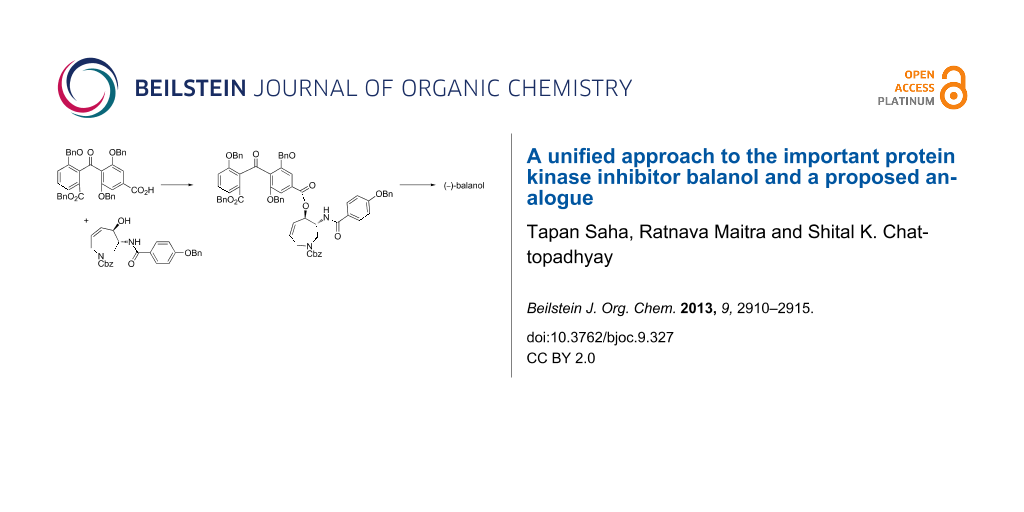
Abstract
A common approach to the important protein kinase inhibitor (−)-balanol and an azepine-ring-modified balanol derivative has been developed using an efficient fragment coupling protocol which proceeded in good overall yield.
Graphical Abstract
Introduction
Protein kinase C (PKC) is a family of phospholipid-dependent kinases that phosphorylate serine and threonine residues of a substrate protein by transferring a phosphate group from ATP to the substrate protein [1-3]. This phosphorylation induces conformational changes of the substrate protein leading to initiation of a number of cellular events including signal transduction [4,5]. The human PKC enzyme comprises of a number of isozymes and inappropriate activation of PKC has been linked to a variety of disorders [6,7]. The development of selective PKC inhibitors as novel therapeutics has therefore remained significant [8-14].
Balanol ((−)-1, Figure 1), a fungal metabolite [15] is known to inhibit a number of PKC isozymes at nanomolar concentrations [16], a finding that has motivated research related to the total- [17-26] or fragment synthesis [27-47] of this important natural product. Based on the information [48,49] that balanol binds to the ATP-docking site of protein kinase, all the three distinct domains present in the natural product such as the benzophenone core [50-52], the azepine core [53-59] and the p-hydroxybenzamide [60,61] unit have been targeted for analogue design in the quest for a more selective drug candidate over the last two decades. Although remarkable achievements have been made, the development of a unified synthetic strategy that would allow access to the natural product itself as well as some of its analogues remains important. A similar target is the closely related natural product ophiocordin (2). Herein, we describe a general approach to some of these targets.
Figure 1: Balanol (1) and ophiocordin (2).
Figure 1: Balanol (1) and ophiocordin (2).
Results and Discussion
The key feature of our retrosynthetic analysis (Figure 2) is the identification of the dehydro derivative of balanol 4 as the unified precursor of balanol (1) and an azepin ring-modified balanol 3. Derivative 4 could be obtained through esterification between the carboxylic acid 5 and the allylic alcohol 6.
Figure 2: Strategic bond disconnections of balanol.
Figure 2: Strategic bond disconnections of balanol.
We thus focused on the synthesis of the two key fragments 5 and 6. The synthesis of the benzophenone unit has previously been achieved by several groups [27-30]. We adopted some of these methodologies with a number of modifications to prepare fragment 5 in its protected form 7 (Scheme 1). At first, the reaction of the known [17] bromo compound 8 with the known [27] aldehyde 9 in the presence of butyllithium effected a smooth conversion to the new benzylic alcohol 10. The latter was oxidized with tetrapropylammonium perruthenate to provide the benzophenone derivative 11 in good yield. Subsequent cleavage of the 1,3-dioxane unit followed by oxidation of the resulting aldehyde 12 furnished carboxylic acid 13 in 73% overall yield over two steps. Concomitant removal of the phenolic MOM ether and the alcoholic TBDPS ether protecting groups in 13 under acidic conditions proceeded without significant loss of product to provide the dihydroxy acid 14 in good yield. Reaction of 14 with an excess of benzyl bromide in the presence of K2CO3 afforded simultaneous protection of the phenolic OH and the carboxylic acid functions leaving the primary alcohol function unprotected, as desired. Compound 15 was then converted following a literature procedure into the known [17] benzophenone 7 through two consecutive oxidations involving the aldehyde 16 as the intermediate. Taken as a hole the described synthesis of 7 from 8 and 9 proceeded in eight linear steps in an overall yield of 22%.
Scheme 1: Synthesis of the benzophenone fragment of balanol.
Scheme 1: Synthesis of the benzophenone fragment of balanol.
The synthesis of the azepine unit [31-47] was achieved following our preliminary report [62]. Thus, reductive amination of Garner’s aldehyde 17 (Scheme 2) with allylamine produced amine 18 which was N-protected with CbzCl to obtain 19 in an overall yield of 89% over three steps. The oxazolidine ring in compound 19 was then cleaved under acidic conditions and the resulting primary alcohol 20 was oxidized carefully under modified Swern conditions [63] to provide the α-chiral aldehyde 21 which was used directly in the next step. Addition of vinylmagnesium bromide to aldehyde 21 under optimized conditions gave a separable mixture of the allylic alcohols 22 and 23 in a combined yield of 64% over two steps. The undesired anti-isomer 23 could be effectively converted to the desired syn-isomer 22 by a Mitsunobu-type inversion [64].
Scheme 2: Synthesis of the hexahydroazepine core of balanol.
Scheme 2: Synthesis of the hexahydroazepine core of balanol.
The major syn-isomer 22 was then acetylated and the resulting diene 24 was subjected to ring-closing metathesis [65] in the presence of Grubbs’ second generation catalyst, benzylidene[1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro-(tricyclohexylphosphine)ruthenium (25). Pleasingly, the desired cycloalkene 26 was obtained in a gratifying yield of 89%. The sequential removal of the O-acetyl group leading to 27 followed by removal of the N-Boc group in the latter was executed under standard conditions to provide amine 28. This was then coupled with 4-benzyloxybenzoic acid using EDC as activating agent to obtain the corresponding amide derivative 29 in an overall yield of 20% over eleven steps from 17. The stereochemical identity of this tetrahydroazepine derivative was confirmed by its selective conversion to the corresponding known azepane derivative 30 which displayed optical and 13C NMR data nearly overlapping with those reported by Nicolaou et al [17].
With the two key fragments 29 and 7 in hand, we next focused on their convergent combination. The esterification of the allylic alcohol 29 with the acid 7 (Scheme 3) proceeded best in the presence of Mukaiyama’s reagent [66], 2-chloro-1-methylpyridinium iodide, to provide the ester 31 in 73% yield. Simultaneous hydrogenolytic removal of the O-benzyl groups and the N-Cbz group under reported conditions finally provided the natural (−)-balanol in a yield of 41%. The product thus obtained displayed spectroscopic and optical data in close agreement to those reported for natural balanol [17].
Scheme 3: Synthesis of balanol and an analogue.
Scheme 3: Synthesis of balanol and an analogue.
We next focused our attention to demonstrate the utility of the intermediate coupled product 31 in a possible synthesis of an azepane ring-modified balanol derivative along the projected pathway. To this end, dihydroxylation of the adduct 31 was next attempted. Pleasingly, the dihydroxylation of 31 proceeded smoothly; however, unfortunately to provide an inseparable mixture of the two possible dihydroxylated isomers 32 in a combined yield of 68%. The isomeric composition of 32 was determined to be 81:19 by HPLC.
Conclusion
In conclusion, we have developed a concise synthetic approach to the naturally occurring (−)-balanol (1) from easily available starting materials and reagents. Most of the synthetic steps proceeded in good to very good overall yield and stereocontrol. The developed synthesis may therefore be a complement to the existing literature. An attempted synthesis of an azepane ring-modified balanol derivative from a common precursor unfortunately was unsuccessful due to difficulty in separating stereoisomeric products. However, the intermediate 31 may prove to be useful in the synthesis of other analogues.
Supporting Information
| Supporting Information File 1: Experimental details and characterization data for the prepared compounds, copies of 1H and 13C NMR spectra of all new compounds, and data for the comparison of 30 and 1 with reported data. | ||
| Format: PDF | Size: 3.6 MB | Download |
References
-
Nishizuka, Y. Nature 1984, 308, 693–698. doi:10.1038/308693a0
Return to citation in text: [1] -
Nishizuka, Y. Science 1986, 233, 305–312. doi:10.1126/science.3014651
Return to citation in text: [1] -
Newton, A. C. J. Biol. Chem. 1995, 270, 28495–28498. doi:10.1074/jbc.270.48.28495
Return to citation in text: [1] -
Nishizuka, Y. FASEB J. 1995, 9, 484–496.
Return to citation in text: [1] -
Roffey, J.; Rosse, C.; Linch, M.; Hibbert, A.; McDonald, N. Q.; Parker, P. J. Curr. Opin. Cell Biol. 2009, 21, 268–279. doi:10.1016/j.ceb.2009.01.019
Return to citation in text: [1] -
Wong, C. F.; Bairy, S. Curr. Pharm. Des. 2013, 19, 4739–4754. doi:10.2174/1381612811319260006
Return to citation in text: [1] -
Mochly-Rosen, D.; Das, K.; Grimes, K. V. Nat. Rev. Drug Discovery 2012, 11, 937–957. doi:10.1038/nrd3871
Return to citation in text: [1] -
Nishizuka, Y. Nature 2002, 334, 661–665. doi:10.1038/334661a0
Return to citation in text: [1] -
Mellor, H.; Parker, P. J. Biochem. J. 1998, 332, 281–292.
Return to citation in text: [1] -
Gomez, D. E.; Skilton, G.; Alonso, D. F.; Kazanietz, M. G. Oncol. Rep. 1999, 6, 1363–1370.
Return to citation in text: [1] -
Lee, M. R.; Duan, W.; Tan, S.-L. Expert Opin. Ther. Targets 2008, 12, 535–552. doi:10.1517/14728222.12.5.535
Return to citation in text: [1] -
Teicher, B. A. Clin. Cancer Res. 2006, 12, 5336–5345. doi:10.1158/1078-0432.CCR-06-0945
Return to citation in text: [1] -
Hofmann, J. Curr. Cancer Drug Targets 2004, 4, 125–146. doi:10.2174/1568009043481579
Return to citation in text: [1] -
Cohen, P. Nat. Rev. Drug Discovery 2002, 1, 309–315. doi:10.1038/nrd773
Return to citation in text: [1] -
Kulanthaivel, P.; Hallock, Y. F.; Boros, C.; Hamilton, S. M.; Janzen, W. P.; Ballas, L. M.; Loomis, C. R.; Jiang, J. B. J. Am. Chem. Soc. 1993, 115, 6452–6453. doi:10.1021/ja00067a087
Return to citation in text: [1] -
Ohshima, S.; Yanagisawa, M.; Katoh, A.; Fujii, T.; Sano, T.; Matsukuma, S.; Furumai, T.; Fujiu, M.; Watanabe, K.; Yokose, K.; Arisawa, M.; Okuda, T. J. Antibiot. 1994, 47, 639–647. doi:10.7164/antibiotics.47.639
Return to citation in text: [1] -
Nicolaou, K. C.; Bunnage, M. E.; Koide, K. J. Am. Chem. Soc. 1994, 116, 8402–8403. doi:10.1021/ja00097a072
Return to citation in text: [1] [2] [3] [4] [5] -
Lampe, J. W.; Hughes, P. F.; Biggers, C. K.; Smith, S. H.; Hu, H. J. Org. Chem. 1994, 59, 5147–5148. doi:10.1021/jo00097a014
Return to citation in text: [1] -
Lampe, J. W.; Hughes, P. F.; Biggers, C. K.; Smith, S. H.; Hu, H. J. Org. Chem. 1996, 61, 4572–4581. doi:10.1021/jo952280k
Return to citation in text: [1] -
Adams, C. P.; Fairway, S. M.; Hardy, C. J.; Hibbs, D. E.; Hursthouse, M. B.; Morley, A. D.; Sharp, B. W.; Vicker, N.; Warner, I. J. Chem. Soc., Perkin Trans. 1 1995, 2355–2362. doi:10.1039/P19950002355
Return to citation in text: [1] -
Tanner, D.; Almario, A.; Högberg, T. Tetrahedron 1995, 51, 6061–6070. doi:10.1016/0040-4020(95)00264-9
Return to citation in text: [1] -
Tanner, D.; Tedenborg, L.; Almario, A.; Pettersson, I.; Csöregh, I.; Kelly, N. M.; Andersson, P. G.; Högberg, T. Tetrahedron 1997, 53, 4857–4868. doi:10.1016/S0040-4020(97)00167-1
Return to citation in text: [1] -
Barbier, P.; Stadlwieser, J. Chimia 1996, 50, 530–532.
Return to citation in text: [1] -
Miyabe, H.; Torieda, M.; Kiguchi, T.; Naito, T. Synlett 1997, 580–582. doi:10.1055/s-1997-3236
Return to citation in text: [1] -
Miyabe, H.; Torieda, M.; Inoue, K.; Tajiri, K.; Kiguchi, T.; Naito, T. J. Org. Chem. 1998, 63, 4397–4407. doi:10.1021/jo980208r
Return to citation in text: [1] -
Srivastava, A. K.; Panda, G. Chem.–Eur. J. 2008, 14, 4675–4688. doi:10.1002/chem.200701991
Return to citation in text: [1] -
Hollinshead, S. P.; Nichols, J. B.; Wilson, J. W. J. Org. Chem. 1994, 59, 6703–6709. doi:10.1021/jo00101a032
Return to citation in text: [1] [2] [3] -
Storm, J. P.; Andersson, C.-M. Org. Lett. 1999, 1, 1451–1453. doi:10.1021/ol9910060
Return to citation in text: [1] [2] -
Laursen, B.; Denieul, M.-P.; Skrydstrup, T. Tetrahedron 2002, 58, 2231–2238. doi:10.1016/S0040-4020(02)00096-0
Return to citation in text: [1] [2] -
Patil, M. L.; Deshpande, V. H.; Ramlingam, S.; Borate, H. B. Tetrahedron 2004, 60, 1869–1873. doi:10.1016/j.tet.2003.12.029
Return to citation in text: [1] [2] -
Oh, H.-S.; Kang, H.-Y. Bull. Korean Chem. Soc. 2012, 33, 3895–3898. doi:10.5012/bkcs.2012.33.11.3895
Return to citation in text: [1] [2] -
Louvel, J.; Chemla, F.; Demont, E.; Ferreira, F.; Pérez-Luna, A.; Voituriez, A. Adv. Synth. Catal. 2011, 353, 2137–2151. doi:10.1002/adsc.201100333
Return to citation in text: [1] [2] -
Hudlický, T. Pure Appl. Chem. 2010, 82, 1785–1796. doi:10.1351/PAC-CON-09-10-07
Return to citation in text: [1] [2] -
Gilmet, J.; Sullivan, B.; Hudlicky, T. Tetrahedron 2009, 65, 212–220. doi:10.1016/j.tet.2008.10.070
Return to citation in text: [1] [2] -
Trost, B. M.; Fandrick, D. R.; Brodmann, T.; Stiles, T. Angew. Chem., Int. Ed. 2007, 46, 6123–6125. doi:10.1002/anie.200700835
Return to citation in text: [1] [2] -
Unthank, M. G.; Hussain, N.; Aggarwal, V. K. Angew. Chem., Int. Ed. 2006, 45, 7066–7069. doi:10.1002/anie.200602782
Return to citation in text: [1] [2] -
Raghavan, S.; Kumar, C. N. Tetrahedron Lett. 2006, 47, 1585–1588. doi:10.1016/j.tetlet.2005.12.123
Return to citation in text: [1] [2] -
Yadav, J. S.; Srinivas, C. Tetrahedron 2003, 59, 10325–10329. doi:10.1016/j.tet.2003.09.089
Return to citation in text: [1] [2] -
Fürstner, A.; Thiel, O. R. J. Org. Chem. 2000, 65, 1738–1742. doi:10.1021/jo991611g
Return to citation in text: [1] [2] -
Masse, C. E.; Morgan, A. J.; Panek, J. S. Org. Lett. 2000, 2, 2571–2573. doi:10.1021/ol0061034
Return to citation in text: [1] [2] -
Riber, D.; Hazell, R.; Skrydstrup, T. J. Org. Chem. 2000, 65, 5382–5390. doi:10.1021/jo000538n
Return to citation in text: [1] [2] -
Phansavath, P.; de Paule, S. D.; Ratovelomanana-Vidal, V.; Genêt, J.-P. Eur. J. Org. Chem. 2000, 3903–3907. doi:10.1002/1099-0690(200012)2000:23<3903::AID-EJOC3903>3.0.CO;2-Q
Return to citation in text: [1] [2] -
Cook, G. R.; Shanker, P. S.; Peterson, S. L. Org. Lett. 1999, 1, 615–618. doi:10.1021/ol990705+
Return to citation in text: [1] [2] -
Wu, M. H.; Jacobsen, E. N. Tetrahedron Lett. 1997, 38, 1693–1696. doi:10.1016/S0040-4039(97)00192-5
Return to citation in text: [1] [2] -
Albertini, E.; Barco, A.; Benetti, S.; Risi, C. D.; Pollini, G. P.; Zanirato, V. Tetrahedron 1997, 53, 17177–17194. doi:10.1016/S0040-4020(97)10139-9
Return to citation in text: [1] [2] -
Naito, T.; Torieda, M.; Tajiri, K.; Ninomiya, I.; Kiguchi, T. Chem. Pharm. Bull. 1996, 44, 624–626. doi:10.1248/cpb.44.624
Return to citation in text: [1] [2] -
Hu, H.; Jagdmann, G. E., Jr.; Hughes, P. F.; Nichols, J. B. Tetrahedron Lett. 1995, 36, 3659–3662. doi:10.1016/0040-4039(95)00623-K
Return to citation in text: [1] [2] -
Narayana, N.; Diller, T. C.; Koide, K.; Bunnage, M. E.; Nicolaou, K. C.; Brunton, L. L.; Xuong, N.-H.; Ten Eyck, L. F.; Taylor, S. S. Biochemistry 1999, 38, 2367–2376. doi:10.1021/bi9820659
Return to citation in text: [1] -
Koide, K.; Bunnage, M. E.; Gomez Paloma, L.; Kanter, J. P.; Taylor, S. S.; Brunton, L. L.; Nicolaou, K. C. Chem. Biol. 1995, 2, 601–608. doi:10.1016/1074-5521(95)90124-8
Return to citation in text: [1] -
Nicolaou, K. C.; Koide, K.; Bunnage, M. E. Chem.–Eur. J. 1995, 1, 454–466. doi:10.1002/chem.19950010711
Return to citation in text: [1] -
Lampe, J. W.; Biggers, C. K.; Defauw, J. M.; Foglesong, R. J.; Hall, S. E.; Heerding, J. M.; Hollinshead, S. P.; Hu, H.; Hughes, P. F.; Jagdmann, G. E., Jr.; Johnson, M. G.; Lai, Y.-S.; Lowden, C. T.; Lynch, M. P.; Mendoza, J. S.; Murphy, M. M.; Wilson, J. W.; Ballas, L. M.; Carter, K.; Darges, J. W.; Davis, J. E.; Hubbard, F. R.; Stamper, M. L. J. Med. Chem. 2002, 45, 2624–2643. doi:10.1021/jm020018f
Return to citation in text: [1] -
Breitenlechner, C. B.; Wegge, T.; Berillon, L.; Graul, K.; Marzenell, K.; Friebe, W.-G.; Thomas, U.; Schumacher, R.; Huber, R.; Engh, R. A.; Masjost, B. J. Med. Chem. 2004, 47, 1375–1390. doi:10.1021/jm0310479
Return to citation in text: [1] -
Lai, Y.-S.; Stamper, M. Bioorg. Med. Chem. Lett. 1995, 5, 2147–2150. doi:10.1016/0960-894X(95)00364-Y
Return to citation in text: [1] -
Lai, Y.-S.; Menaldino, D. S.; Nichols, J. B.; Jagdmann, G. E., Jr..; Mylott, F.; Gillespie, J.; Hall, S. E. Bioorg. Med. Chem. Lett. 1995, 5, 2151–2154. doi:10.1016/0960-894X(95)00365-Z
Return to citation in text: [1] -
Mendoza, J. S.; Jagdmann, G. E., Jr.; Gosnell, P. A. Bioorg. Med. Chem. Lett. 1995, 5, 2211–2216. doi:10.1016/0960-894X(95)00382-4
Return to citation in text: [1] -
Crane, H. M.; Menaldino, D. S.; Jagdmann, G. E., Jr.; Darges, J. W.; Buben, J. A. Bioorg. Med. Chem. Lett. 1995, 5, 2133–2138. doi:10.1016/0960-894X(95)00361-V
Return to citation in text: [1] -
Defauw, J. M.; Murphy, M. M.; Jagdmann, G. E., Jr.; Hu, H.; Lampe, J. W.; Hollinshead, S. P.; Mitchell, T. J.; Crane, H. M.; Heerding, J. M.; Mendoza, J. S.; Davis, J. E.; Darges, J. W.; Hubbard, F. R.; Hall, S. E. J. Med. Chem. 1996, 39, 5215–5227. doi:10.1021/jm960581w
Return to citation in text: [1] -
Hu, H.; Hollinshead, S. P.; Hall, S. E.; Kalter, K.; Ballas, L. M. Bioorg. Med. Chem. Lett. 1996, 6, 973–978. doi:10.1016/0960-894X(96)00151-5
Return to citation in text: [1] -
Lai, Y.-S.; Mendoza, J. S.; Jagdmann, G. E., Jr.; Menaldino, D. S.; Biggers, C. K.; Heerding, J. M.; Wilson, J. W.; Hall, S. E.; Jiang, J. B.; Janzen, W. P.; Ballas, L. M. J. Med. Chem. 1997, 40, 226–235. doi:10.1021/jm960497g
Return to citation in text: [1] -
Jagdmann, G. E., Jr.; Dafauw, J. M.; Lampe, J. W.; Darges, J. W.; Kalter, K. Bioorg. Med. Chem. Lett. 1996, 6, 1759–1764. doi:10.1016/0960-894X(96)00311-3
Return to citation in text: [1] -
Hu, H.; Mendoza, J. S.; Lowden, C. T.; Ballas, L. M.; Janzen, W. P. Bioorg. Med. Chem. 1997, 5, 1873–1882. doi:10.1016/S0968-0896(97)00125-9
Return to citation in text: [1] -
Roy, S. P.; Chattopadhyay, S. K. Tetrahedron Lett. 2008, 49, 5498–5501. doi:10.1016/j.tetlet.2008.07.031
Return to citation in text: [1] -
Mancuso, A. J.; Swern, D. Synthesis 1981, 165–185. doi:10.1055/s-1981-29377
Return to citation in text: [1] -
Grubbs, R. H.; Schrock, R. R.; Fürstner, A., Eds. Olefin Metathesis. Adv. Synth. Catal. 2007, 349, 1–265. doi:10.1002/adsc.200790000
Return to citation in text: [1] -
Mukaiyama, T. Angew. Chem., Int. Ed. Engl. 1979, 18, 707–721. doi:10.1002/anie.197907073
Return to citation in text: [1] -
Mitsunobu, O. Synthesis 1981, 1–28. doi:10.1055/s-1981-29317
Return to citation in text: [1]
| 31. | Oh, H.-S.; Kang, H.-Y. Bull. Korean Chem. Soc. 2012, 33, 3895–3898. doi:10.5012/bkcs.2012.33.11.3895 |
| 32. | Louvel, J.; Chemla, F.; Demont, E.; Ferreira, F.; Pérez-Luna, A.; Voituriez, A. Adv. Synth. Catal. 2011, 353, 2137–2151. doi:10.1002/adsc.201100333 |
| 33. | Hudlický, T. Pure Appl. Chem. 2010, 82, 1785–1796. doi:10.1351/PAC-CON-09-10-07 |
| 34. | Gilmet, J.; Sullivan, B.; Hudlicky, T. Tetrahedron 2009, 65, 212–220. doi:10.1016/j.tet.2008.10.070 |
| 35. | Trost, B. M.; Fandrick, D. R.; Brodmann, T.; Stiles, T. Angew. Chem., Int. Ed. 2007, 46, 6123–6125. doi:10.1002/anie.200700835 |
| 36. | Unthank, M. G.; Hussain, N.; Aggarwal, V. K. Angew. Chem., Int. Ed. 2006, 45, 7066–7069. doi:10.1002/anie.200602782 |
| 37. | Raghavan, S.; Kumar, C. N. Tetrahedron Lett. 2006, 47, 1585–1588. doi:10.1016/j.tetlet.2005.12.123 |
| 38. | Yadav, J. S.; Srinivas, C. Tetrahedron 2003, 59, 10325–10329. doi:10.1016/j.tet.2003.09.089 |
| 39. | Fürstner, A.; Thiel, O. R. J. Org. Chem. 2000, 65, 1738–1742. doi:10.1021/jo991611g |
| 40. | Masse, C. E.; Morgan, A. J.; Panek, J. S. Org. Lett. 2000, 2, 2571–2573. doi:10.1021/ol0061034 |
| 41. | Riber, D.; Hazell, R.; Skrydstrup, T. J. Org. Chem. 2000, 65, 5382–5390. doi:10.1021/jo000538n |
| 42. | Phansavath, P.; de Paule, S. D.; Ratovelomanana-Vidal, V.; Genêt, J.-P. Eur. J. Org. Chem. 2000, 3903–3907. doi:10.1002/1099-0690(200012)2000:23<3903::AID-EJOC3903>3.0.CO;2-Q |
| 43. | Cook, G. R.; Shanker, P. S.; Peterson, S. L. Org. Lett. 1999, 1, 615–618. doi:10.1021/ol990705+ |
| 44. | Wu, M. H.; Jacobsen, E. N. Tetrahedron Lett. 1997, 38, 1693–1696. doi:10.1016/S0040-4039(97)00192-5 |
| 45. | Albertini, E.; Barco, A.; Benetti, S.; Risi, C. D.; Pollini, G. P.; Zanirato, V. Tetrahedron 1997, 53, 17177–17194. doi:10.1016/S0040-4020(97)10139-9 |
| 46. | Naito, T.; Torieda, M.; Tajiri, K.; Ninomiya, I.; Kiguchi, T. Chem. Pharm. Bull. 1996, 44, 624–626. doi:10.1248/cpb.44.624 |
| 47. | Hu, H.; Jagdmann, G. E., Jr.; Hughes, P. F.; Nichols, J. B. Tetrahedron Lett. 1995, 36, 3659–3662. doi:10.1016/0040-4039(95)00623-K |
| 62. | Roy, S. P.; Chattopadhyay, S. K. Tetrahedron Lett. 2008, 49, 5498–5501. doi:10.1016/j.tetlet.2008.07.031 |
| 1. | Nishizuka, Y. Nature 1984, 308, 693–698. doi:10.1038/308693a0 |
| 2. | Nishizuka, Y. Science 1986, 233, 305–312. doi:10.1126/science.3014651 |
| 3. | Newton, A. C. J. Biol. Chem. 1995, 270, 28495–28498. doi:10.1074/jbc.270.48.28495 |
| 15. | Kulanthaivel, P.; Hallock, Y. F.; Boros, C.; Hamilton, S. M.; Janzen, W. P.; Ballas, L. M.; Loomis, C. R.; Jiang, J. B. J. Am. Chem. Soc. 1993, 115, 6452–6453. doi:10.1021/ja00067a087 |
| 27. | Hollinshead, S. P.; Nichols, J. B.; Wilson, J. W. J. Org. Chem. 1994, 59, 6703–6709. doi:10.1021/jo00101a032 |
| 8. | Nishizuka, Y. Nature 2002, 334, 661–665. doi:10.1038/334661a0 |
| 9. | Mellor, H.; Parker, P. J. Biochem. J. 1998, 332, 281–292. |
| 10. | Gomez, D. E.; Skilton, G.; Alonso, D. F.; Kazanietz, M. G. Oncol. Rep. 1999, 6, 1363–1370. |
| 11. | Lee, M. R.; Duan, W.; Tan, S.-L. Expert Opin. Ther. Targets 2008, 12, 535–552. doi:10.1517/14728222.12.5.535 |
| 12. | Teicher, B. A. Clin. Cancer Res. 2006, 12, 5336–5345. doi:10.1158/1078-0432.CCR-06-0945 |
| 13. | Hofmann, J. Curr. Cancer Drug Targets 2004, 4, 125–146. doi:10.2174/1568009043481579 |
| 14. | Cohen, P. Nat. Rev. Drug Discovery 2002, 1, 309–315. doi:10.1038/nrd773 |
| 17. | Nicolaou, K. C.; Bunnage, M. E.; Koide, K. J. Am. Chem. Soc. 1994, 116, 8402–8403. doi:10.1021/ja00097a072 |
| 6. | Wong, C. F.; Bairy, S. Curr. Pharm. Des. 2013, 19, 4739–4754. doi:10.2174/1381612811319260006 |
| 7. | Mochly-Rosen, D.; Das, K.; Grimes, K. V. Nat. Rev. Drug Discovery 2012, 11, 937–957. doi:10.1038/nrd3871 |
| 27. | Hollinshead, S. P.; Nichols, J. B.; Wilson, J. W. J. Org. Chem. 1994, 59, 6703–6709. doi:10.1021/jo00101a032 |
| 28. | Storm, J. P.; Andersson, C.-M. Org. Lett. 1999, 1, 1451–1453. doi:10.1021/ol9910060 |
| 29. | Laursen, B.; Denieul, M.-P.; Skrydstrup, T. Tetrahedron 2002, 58, 2231–2238. doi:10.1016/S0040-4020(02)00096-0 |
| 30. | Patil, M. L.; Deshpande, V. H.; Ramlingam, S.; Borate, H. B. Tetrahedron 2004, 60, 1869–1873. doi:10.1016/j.tet.2003.12.029 |
| 17. | Nicolaou, K. C.; Bunnage, M. E.; Koide, K. J. Am. Chem. Soc. 1994, 116, 8402–8403. doi:10.1021/ja00097a072 |
| 4. | Nishizuka, Y. FASEB J. 1995, 9, 484–496. |
| 5. | Roffey, J.; Rosse, C.; Linch, M.; Hibbert, A.; McDonald, N. Q.; Parker, P. J. Curr. Opin. Cell Biol. 2009, 21, 268–279. doi:10.1016/j.ceb.2009.01.019 |
| 17. | Nicolaou, K. C.; Bunnage, M. E.; Koide, K. J. Am. Chem. Soc. 1994, 116, 8402–8403. doi:10.1021/ja00097a072 |
| 48. | Narayana, N.; Diller, T. C.; Koide, K.; Bunnage, M. E.; Nicolaou, K. C.; Brunton, L. L.; Xuong, N.-H.; Ten Eyck, L. F.; Taylor, S. S. Biochemistry 1999, 38, 2367–2376. doi:10.1021/bi9820659 |
| 49. | Koide, K.; Bunnage, M. E.; Gomez Paloma, L.; Kanter, J. P.; Taylor, S. S.; Brunton, L. L.; Nicolaou, K. C. Chem. Biol. 1995, 2, 601–608. doi:10.1016/1074-5521(95)90124-8 |
| 53. | Lai, Y.-S.; Stamper, M. Bioorg. Med. Chem. Lett. 1995, 5, 2147–2150. doi:10.1016/0960-894X(95)00364-Y |
| 54. | Lai, Y.-S.; Menaldino, D. S.; Nichols, J. B.; Jagdmann, G. E., Jr..; Mylott, F.; Gillespie, J.; Hall, S. E. Bioorg. Med. Chem. Lett. 1995, 5, 2151–2154. doi:10.1016/0960-894X(95)00365-Z |
| 55. | Mendoza, J. S.; Jagdmann, G. E., Jr.; Gosnell, P. A. Bioorg. Med. Chem. Lett. 1995, 5, 2211–2216. doi:10.1016/0960-894X(95)00382-4 |
| 56. | Crane, H. M.; Menaldino, D. S.; Jagdmann, G. E., Jr.; Darges, J. W.; Buben, J. A. Bioorg. Med. Chem. Lett. 1995, 5, 2133–2138. doi:10.1016/0960-894X(95)00361-V |
| 57. | Defauw, J. M.; Murphy, M. M.; Jagdmann, G. E., Jr.; Hu, H.; Lampe, J. W.; Hollinshead, S. P.; Mitchell, T. J.; Crane, H. M.; Heerding, J. M.; Mendoza, J. S.; Davis, J. E.; Darges, J. W.; Hubbard, F. R.; Hall, S. E. J. Med. Chem. 1996, 39, 5215–5227. doi:10.1021/jm960581w |
| 58. | Hu, H.; Hollinshead, S. P.; Hall, S. E.; Kalter, K.; Ballas, L. M. Bioorg. Med. Chem. Lett. 1996, 6, 973–978. doi:10.1016/0960-894X(96)00151-5 |
| 59. | Lai, Y.-S.; Mendoza, J. S.; Jagdmann, G. E., Jr.; Menaldino, D. S.; Biggers, C. K.; Heerding, J. M.; Wilson, J. W.; Hall, S. E.; Jiang, J. B.; Janzen, W. P.; Ballas, L. M. J. Med. Chem. 1997, 40, 226–235. doi:10.1021/jm960497g |
| 17. | Nicolaou, K. C.; Bunnage, M. E.; Koide, K. J. Am. Chem. Soc. 1994, 116, 8402–8403. doi:10.1021/ja00097a072 |
| 27. | Hollinshead, S. P.; Nichols, J. B.; Wilson, J. W. J. Org. Chem. 1994, 59, 6703–6709. doi:10.1021/jo00101a032 |
| 28. | Storm, J. P.; Andersson, C.-M. Org. Lett. 1999, 1, 1451–1453. doi:10.1021/ol9910060 |
| 29. | Laursen, B.; Denieul, M.-P.; Skrydstrup, T. Tetrahedron 2002, 58, 2231–2238. doi:10.1016/S0040-4020(02)00096-0 |
| 30. | Patil, M. L.; Deshpande, V. H.; Ramlingam, S.; Borate, H. B. Tetrahedron 2004, 60, 1869–1873. doi:10.1016/j.tet.2003.12.029 |
| 31. | Oh, H.-S.; Kang, H.-Y. Bull. Korean Chem. Soc. 2012, 33, 3895–3898. doi:10.5012/bkcs.2012.33.11.3895 |
| 32. | Louvel, J.; Chemla, F.; Demont, E.; Ferreira, F.; Pérez-Luna, A.; Voituriez, A. Adv. Synth. Catal. 2011, 353, 2137–2151. doi:10.1002/adsc.201100333 |
| 33. | Hudlický, T. Pure Appl. Chem. 2010, 82, 1785–1796. doi:10.1351/PAC-CON-09-10-07 |
| 34. | Gilmet, J.; Sullivan, B.; Hudlicky, T. Tetrahedron 2009, 65, 212–220. doi:10.1016/j.tet.2008.10.070 |
| 35. | Trost, B. M.; Fandrick, D. R.; Brodmann, T.; Stiles, T. Angew. Chem., Int. Ed. 2007, 46, 6123–6125. doi:10.1002/anie.200700835 |
| 36. | Unthank, M. G.; Hussain, N.; Aggarwal, V. K. Angew. Chem., Int. Ed. 2006, 45, 7066–7069. doi:10.1002/anie.200602782 |
| 37. | Raghavan, S.; Kumar, C. N. Tetrahedron Lett. 2006, 47, 1585–1588. doi:10.1016/j.tetlet.2005.12.123 |
| 38. | Yadav, J. S.; Srinivas, C. Tetrahedron 2003, 59, 10325–10329. doi:10.1016/j.tet.2003.09.089 |
| 39. | Fürstner, A.; Thiel, O. R. J. Org. Chem. 2000, 65, 1738–1742. doi:10.1021/jo991611g |
| 40. | Masse, C. E.; Morgan, A. J.; Panek, J. S. Org. Lett. 2000, 2, 2571–2573. doi:10.1021/ol0061034 |
| 41. | Riber, D.; Hazell, R.; Skrydstrup, T. J. Org. Chem. 2000, 65, 5382–5390. doi:10.1021/jo000538n |
| 42. | Phansavath, P.; de Paule, S. D.; Ratovelomanana-Vidal, V.; Genêt, J.-P. Eur. J. Org. Chem. 2000, 3903–3907. doi:10.1002/1099-0690(200012)2000:23<3903::AID-EJOC3903>3.0.CO;2-Q |
| 43. | Cook, G. R.; Shanker, P. S.; Peterson, S. L. Org. Lett. 1999, 1, 615–618. doi:10.1021/ol990705+ |
| 44. | Wu, M. H.; Jacobsen, E. N. Tetrahedron Lett. 1997, 38, 1693–1696. doi:10.1016/S0040-4039(97)00192-5 |
| 45. | Albertini, E.; Barco, A.; Benetti, S.; Risi, C. D.; Pollini, G. P.; Zanirato, V. Tetrahedron 1997, 53, 17177–17194. doi:10.1016/S0040-4020(97)10139-9 |
| 46. | Naito, T.; Torieda, M.; Tajiri, K.; Ninomiya, I.; Kiguchi, T. Chem. Pharm. Bull. 1996, 44, 624–626. doi:10.1248/cpb.44.624 |
| 47. | Hu, H.; Jagdmann, G. E., Jr.; Hughes, P. F.; Nichols, J. B. Tetrahedron Lett. 1995, 36, 3659–3662. doi:10.1016/0040-4039(95)00623-K |
| 60. | Jagdmann, G. E., Jr.; Dafauw, J. M.; Lampe, J. W.; Darges, J. W.; Kalter, K. Bioorg. Med. Chem. Lett. 1996, 6, 1759–1764. doi:10.1016/0960-894X(96)00311-3 |
| 61. | Hu, H.; Mendoza, J. S.; Lowden, C. T.; Ballas, L. M.; Janzen, W. P. Bioorg. Med. Chem. 1997, 5, 1873–1882. doi:10.1016/S0968-0896(97)00125-9 |
| 17. | Nicolaou, K. C.; Bunnage, M. E.; Koide, K. J. Am. Chem. Soc. 1994, 116, 8402–8403. doi:10.1021/ja00097a072 |
| 18. | Lampe, J. W.; Hughes, P. F.; Biggers, C. K.; Smith, S. H.; Hu, H. J. Org. Chem. 1994, 59, 5147–5148. doi:10.1021/jo00097a014 |
| 19. | Lampe, J. W.; Hughes, P. F.; Biggers, C. K.; Smith, S. H.; Hu, H. J. Org. Chem. 1996, 61, 4572–4581. doi:10.1021/jo952280k |
| 20. | Adams, C. P.; Fairway, S. M.; Hardy, C. J.; Hibbs, D. E.; Hursthouse, M. B.; Morley, A. D.; Sharp, B. W.; Vicker, N.; Warner, I. J. Chem. Soc., Perkin Trans. 1 1995, 2355–2362. doi:10.1039/P19950002355 |
| 21. | Tanner, D.; Almario, A.; Högberg, T. Tetrahedron 1995, 51, 6061–6070. doi:10.1016/0040-4020(95)00264-9 |
| 22. | Tanner, D.; Tedenborg, L.; Almario, A.; Pettersson, I.; Csöregh, I.; Kelly, N. M.; Andersson, P. G.; Högberg, T. Tetrahedron 1997, 53, 4857–4868. doi:10.1016/S0040-4020(97)00167-1 |
| 23. | Barbier, P.; Stadlwieser, J. Chimia 1996, 50, 530–532. |
| 24. | Miyabe, H.; Torieda, M.; Kiguchi, T.; Naito, T. Synlett 1997, 580–582. doi:10.1055/s-1997-3236 |
| 25. | Miyabe, H.; Torieda, M.; Inoue, K.; Tajiri, K.; Kiguchi, T.; Naito, T. J. Org. Chem. 1998, 63, 4397–4407. doi:10.1021/jo980208r |
| 26. | Srivastava, A. K.; Panda, G. Chem.–Eur. J. 2008, 14, 4675–4688. doi:10.1002/chem.200701991 |
| 64. | Grubbs, R. H.; Schrock, R. R.; Fürstner, A., Eds. Olefin Metathesis. Adv. Synth. Catal. 2007, 349, 1–265. doi:10.1002/adsc.200790000 |
| 16. | Ohshima, S.; Yanagisawa, M.; Katoh, A.; Fujii, T.; Sano, T.; Matsukuma, S.; Furumai, T.; Fujiu, M.; Watanabe, K.; Yokose, K.; Arisawa, M.; Okuda, T. J. Antibiot. 1994, 47, 639–647. doi:10.7164/antibiotics.47.639 |
| 50. | Nicolaou, K. C.; Koide, K.; Bunnage, M. E. Chem.–Eur. J. 1995, 1, 454–466. doi:10.1002/chem.19950010711 |
| 51. | Lampe, J. W.; Biggers, C. K.; Defauw, J. M.; Foglesong, R. J.; Hall, S. E.; Heerding, J. M.; Hollinshead, S. P.; Hu, H.; Hughes, P. F.; Jagdmann, G. E., Jr.; Johnson, M. G.; Lai, Y.-S.; Lowden, C. T.; Lynch, M. P.; Mendoza, J. S.; Murphy, M. M.; Wilson, J. W.; Ballas, L. M.; Carter, K.; Darges, J. W.; Davis, J. E.; Hubbard, F. R.; Stamper, M. L. J. Med. Chem. 2002, 45, 2624–2643. doi:10.1021/jm020018f |
| 52. | Breitenlechner, C. B.; Wegge, T.; Berillon, L.; Graul, K.; Marzenell, K.; Friebe, W.-G.; Thomas, U.; Schumacher, R.; Huber, R.; Engh, R. A.; Masjost, B. J. Med. Chem. 2004, 47, 1375–1390. doi:10.1021/jm0310479 |
| 65. | Mukaiyama, T. Angew. Chem., Int. Ed. Engl. 1979, 18, 707–721. doi:10.1002/anie.197907073 |
© 2013 Saha et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)