 15, 894–897. doi:10.1021/ol400032g |
| 42. |
Tlili, A.; Billard, T. Angew. Chem. 2013, 125, 6952–6954. doi:10.1002/ange.201301438
Angew. Chem., Int. Ed. 2013, 52, 6818–6819. doi:10.1002/anie.201301438 |
| 43. | Yang, Y.; Jiang, X.; Qing, F.-L. J. Org. Chem. 2012, 77, 7538–7547. doi:10.1021/jo3013385 |
| 44. |
Alazet, S.; Zimmer, L.; Billard, T. Angew. Chem. 2013, 125, 11014–11017. doi:10.1002/ange.201305179
Angew. Chem., Int. Ed. 2013, 52, 10814–10817. doi:10.1002/anie.201305179 |
| 37. | Ferry, A.; Billard, T.; Langlois, B. R.; Bacqué, E. J. Org. Chem. 2008, 73, 9362–9365. doi:10.1021/jo8018544 |
| 37. | Ferry, A.; Billard, T.; Langlois, B. R.; Bacqué, E. J. Org. Chem. 2008, 73, 9362–9365. doi:10.1021/jo8018544 |
| 36. | Various nomenclatures have been used for these compounds in previous publications, including our own.According to the IUPAC rules it appears that the true name must be trifluoromethanesulfenamide. Alternatively, trifluoromethylsulfanylamine could be used. |
| 16. | Firestone, R. A.; Barker, P. L.; Pisano, J. M.; Ashe, B. M.; Dahlgren, M. E. Tetrahedron 1990, 46, 2255–2262. doi:10.1016/S0040-4020(01)82006-8 |
| 22. | Borowski, H. E.; Haas, A. Chem. Ber. 1982, 115, 523–532. doi:10.1002/cber.19821150215 |
| 23. | Ceacareanu, D. M.; Gerstenberger, M. R. C.; Haas, A. Chem. Ber. 1983, 116, 3325–3331. doi:10.1002/cber.19831161008 |
| 24. | Geisel, M.; Mews, R. Chem. Ber. 1987, 120, 1675–1677. doi:10.1002/cber.19871201011 |
| 25. | Gerstenberger, M. R. C.; Haas, A.; Wille, R.; Yazdanbakhsch, M. Rev. Chim. Miner. 1986, 23, 485–496. |
| 26. | Gupta, O. D.; Kamil, W. A.; Shreeve, J. M. Inorg. Chem. 1985, 24, 2126–2129. doi:10.1021/ic00208a004 |
| 27. | Haas, A.; Lieb, M. J. Heterocycl. Chem. 1986, 23, 1079–1084. doi:10.1002/jhet.5570230423 |
| 28. | Haas, A.; Lieb, M.; Schwederski, B. Rev. Roum. Chim. 1987, 32, 1219–1224. |
| 29. | Kolasa, A.; Lieb, M. J. Fluorine Chem. 1995, 70, 45–47. doi:10.1016/0022-1139(94)03086-F |
| 30. | Kost, D.; Egozy, H. J. Org. Chem. 1989, 54, 4909–4913. doi:10.1021/jo00281a038 |
| 31. | Munavalli, S.; Rohrbaugh, D. K.; Rossman, D. I.; Berg, F. J.; Wagner, G. W.; Durst, H. D. Synth. Commun. 2000, 30, 2847–2854. doi:10.1080/00397910008087435 |
| 32. | Munavalli, S.; Rohrbaugh, D. K.; Rossman, D. I.; Durst, H. D. Phosphorus, Sulfur Silicon Relat. Elem. 2002, 177, 1109–1116. doi:10.1080/10426500211704 |
| 33. | Munavalli, S.; Rohrbaugh, D. K.; Rossman, D. I.; Wagner, W. G.; Durst, H. D. Phosphorus, Sulfur Silicon Relat. Elem. 2003, 178, 107–113. doi:10.1080/10426500307779 |
| 34. | Peach, M. E. Can. J. Chem. 1967, 45, 429–432. doi:10.1139/v67-076 |
| 35. | Yagupol'skii, L. M.; Bezdudnyi, A. V.; Yagupol'skii, Yu. L. Russ. J. Org. Chem. 2006, 42, 1275–1279. doi:10.1134/S1070428006090041 |
| 38. |
Baert, F.; Colomb, J.; Billard, T. Angew. Chem. 2012, 124, 10528–10531. doi:10.1002/ange.201205156
Angew. Chem., Int. Ed. 2012, 51, 10382–10385. doi:10.1002/anie.201205156 |
| 44. |
Alazet, S.; Zimmer, L.; Billard, T. Angew. Chem. 2013, 125, 11014–11017. doi:10.1002/ange.201305179
Angew. Chem., Int. Ed. 2013, 52, 10814–10817. doi:10.1002/anie.201305179 |
© 2013 Alazet et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)