Abstract
Novel dihydro-1,3-oxazinoporphyrins and naphtho[e]bis(dihydro-1,3-oxazinoporphyrin) derivatives, in which the porphyrin macrocycle is covalently linked to the dihydro-1,3-oxazine ring system were successfully synthesized from 5-(4-aminophenyl)-10,15,20-triphenylporphyrin in good yields. The structures of the target products were established on the basis of spectral data and elemental analyses.
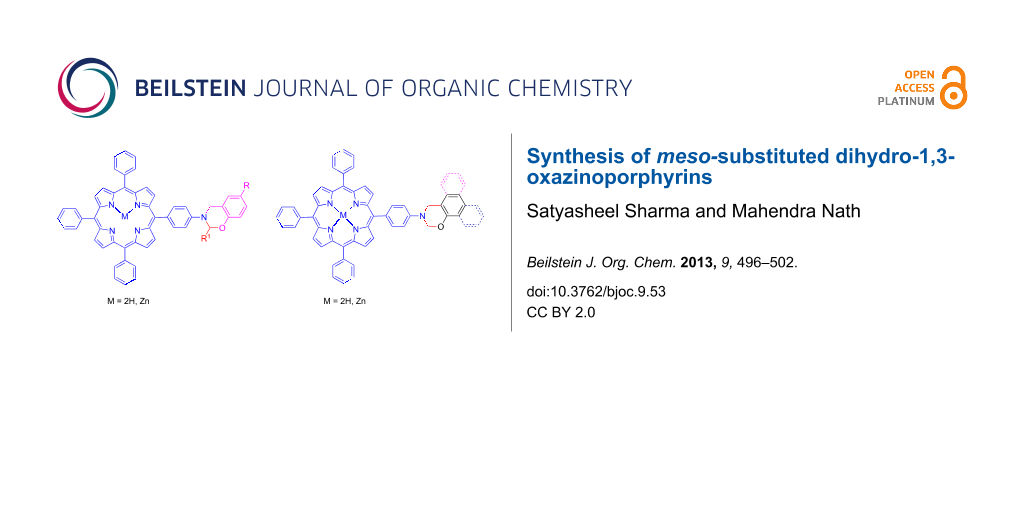
Graphical Abstract
Introduction
Porphyrin macrocycles are of crucial interest for their potential applications in diverse fields such as biomimetic models for photosynthesis [1,2], electronic materials [3], catalysis [4] and medicine [5,6]. In the past few decades, the synthesis of porphyrin derivatives has emerged as one of the major areas of research due to the success of these molecules for the eradication of malignant cells by photodynamic therapy (PDT) after their selective accumulation [7-10] in neoplastic tissues. In addition, the low dark-toxicity profile, easy removal from the tissue, and efficiency in generating reactive oxygen species by the absorption of photons in the visible or near IR region make them ideal candidates for developing effective photodynamic agents. These findings have encouraged researchers to design and synthesize potential targeting anticancer drugs derived from porphyrins [11,12]. Previously, a large number of these molecules have been synthesized by the coupling of diverse pharmaceutically important moieties, such as carbohydrates [13-15], amino acid residues [16-19], steroids [20,21], glycosides [22-24], nitroxyl derivatives [25], pyrrolidinone [26], pyrrolidine [27] and piperazine [28], to the porphyrin periphery. In addition, many porphyrin dimers and trimers have displayed significant biological efficacy [29] and some of these are used as photosensitizers in PDT applications for the treatment of various types of cancers [30].
Thorough literature search revealed that heterocycles containing a dihydro-1,3-oxazine ring system exhibit a wide spectrum of pharmacological activities, for example, acting as antimicrobial [31-33], anti-HIV [34], antimalarial [35] or antitumor agents [36,37]. By considering the anticancer significance of these two classes of molecules, it was contemplated to construct new dihydro-1,3-oxazinoporphyrins combining the porphyrin and dihydro-1,3-oxazine moieties in a single molecular framework. Such hybrid compounds may prove useful for pharmacological studies or in the development of new phototherapeutic agents. Therefore, in continuation of our efforts towards the synthesis of diverse porphyrin analogues [38-41] through peripheral functionalization of easily accessible meso-tetraarylporphyrins, we now report herein the first synthesis and spectroscopic characterization of a novel series of dihydro-1,3-oxazinoporphyrins.
Results and Discussion
The targeted dihydro-1,3-benzoxazinoporphyrins 6–9 were prepared in a three step procedure, starting from 5-(4-aminophenyl)-10,15,20-triphenylporphyrin (1), which was obtained by the reduction of 5-(4-nitrophenyl)-10,15,20-triphenylporphyrin using SnCl2 under acidic conditions [41,42]. Firstly, meso-(4-aminophenyl)porphyrin 1 was reacted with salicylaldehyde or 5-chlorosalicylaldehyde in the presence of La(OTf)3 as a Lewis acid catalyst in toluene under reflux to afford the corresponding iminoporphyrins 2 and 3, which on reduction by NaBH4 in a chloroform/methanol mixture at 25 °C produced meso-substituted aminoporphyrins 4 and 5, respectively. In the final step, these aminoporphyrins underwent a condensation cyclization reaction with aldehydes in THF under reflux to form new dihydro-1,3-benzoxazinoporphyrins 6–9 in good yields. Further, these free-base porphyrins were successfully converted to their zinc(II) analogues 10–13 by using Zn(OAc)2·2H2O as outlined in Scheme 1.
Scheme 1: Synthesis of dihydro-1,3-benzoxazinoporphyrins.
Scheme 1: Synthesis of dihydro-1,3-benzoxazinoporphyrins.
In contrast, the synthesis of new dihydro-1,3-naphthoxazinoporphyrins 14 and 16 was achieved in 75–85% yields in a one-pot three-component Mannich type condensation–cyclization reaction of 5-(4-aminophenyl)-10,15,20-triphenylporphyrin (1) with α- or β-naphthol and formaldehyde in THF under reflux. After reaction with Zn(OAc)2·2H2O in CHCl3/MeOH mixture, the free-base naphthoxazinoporphyrins 14 and 16 underwent zinc insertion to afford zinc (II) dihydro-1,3-naphthoxazinoporphyrins 15 and 17 in 90–92% yields as depicted in Scheme 2.
Scheme 2: Synthesis of dihydro-1,3-naphthoxazinoporphyrins.
Scheme 2: Synthesis of dihydro-1,3-naphthoxazinoporphyrins.
In addition, the dimeric naphthoxazinoporphyrins 18–20 were also prepared in moderate yields through one-pot Mannich-type condensation–cyclization reaction of 5-(4-aminophenyl)-10,15,20-triphenylporphyrin (1) with α,α- or β,β- or α,β-dihydroxynaphthalenes and formaldehyde in THF under reflux (Scheme 3). Attempts have also been made to prepare the corresponding zinc naphthoxazinoporphyrin dyads, but always impure products were obtained even after repeated column chromatography.
Scheme 3: Synthesis of naphtho[e]bis(dihydro-1,3-oxazinoporphyrin) derivatives.
Scheme 3: Synthesis of naphtho[e]bis(dihydro-1,3-oxazinoporphyrin) derivatives.
The newly synthesized porphyrins were characterized on the basis of 1H NMR, IR, mass and UV–vis data. The 1H NMR spectra of all the free-base porphyrins showed a singlet around δ −2.7 ppm corresponding to the internal NH protons. Iminoporphyrins 2 and 3 showed the OH and N=CH protons as singlets around 13.4 and 8.9 ppm, respectively. For the aminoporphyrins 4 and 5, the hydroxy group and NH protons appeared as broad singlets at 8.4 ppm and 4.3 ppm, respectively. The protons of the CH2 group appeared as a singlet at 4.6 ppm. The two characteristic peaks for the dihydro-1,3-oxazinoporphyrins 6, 7, 10, 11 and 14–20 corresponding to N–CH2–Ar and N–CH2–O groups appeared as two singlets between 4 and 6 ppm. In contrast, the proton NMR spectra of dihydro-1,3-oxazinoporphyrins 8, 9, 12 and 13 showed two doublets between 4 and 5 ppm corresponding to one proton each with a coupling constant of 16.5 Hz due to the coupling between two geminal protons of the N–CH2–Ar group, and a characteristic singlet around 6–7 ppm for the N–CH–O proton of the oxazine ring. The IR spectra of the porphyrins 2–5 showed a peak around 3400 cm−1 corresponding to the hydroxy group. Further, the disappearance of the hydroxy peak in the IR spectra of porphyrins 6–13 confirmed the formation of the oxazine ring. Further, the electronic absorption and emission data of all the synthesized porphyrins are presented in Table 1.
Table 1: Electronic absorption and emission data of porphyrins (2–20).
| Compound | Absorptiona λmax, nm (ε × 10−4 , M−1 cm−1) | Fluorescencea,b (λem/nm) |
|---|---|---|
| 2 | 421 (34.68), 518 (1.19), 554 (1.03), 593 (0.60), 648 (0.51) | 652, 717 |
| 3 | 421 (36.60), 517 (1.91), 553 (1.15), 592 (0.82), 647 (0.68) | 652, 718 |
| 4 | 421 (46.32), 518 (2.20), 553 (1.25), 592 (0.82), 648 (0.68) | 653, 717 |
| 5 | 421 (44.42), 518 (1.92), 555 (1.02), 592 (0.59), 648 (0.48) | 652, 718 |
| 6 | 421 (46.82), 518 (2.12), 554 (1.21), 592 (0.78), 649 (0.66) | 652, 717 |
| 7 | 421 (36.84), 518 (1.90), 554 (1.12), 592 (0.78), 648 (0.66) | 652, 717 |
| 8 | 421 (52.42), 518 (2.11), 553 (1.36), 592 (0.85), 648 (0.72) | 652, 718 |
| 9 | 421 (37.75), 518 (1.83), 553 (1.05), 592 (0.72), 647 (0.65) | 653, 718 |
| 10 | 426 (42.69), 556 (1.81), 597 (0.77) | 603, 652 |
| 11 | 426 (54.53), 555 (2.26), 597 (0.94) | 604, 654 |
| 12 | 426 (44.70), 556 (1.97), 597 (0.88) | 603, 654 |
| 13 | 426 (52.75), 555 (2.35), 597 (1.01) | 603, 652 |
| 14 | 421 (44.63), 518 (1.90), 554 (1.03), 593 (0.60), 648 (0.51) | 654, 717 |
| 15 | 426 (41.44), 556 (1.93), 597 (0.90) | 605, 655 |
| 16 | 421 (44.57), 518 (2.11), 555 (1.22), 592 (0.79), 648 (0.69) | 652, 718 |
| 17 | 426 (47.83), 556 (2.27), 597 (1.02) | 603, 652 |
| 18 | 421 (49.94), 518 (2.43), 554 (1.39), 593 (0.79), 649 (0.59) | 654, 719 |
| 19 | 421 (53.58), 518 (2.59), 555 (1.47), 592 (0.89), 648 (0.74) | 654, 718 |
| 20 | 421 (46.01), 518 (2.79), 554 (1.68), 592 (1.17), 650 (1.09) | 654, 720 |
aAbsorption and emission data were measured for CHCl3 solutions of porphyrins at 298 K. bExcitation wavelength for the emission data is 420 nm.
The electronic absorption spectra of all the free-base dihydro-1,3-oxazinoporphyrins exhibited a typical Soret band at 421 nm and four weaker Q bands at ~518, 553, 592 and 648 nm. In contrast, the zinc(II) dihydro-1,3-oxazinoporphyrins showed the Soret band at 426 nm and two Q bands at ~555 and 597 nm. In addition, the newly prepared free-base porphyrins displayed typical emission bands at ~652 and 717 nm, whereas their zinc(II) analogues showed fluorescence bands near 603 and 652 nm. The UV–vis and fluorescence spectra of selected free-base dihydro-1,3-oxazinoporphyrins 6, 8, 14, 16 and 18 and zinc(II) dihydro-1,3-oxazinoporphyrins 10, 12, 15 and 17 are shown in Figure 1.
![[1860-5397-9-53-1]](/bjoc/content/figures/1860-5397-9-53-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: (a) Electronic absorption spectra of free-base porphyrins 6, 8, 14, 16 and 18 in CHCl3 at 298 K. (b) Electronic absorption spectra of zinc porphyrins 10, 12, 15 and 17 in CHCl3 at 298 K. Inset in both (a) and (b) shows the Q bands. (c) Fluorescence spectra of free-base porphyrins 6, 8, 14, 16 and 18 in CHCl3 (2 × 10−6 mol L−1) at 298 K, λex = 420 nm. (d) Fluorescence spectra of zinc porphyrins 10, 12, 15 and 17 in CHCl3 (2 × 10−6 mol L−1) at 298 K, λex = 420 nm.
Figure 1: (a) Electronic absorption spectra of free-base porphyrins 6, 8, 14, 16 and 18 in CHCl3 at 298 K. (b...
Conclusion
In summary, we have developed a convenient synthetic protocol for the construction of a new series of dihydro-1,3-oxazinoporphyrins in moderate to good yields. These novel porphyrin-dihydro-1,3-oxazine hybrids may be considered as potential candidates not only for biological evaluations but also for the development of newer π-conjugated molecules for various material applications.
Supporting Information
| Supporting Information File 1: Experimental details and characterization data | ||
| Format: PDF | Size: 331.2 KB | Download |
References
-
Aratani, N.; Kim, D.; Osuka, A. Acc. Chem. Res. 2009, 42, 1922–1934. doi:10.1021/ar9001697
Return to citation in text: [1] -
Balaban, T. S. Acc. Chem. Res. 2005, 38, 612–623. doi:10.1021/ar040211z
Return to citation in text: [1] -
Drain, C. M.; Varotto, A.; Radivojevic, I. Chem. Rev. 2009, 109, 1630–1658. doi:10.1021/cr8002483
Return to citation in text: [1] -
Liu, W.; Groves, J. T. J. Am. Chem. Soc. 2010, 132, 12847–12849. doi:10.1021/ja105548x
Return to citation in text: [1] -
Hiramatsu, R.; Kawabata, S.; Miyatake, S.-I.; Kuroiwa, T.; Easson, M. W.; Vicente, M. G. H. Lasers Surg. Med. 2011, 43, 52–58. doi:10.1002/lsm.21026
Return to citation in text: [1] -
Gianferrara, T.; Bergamo, A.; Bratos, I.; Milani, B.; Spagnul, C.; Sava, G.; Alessio, E. J. Med. Chem. 2010, 53, 4678–4690. doi:10.1021/jm1002588
Return to citation in text: [1] -
Winkelman, J.; Slater, G.; Grossman, J. Cancer Res. 1967, 27, 2060–2064.
Return to citation in text: [1] -
Zhang, J.-X.; Zhou, J.-W.; Chan, C.-F.; Lau, T. C.-K.; Kwong, D. W. J.; Tam, H.-L.; Mak, N.-K.; Wong, K.-L.; Wong, W.-K. Bioconjugate Chem. 2012, 23, 1623–1638. doi:10.1021/bc300201h
Return to citation in text: [1] -
Wild, P. J.; Krieg, R. C.; Seidl, J.; Stoehr, R.; Reher, K.; Hofmann, C.; Louhelainen, J.; Rosenthal, A.; Hartmann, A.; Pilarsky, C.; Bosserhoff, A. K.; Knuechel, R. Mol. Cancer Ther. 2005, 4, 516–528. doi:10.1158/1535-7163.MCT-04-0141
Return to citation in text: [1] -
Robinson, G. D., Jr.; Alavi, A.; Vaum, R.; Staum, M. J. Nucl. Med. 1986, 27, 239–242.
Return to citation in text: [1] -
Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R. K. Chem. Soc. Rev. 2011, 40, 340–362. doi:10.1039/b915149b
Return to citation in text: [1] -
Celli, J. P.; Spring, B. Q.; Rizvi, I.; Evans, C. L.; Samkoe, K. S.; Verma, S.; Pogue, B. W.; Hasan, T. Chem. Rev. 2010, 110, 2795–2838. doi:10.1021/cr900300p
Return to citation in text: [1] -
Hombrecher, H. K.; Ohm, S.; Koll, D. Tetrahedron 1996, 52, 5441–5448. doi:10.1016/0040-4020(96)00174-3
Return to citation in text: [1] -
Mikata, Y.; Onchi, Y.; Tabata, K.; Ogura, S.-i.; Okura, I.; Ono, H.; Yano, S. Tetrahedron Lett. 1998, 39, 4505–4508. doi:10.1016/S0040-4039(98)00801-6
Return to citation in text: [1] -
Sol, V.; Blais, J. C.; Carré, V.; Granet, R.; Guilloton, M.; Spiro, M.; Krausz, P. J. Org. Chem. 1999, 64, 4431–4444. doi:10.1021/jo982499+
Return to citation in text: [1] -
Wang, H. M.; Jiang, J. Q.; Xiao, J. H.; Gao, R. L.; Lin, F. Y.; Liu, X. Y. Chem.-Biol. Interact. 2008, 172, 154–158. doi:10.1016/j.cbi.2007.11.016
Return to citation in text: [1] -
Weimin, S.; Gen, Z.; Guifu, D.; Yunxiao, Z.; Jin, Z.; Jingchao, T. Bioorg. Med. Chem. 2008, 16, 5665–5671. doi:10.1016/j.bmc.2008.03.063
Return to citation in text: [1] -
Shi, W. M.; Wu, J.; Wu, Y. F.; Qian, K. X. Chin. Chem. Lett. 2004, 15, 1427–1429.
Return to citation in text: [1] -
Hamblin, M. R.; Newman, E. L. J. Photochem. Photobiol., B 1994, 26, 45–56. doi:10.1016/1011-1344(94)85035-6
Return to citation in text: [1] -
Viola, A.; Mannoni, P.; Chanon, M.; Julliard, M.; Mehta, G.; Maiya, B. G.; Muthusamy, S.; Sambaiah, T. J. Photochem. Photobiol., B 1997, 40, 263–273. doi:10.1016/S1011-1344(97)00067-5
Return to citation in text: [1] -
Hombrecher, H. K.; Schell, C. Bioorg. Med. Chem. Lett. 1996, 6, 1199–1202. doi:10.1016/0960-894X(96)00201-6
Return to citation in text: [1] -
Ibrahim, H.; Kasselouri, A.; You, C.; Maillard, P.; Rosilio, V.; Pansu, R.; Prognon, P. J. Photochem. Photobiol., A 2011, 217, 10–21. doi:10.1016/j.jphotochem.2010.09.008
Return to citation in text: [1] -
Sol, V.; Chaleix, V.; Champavier, Y.; Granet, R.; Huang, Y.-M.; Krausz, P. Bioorg. Med. Chem. 2006, 14, 7745–7760. doi:10.1016/j.bmc.2006.08.004
Return to citation in text: [1] -
Liang, G.; Wang, L.; Yang, Z.; Koon, H.; Mak, N.; Chang, C. K.; Xu, B. Chem. Commun. 2006, 5021–5023. doi:10.1039/b611557h
Return to citation in text: [1] -
Koo, M.-S.; Ozawa, T.; Santos, R. A.; Lamborn, K. R.; Bollen, A. W.; Deen, D. F.; Kahl, S. B. J. Med. Chem. 2007, 50, 820–827. doi:10.1021/jm060895b
Return to citation in text: [1] -
Cheng, H.; Ma, J. S.; Zhang, D. H.; Chen, Q. Q. Synth. Commun. 2001, 31, 1941–1945. doi:10.1081/SCC-100104409
Return to citation in text: [1] -
Silva, A. M. G.; Lacerda, P. S. S.; Tomé, A. C.; Neves, M. G. P. M. S.; Silva, A. M. S.; Cavaleiro, J. A. S.; Makarova, E. A.; Lukyanets, E. A. J. Org. Chem. 2006, 71, 8352–8356. doi:10.1021/jo0611770
Return to citation in text: [1] -
Guo, C.-C.; Li, H.-P.; Zhang, X.-B. Bioorg. Med. Chem. 2003, 11, 1745–1751. doi:10.1016/S0968-0896(03)00027-0
Return to citation in text: [1] -
Cavaleiro, J. A. S.; Neves, M. G. P. M.; Tomé, A. C.; Silva, A. M. S.; Faustino, M. A. F.; Lacerda, P. S.; Silva, A. M. G. J. Heterocycl. Chem. 2000, 37, 527–534. doi:10.1002/jhet.5570370310
Return to citation in text: [1] -
Pandey, R. K.; Shiau, F.-Y.; Medforth, C. J.; Dougherty, T. J.; Smith, K. M. Tetrahedron Lett. 1990, 31, 7399–7402. doi:10.1016/S0040-4039(00)88499-3
Return to citation in text: [1] -
Mathew, B. P.; Kumar, A.; Sharma, S.; Shukla, P. K.; Nath, M. Eur. J. Med. Chem. 2010, 45, 1502–1507. doi:10.1016/j.ejmech.2009.12.058
Return to citation in text: [1] -
Chylińska, J. B.; Janowiec, M.; Urbański, T. Br. J. Pharmacol. 1971, 43, 649–657. doi:10.1111/j.1476-5381.1971.tb07194.x
Return to citation in text: [1] -
Latif, N.; Mishriky, N.; Assad, F. M. Aust. J. Chem. 1982, 35, 1037–1043. doi:10.1071/CH9821037
Return to citation in text: [1] -
Cocuzza, A. J.; Chidester, D. R.; Cordova, B. C.; Jeffrey, S.; Parsons, R. L.; Bacheler, L. T.; Erickson-Viitanen, S.; Trainor, G. L.; Ko, S. S. Bioorg. Med. Chem. Lett. 2001, 11, 1177–1179. doi:10.1016/S0960-894X(01)00192-5
Return to citation in text: [1] -
Duffin, W. M.; Rollo, I. M. Br. J. Pharmacol. Chemother. 1957, 12, 171–175. doi:10.1111/j.1476-5381.1957.tb00116.x
Return to citation in text: [1] -
Kuehne, M. E.; Konopka, E. A. J. Med. Chem. 1962, 5, 257–280. doi:10.1021/jm01237a005
Return to citation in text: [1] -
Chylińska, J. B.; Urbański, T.; Mordarski, M. J. Med. Chem. 1963, 6, 484–487. doi:10.1021/jm00341a004
Return to citation in text: [1] -
Sharma, S.; Nath, M. New J. Chem. 2011, 35, 1630–1639. doi:10.1039/c1nj20248k
Return to citation in text: [1] -
Sharma, S.; Nath, M. J. Heterocycl. Chem. 2012, 49, 88–92. doi:10.1002/jhet.664
Return to citation in text: [1] -
Sharma, S.; Nath, M. Dyes Pigm. 2012, 92, 1241–1249. doi:10.1016/j.dyepig.2011.07.022
Return to citation in text: [1] -
Bhatt, R. K.; Sharma, S.; Nath, M. Monatsh. Chem. 2012, 143, 309–316. doi:10.1007/s00706-011-0625-0
Return to citation in text: [1] [2] -
Kruper, W. J., Jr.; Chamberlin, A. T.; Kochanny, M. J. Org. Chem. 1989, 54, 2753–2756. doi:10.1021/jo00272a057
Return to citation in text: [1]
| 35. | Duffin, W. M.; Rollo, I. M. Br. J. Pharmacol. Chemother. 1957, 12, 171–175. doi:10.1111/j.1476-5381.1957.tb00116.x |
| 31. | Mathew, B. P.; Kumar, A.; Sharma, S.; Shukla, P. K.; Nath, M. Eur. J. Med. Chem. 2010, 45, 1502–1507. doi:10.1016/j.ejmech.2009.12.058 |
| 32. | Chylińska, J. B.; Janowiec, M.; Urbański, T. Br. J. Pharmacol. 1971, 43, 649–657. doi:10.1111/j.1476-5381.1971.tb07194.x |
| 33. | Latif, N.; Mishriky, N.; Assad, F. M. Aust. J. Chem. 1982, 35, 1037–1043. doi:10.1071/CH9821037 |
| 34. | Cocuzza, A. J.; Chidester, D. R.; Cordova, B. C.; Jeffrey, S.; Parsons, R. L.; Bacheler, L. T.; Erickson-Viitanen, S.; Trainor, G. L.; Ko, S. S. Bioorg. Med. Chem. Lett. 2001, 11, 1177–1179. doi:10.1016/S0960-894X(01)00192-5 |
| 1. | Aratani, N.; Kim, D.; Osuka, A. Acc. Chem. Res. 2009, 42, 1922–1934. doi:10.1021/ar9001697 |
| 2. | Balaban, T. S. Acc. Chem. Res. 2005, 38, 612–623. doi:10.1021/ar040211z |
| 7. | Winkelman, J.; Slater, G.; Grossman, J. Cancer Res. 1967, 27, 2060–2064. |
| 8. | Zhang, J.-X.; Zhou, J.-W.; Chan, C.-F.; Lau, T. C.-K.; Kwong, D. W. J.; Tam, H.-L.; Mak, N.-K.; Wong, K.-L.; Wong, W.-K. Bioconjugate Chem. 2012, 23, 1623–1638. doi:10.1021/bc300201h |
| 9. | Wild, P. J.; Krieg, R. C.; Seidl, J.; Stoehr, R.; Reher, K.; Hofmann, C.; Louhelainen, J.; Rosenthal, A.; Hartmann, A.; Pilarsky, C.; Bosserhoff, A. K.; Knuechel, R. Mol. Cancer Ther. 2005, 4, 516–528. doi:10.1158/1535-7163.MCT-04-0141 |
| 10. | Robinson, G. D., Jr.; Alavi, A.; Vaum, R.; Staum, M. J. Nucl. Med. 1986, 27, 239–242. |
| 29. | Cavaleiro, J. A. S.; Neves, M. G. P. M.; Tomé, A. C.; Silva, A. M. S.; Faustino, M. A. F.; Lacerda, P. S.; Silva, A. M. G. J. Heterocycl. Chem. 2000, 37, 527–534. doi:10.1002/jhet.5570370310 |
| 5. | Hiramatsu, R.; Kawabata, S.; Miyatake, S.-I.; Kuroiwa, T.; Easson, M. W.; Vicente, M. G. H. Lasers Surg. Med. 2011, 43, 52–58. doi:10.1002/lsm.21026 |
| 6. | Gianferrara, T.; Bergamo, A.; Bratos, I.; Milani, B.; Spagnul, C.; Sava, G.; Alessio, E. J. Med. Chem. 2010, 53, 4678–4690. doi:10.1021/jm1002588 |
| 30. | Pandey, R. K.; Shiau, F.-Y.; Medforth, C. J.; Dougherty, T. J.; Smith, K. M. Tetrahedron Lett. 1990, 31, 7399–7402. doi:10.1016/S0040-4039(00)88499-3 |
| 4. | Liu, W.; Groves, J. T. J. Am. Chem. Soc. 2010, 132, 12847–12849. doi:10.1021/ja105548x |
| 27. | Silva, A. M. G.; Lacerda, P. S. S.; Tomé, A. C.; Neves, M. G. P. M. S.; Silva, A. M. S.; Cavaleiro, J. A. S.; Makarova, E. A.; Lukyanets, E. A. J. Org. Chem. 2006, 71, 8352–8356. doi:10.1021/jo0611770 |
| 3. | Drain, C. M.; Varotto, A.; Radivojevic, I. Chem. Rev. 2009, 109, 1630–1658. doi:10.1021/cr8002483 |
| 28. | Guo, C.-C.; Li, H.-P.; Zhang, X.-B. Bioorg. Med. Chem. 2003, 11, 1745–1751. doi:10.1016/S0968-0896(03)00027-0 |
| 20. | Viola, A.; Mannoni, P.; Chanon, M.; Julliard, M.; Mehta, G.; Maiya, B. G.; Muthusamy, S.; Sambaiah, T. J. Photochem. Photobiol., B 1997, 40, 263–273. doi:10.1016/S1011-1344(97)00067-5 |
| 21. | Hombrecher, H. K.; Schell, C. Bioorg. Med. Chem. Lett. 1996, 6, 1199–1202. doi:10.1016/0960-894X(96)00201-6 |
| 25. | Koo, M.-S.; Ozawa, T.; Santos, R. A.; Lamborn, K. R.; Bollen, A. W.; Deen, D. F.; Kahl, S. B. J. Med. Chem. 2007, 50, 820–827. doi:10.1021/jm060895b |
| 41. | Bhatt, R. K.; Sharma, S.; Nath, M. Monatsh. Chem. 2012, 143, 309–316. doi:10.1007/s00706-011-0625-0 |
| 42. | Kruper, W. J., Jr.; Chamberlin, A. T.; Kochanny, M. J. Org. Chem. 1989, 54, 2753–2756. doi:10.1021/jo00272a057 |
| 16. | Wang, H. M.; Jiang, J. Q.; Xiao, J. H.; Gao, R. L.; Lin, F. Y.; Liu, X. Y. Chem.-Biol. Interact. 2008, 172, 154–158. doi:10.1016/j.cbi.2007.11.016 |
| 17. | Weimin, S.; Gen, Z.; Guifu, D.; Yunxiao, Z.; Jin, Z.; Jingchao, T. Bioorg. Med. Chem. 2008, 16, 5665–5671. doi:10.1016/j.bmc.2008.03.063 |
| 18. | Shi, W. M.; Wu, J.; Wu, Y. F.; Qian, K. X. Chin. Chem. Lett. 2004, 15, 1427–1429. |
| 19. | Hamblin, M. R.; Newman, E. L. J. Photochem. Photobiol., B 1994, 26, 45–56. doi:10.1016/1011-1344(94)85035-6 |
| 26. | Cheng, H.; Ma, J. S.; Zhang, D. H.; Chen, Q. Q. Synth. Commun. 2001, 31, 1941–1945. doi:10.1081/SCC-100104409 |
| 13. | Hombrecher, H. K.; Ohm, S.; Koll, D. Tetrahedron 1996, 52, 5441–5448. doi:10.1016/0040-4020(96)00174-3 |
| 14. | Mikata, Y.; Onchi, Y.; Tabata, K.; Ogura, S.-i.; Okura, I.; Ono, H.; Yano, S. Tetrahedron Lett. 1998, 39, 4505–4508. doi:10.1016/S0040-4039(98)00801-6 |
| 15. | Sol, V.; Blais, J. C.; Carré, V.; Granet, R.; Guilloton, M.; Spiro, M.; Krausz, P. J. Org. Chem. 1999, 64, 4431–4444. doi:10.1021/jo982499+ |
| 36. | Kuehne, M. E.; Konopka, E. A. J. Med. Chem. 1962, 5, 257–280. doi:10.1021/jm01237a005 |
| 37. | Chylińska, J. B.; Urbański, T.; Mordarski, M. J. Med. Chem. 1963, 6, 484–487. doi:10.1021/jm00341a004 |
| 11. | Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R. K. Chem. Soc. Rev. 2011, 40, 340–362. doi:10.1039/b915149b |
| 12. | Celli, J. P.; Spring, B. Q.; Rizvi, I.; Evans, C. L.; Samkoe, K. S.; Verma, S.; Pogue, B. W.; Hasan, T. Chem. Rev. 2010, 110, 2795–2838. doi:10.1021/cr900300p |
| 22. | Ibrahim, H.; Kasselouri, A.; You, C.; Maillard, P.; Rosilio, V.; Pansu, R.; Prognon, P. J. Photochem. Photobiol., A 2011, 217, 10–21. doi:10.1016/j.jphotochem.2010.09.008 |
| 23. | Sol, V.; Chaleix, V.; Champavier, Y.; Granet, R.; Huang, Y.-M.; Krausz, P. Bioorg. Med. Chem. 2006, 14, 7745–7760. doi:10.1016/j.bmc.2006.08.004 |
| 24. | Liang, G.; Wang, L.; Yang, Z.; Koon, H.; Mak, N.; Chang, C. K.; Xu, B. Chem. Commun. 2006, 5021–5023. doi:10.1039/b611557h |
| 38. | Sharma, S.; Nath, M. New J. Chem. 2011, 35, 1630–1639. doi:10.1039/c1nj20248k |
| 39. | Sharma, S.; Nath, M. J. Heterocycl. Chem. 2012, 49, 88–92. doi:10.1002/jhet.664 |
| 40. | Sharma, S.; Nath, M. Dyes Pigm. 2012, 92, 1241–1249. doi:10.1016/j.dyepig.2011.07.022 |
| 41. | Bhatt, R. K.; Sharma, S.; Nath, M. Monatsh. Chem. 2012, 143, 309–316. doi:10.1007/s00706-011-0625-0 |
© 2013 Sharma and Nath; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)