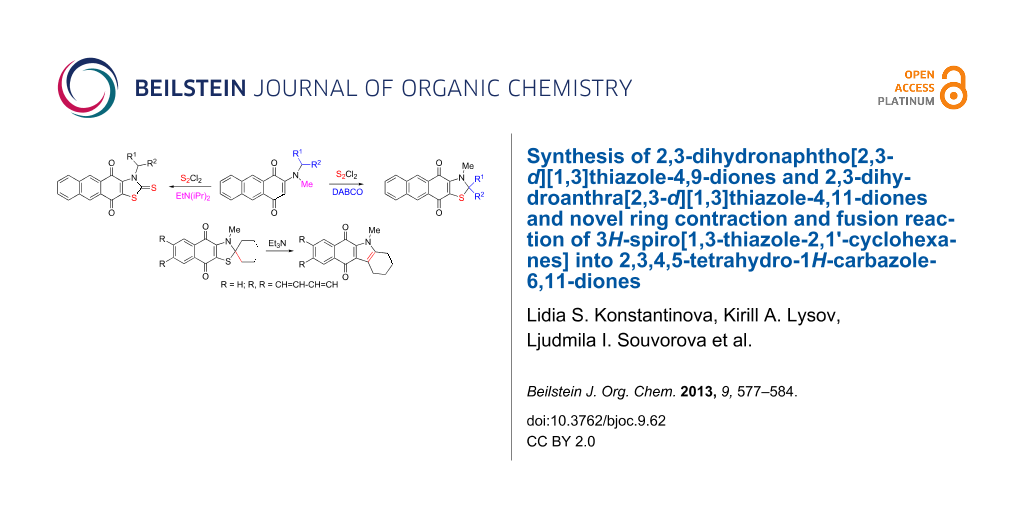
Abstract
Treatment of N-substituted 2-(methylamino)naphthoquinones 3 and -anthracene-1,4-diones 4 with S2Cl2 and DABCO in chlorobenzene gave the corresponding 2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-diones 1 and 2,3-dihydroanthra[2,3-d][1,3]thiazole-4,11-diones 2 by triethylamine addition, in high to moderate yields. The DABCO replacement for N-ethyldiisopropylamine in the reaction of anthracene-1,4-diones 4 led unexpectedly to the corresponding 2-thioxo-2,3-dihydroanthra[2,3-d][1,3]thiazole-4,11-diones 10. The reaction of 3H-spiro[1,3-thiazole-2,1'-cyclohexanes] 1d, 2d with Et3N in chlorobenzene under reflux yielded 2,3,4,5-tetrahydro-1H-carbazole-6,11-diones 15, 16, i.e., ring contraction and fusion products. A plausible mechanism was proposed for the formation of the products.
Graphical Abstract
Introduction
The 1,4-naphthoquinone structure is common for various natural products. It is associated with numerous biological activities, such as enzyme-inhibitory, antifungal, antibacterial, anticancer, antiproliferative, antiplatelet, anti-inflammatory, antiallergic, and antimalarial ones. Benzoquinones fused with heterocycles containing nitrogen in position 2 are the most promising ones for furthering clinical applications [1]. Meanwhile, in terms of further structural and chemical system modifications, the introduction of other heteroatoms (e.g., sulfur) through the heterocyclic ring incorporation to the quinone system has been one of the most interesting modifications. In fact, 2-alkylthio-1,4-naphthoquinones were found to show cell-growth inhibitory properties [2], and dihydrothienonaphthoquinones appeared to be potent antitumour compounds [3].
In searching for agents with better pharmacological properties, wider activity range, and low side effects it seemed quite promising to incorporate two heteroatoms into the heterocycle attached to the naphthoquinone (e.g., thiazole) core. Despite continuous interest in 1,4-naphthoquinones fused with heterocycles, only a limited number of thiazolonaphthoquinones have been known so far, no general approach to their synthesis has been proposed, and yields are often low. 3-Methyl-2-thioxo-2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-dione has been shown to possess a fungicidal activity to Pyricularia Oryzae [4,5]. A family of thiazolonaphthoquinones fused on a chain with nitrogen heterocycles (pyrrole [6,7], and triazoles [8]) were found to possess antifungal and antitumor activity toward a number of species causing fungal diseases and toward Walker 256 carcinoma cell lines. The compounds with the thiazoloanthroquinone structure have not been reported in the literature so far. We were interested in developing a general method for the synthesis of naphtho- and anthraquinones from readily available 2-aminonaphtho- and anthroquinones as well as in the study of their chemical properties and side reactions.
A retrosynthetic analysis of naphtho- and anthraquinones 1 and 2 led us to a conclusion that the most reliable route would be a reaction of sulfur monochloride with dialkylamino derivatives 3 and 4 (Scheme 1). According to the classification of the synthesis of sulfur-containing heterocycles from organic substrates and S2Cl2, thiazoles 1 and 2 should be generated from the four-member group shown in red [9]. In turn, dialkylamino derivatives 3 and 4 with two C–H groups activated for the electrophilic attack, one in the α-position to the carbonyl group [10,11] and another located near the nitrogen atom [12], could be prepared from the corresponding naphtho- 5 or anthraquinones 6.
Scheme 1: Retrosynthetic analysis of 2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-diones and 2,3-dihydroanthra[2,3-d][1,3]thiazole-4,11-diones.
Scheme 1: Retrosynthetic analysis of 2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-diones and 2,3-dihydroanthra[...
In this paper we report a study of a reaction between dialkylaminonaphtho- and anthraquinones and sulfur monochloride in the presence of tertiary amines, a selective synthesis of fused thiazoles, and some of their chemical transformations.
Results and Discussion
We examined in detail the reaction of 2-[butyl(methyl)amino]naphthoquinone 3a with sulfur monochloride and tertiary amines [N-ethyldiisopropylamine (Hünig’s base) and 1,4-diazabicyclooctane (DABCO)]. Treatment of naphthoquinone 3a with S2Cl2 (9 equiv) and Hünig’s base (5 equiv) in THF at 0 °C for 72 h with subsequent heating under reflux for 2 h (these reaction conditions had been used in the synthesis of 3,8-dichloroindeno-2H-[2,1-b]thiophen-2-one from indenylacetic acid [13]) gave with a high yield (81%) only the chlorinated product 7a. No sulfurated heterocycles were detected in the reaction mixture. The best (practically quantitative) yield of chloride 7a was achieved by using a 15-fold S2Cl2 excess (Scheme 2). Reactions of naphthoquinone 3a with S2Cl2 and Hünig’s base in other solvents, such as chlorobenzene or acetonitrile, after 1 h under reflux led to the same chlorinated product 7a albeit in lower yields (70% and 65%, respectively). Sulfur monochloride is known as a powerful chlorinating agent for chlorination of aromatic and heteroaromatic compounds [14,15].
Scheme 2: Reaction of 2-[butyl(methyl)amino]naphthoquinone 3a with S2Cl2 and Hünig’s base.
Scheme 2: Reaction of 2-[butyl(methyl)amino]naphthoquinone 3a with S2Cl2 and Hünig’s base.
In an attempt to increase the sulfurating ability of S2Cl2 the complex 8 obtained from S2Cl2 and 2 equiv of DABCO [16] was used. We have recently shown that this complex can convert N-substituted 2-methyl-1H-indoles to [1,2]dithiolo[4,3-b]indole-3(4H)-thiones [15].
A reaction of naphthoquinone 3a with a fivefold excess of complex 8 in chlorobenzene for 0.5 h at −20 °C with subsequent treatment with Et3N and heating at 100 °C for another 0.5 h, led to a blue solid, mp 71–74 °C. Microanalysis and mass spectrometry data allowed the establishment of its molecular formula as C15H15NO2S. The 1H NMR spectrum showed the presence of four aromatic protons (7.05–7.58 ppm), two methyl groups (0.98, 3.40 ppm), two methylene groups (1.48, 1.87 ppm) and one methyne group (5.14 ppm) linked to the methylene group (J = 5.5 Hz). The remaining N-methyl group (3.40 ppm), loss of one cyclohexandione C–H proton, and butyl CH2 fragment conversion to the methyne group undoubtedly supported a novel thiazole structure, 1a (Scheme 3). The 13C NMR spectrum confirmed these observations.
Scheme 3: Synthesis of 2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-diones 1.
Scheme 3: Synthesis of 2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-diones 1.
Further on, we extended this reaction to other naphthoquinones 3. Fused thiazoles 1 were obtained with yields from moderate to high. NMR spectroscopy of thiazoles 1 showed that in all the cases either the primary, secondary or tertiary carbon atom attached to the nitrogen atom was included in the thiazole cycle whereas the N-methyl group remained intact, except for dimethylamino derivative 3c where the N-methyl group reacted exactly under the same conditions as with other naphthoquinones 3, giving 3-methyl-2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-dione (1c) in a moderate yield.
The above conditions were further applied to anthraquinones 4 structurally similar to naphthoquinones 3. Anthraquinonothiazoles 2 were isolated after treatment of dialkylamino derivatives 4 with complex 8 and triethylamine in chlorobenzene (Scheme 4).
Scheme 4: Synthesis of 2,3-dihydroanthra[2,3-d][1,3]thiazole-4,11-diones 2.
Scheme 4: Synthesis of 2,3-dihydroanthra[2,3-d][1,3]thiazole-4,11-diones 2.
In order to compare the reactivity of anthraquinones 4 and naphthoquinones 3 we studied a reaction of 4 with a mixture of S2Cl2 and Hünig’s base in THF. After treatment of anthraquinone 4a with S2Cl2 (9 equiv) and Hünig’s base (5 equiv) in THF at 0 °C for 72 h with subsequent 2 h heating under reflux we isolated, along with the chlorinated product 9a, orange crystals 10a, mp 183–185 °C. Microanalysis and mass-spectrometry data allowed the establishment of its molecular formula as C19H15NO2S2, suggesting the presence of two sulfur atoms inserted into the final product instead of four hydrogen atoms. The 1H NMR spectrum showed that both the anthraquinone ring and butyl group remained intact whereas three protons of the methyl group and one of the quinone ring disappeared. These observations are in agreement with the thiazole-2-thione structure 10a (Scheme 5). The 13C NMR spectrum confirmed this structure by a characteristic C=S signal at 189.8 ppm. The reaction was then extended to other anthraquinone derivatives 4. Fused anthraquinonothiazole-2-thiones 10 were isolated in yields ranging from moderate to low together with chlorinated products 9 (with higher yields in most cases).
Scheme 5: Reaction of N-substituted 2-(methylamino)anthracene-1,4-diones 4 with S2Cl2 and Hünig’s base.
Scheme 5: Reaction of N-substituted 2-(methylamino)anthracene-1,4-diones 4 with S2Cl2 and Hünig’s base.
To confirm the structure of anthraquinonothiazoles 10 we treated thiazole 2c with complex 8 in chlorobenzene at 115 °C for 3 h. Thione 10c identical to the sample prepared from anthraquinone 4 was isolated in moderate yield (67%). Naphthoquinonothiazole 1c reacted with complex 8 by the same pathway yielding corresponding thiazole-2-thione 11 (Scheme 6).
Scheme 6: Synthesis of thiazole-2-thiones 10c and 11 from quinonothiazoles 1c and 2c.
Scheme 6: Synthesis of thiazole-2-thiones 10c and 11 from quinonothiazoles 1c and 2c.
To the best of our knowledge, there exists only a single example of the thiazole conversion to thiazole-2-thione, i.e., heating of 3-methyl-2,3-dihydro-1,3-benzothiazole with elemental sulfur at 200 °C for 0.5 h giving 3-methyl-1,3-benzothiazole-2(3H)-thione [17]. We described a similar conversion of the methylene group to thioketone by the action of sulfur monochloride in the presence of DABCO [18,19]. A plausible mechanism includes the addition of complex 8 to the activated methylene group with formation of S-S-DABCO derivative 12 followed by elimination of a sulfur atom and an HCl molecule [19].
We believe that naphtho- (1) and anthraquinonothiazoles (2) are generated by the same route. The most plausible mechanism is shown in Scheme 7.
Scheme 7: A plausible mechanism for the formation of naphtho- and anthraquinonothiazoles.
Scheme 7: A plausible mechanism for the formation of naphtho- and anthraquinonothiazoles.
The key step is assumed to be the insertion of two sulfur atoms of complex 8 between two activated CH groups, the first one being adjacent to the carbonyl group of the quinone ring and the second to the methyl or methyne group attached to the nitrogen atom with the formation of the six-membered dithiazine ring in 14, similarly to that proposed for 1,2-dithioles prepared from isopropylamines [14]. Ultimate sulfur extrusion (cf. reference [13]) would then give stable and planar products 1 and 2.
However, the S2Cl2 and Hünig’s base combination in THF is preferable in the case of the cyclization of anthraquinone derivatives 4 of the thiazole ring using the N-methyl group. It is possible that sulfur in intermediate 13, which contains the bulkier Y substituent EtN(iPr)2+ compared to DABCO, attacks the less bulky methyl group giving thiazoles, which are subsequently transformed to thiazole-2-thiones 10 by the further action of S2Cl2 and Hünig’s base.
Further study of the reaction between 2-[cyclohexyl(methyl)amino]naphthoquinone 1d and complex 8 in chlorobenzene allowed us to identify the stable byproduct 15 whose quantity increased by prolonged heating of the reaction mixture, whereas the quantity of thiazole 1d decreased. This was assumed to be a thermal conversion of 1d to 15. Base (e.g., triethylamine) was found to catalyze this reaction and compound 15 was isolated by the heating of 1d in chlorobenzene at 120 °C for 12 h in 78% yield (Scheme 8). According to mass spectrometry and elemental analysis, this is formally a product of H2S elimination, which was confirmed by isolation of triethylammonium hydrogen sulfide in practically quantitative yield from the reaction mixture. The 1H and 13C spectra showed that the unchanged naphthoquinone cycle and N-methyl group are unchanged, while one of the α-methylene groups in the cyclohexane ring was transformed to the tertiary sp2 carbon atom. All data are in agreement with the structure of 5-methyl-2,3,4,5-tetrahydro-1H-benzo[b]carbazole-6,11-dione (15) [20].
Scheme 8: Synthesis of 5-methyl-2,3,4,5-tetrahydro-1H-benzo[b]carbazole-6,11-dione (15) and 5-methyl-2,3,4,5-tetrahydro-1H-naphtho[2,3-b]carbazole-6,13-dione (16).
Scheme 8: Synthesis of 5-methyl-2,3,4,5-tetrahydro-1H-benzo[b]carbazole-6,11-dione (15) and 5-methyl-2,3,4,5-...
Anthraquinonothiazole 2d reacted with triethylamine in the same way as naphthoquinone 1d giving fused pyrrole 16 with the same yield. The most plausible pathway for this reaction is given in Scheme 9.
Scheme 9: A plausible mechanism for the conversion of spiro compounds 1d or 2d into carbazolediones 15 and 16.
Scheme 9: A plausible mechanism for the conversion of spiro compounds 1d or 2d into carbazolediones 15 and 16....
The key steps are assumed to be the thiazole ring opening with the formation of thiol 17 followed by the dihydropyrrole ring closure under the impact of both quinone and amine groups and the generation of the aromatic pyrrole cycle with hydrogen sulfide extrusion by the action of base (triethylamine). Although, to the best of our knowledge, the transformation of 3H-spiro(thiazol-2,1'-cyclohexanes) to tetrahydroindoles has been so far unknown, similar dehydration of spirooxazolocyclohexane to tetrahydroindoles by treatment with base has been previously described [21,22].
Conclusion
The reaction of N-substituted 2-(methylamino)naphthoquinones and -anthracene-1,4-diones with S2Cl2 provides a short and convenient route to 2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-diones and 2,3-dihydroanthra[2,3-d][1,3]thiazole-4,11-diones, which are of special interest as biologically active compounds. A striking difference in the influence of 1,4-diazabicyclooctane and N-ethyldiisopropylamine in the reaction of 2-(methylamino)anthracene-1,4-diones with sulfur monochloride was discovered and explained. 3H-Spiro(thiazol-2,1'-cyclohexanes) underwent a new ring contraction and fusion reaction resulting in the formation of tetrahydroindoles.
Experimental
Melting points were determined on a Kofler hot-stage apparatus and are uncorrected. IR spectra were recorded on a Specord M-80 instrument in KBr pellets. 1H NMR were recorded on a Bruker WM 250 spectrometer (250 MHz), and 13C NMR spectra were recorded on a Bruker AM 300 (75.5 MHz) in CDCl3 solution. J-values are given in hertz (Hz). Mass spectra were recorded on a Finnigan MAT INCOS 50 instrument by using electron impact ionization. High-resolution mass spectra (HRMS) were measured on a Bruker micrOTOF II instrument by using electrospray ionization (ESI) [23]. The measurements were done in a positive-ion mode (interface capillary voltage – 4500 V) or in a negative-ion mode (3200 V); mass range from m/z 50 to 3000 Da; external or internal calibration was done with Electrospray Calibrant Solution (Fluka). A syringe injection was used for solutions in acetonitrile, methanol or water (flow rate 3 μL/min). Nitrogen was applied as dry gas; the interface temperature was set at 180 °C. 2-(Dialkylamino)naphthoquinones 3 were prepared as described [24]. 2-(Dialkylamino)anthracene-1,4-diones 4 were prepared from anthracene-1,4-dione by a similar procedure [25].
General procedure for the synthesis of 3-methyl-2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-diones 1 and 3-methyl-2,3-dihydroanthra[2,3-d][1,3]thiazole-4,11-diones 2: Sulfur monochloride (0.40 mL, 5.00 mmol) was added dropwise to a stirred solution of 1,4-diazabicyclooctane (1.12 g, 10.00 mmol) in chlorobenzene (80 mL) at −30 °C. The reaction mixture was stirred for 1 h at room temperature, and a solution of the appropriate 2-(dialkylamino)naphthoquinone 3 or 2-(dialkylamino)anthracene-1,4-dione 4 (1.00 mmol) in chlorobenzene (40 mL) was added at −30 °C. The reaction mixture was stirred for 0.5 h at −20 °C, and triethylamine (1.4 mL, 10 mmol) was added at this temperature. The reaction mixture was stirred for 0.5 h at 100 °C and filtered, the solvent was evaporated under reduced pressure, and the residue was separated by column chromatography (Silica gel Merck 60 pretreated with triethylamine, hexane to hexane/CH2Cl2 mixtures).
General procedure for the reaction of 2-(dialkylamino)anthracene-1,4-diones 4, sulfur monochloride and Hünig’s base: Sulfur monochloride (0.72 mL, 9.00 mmol) was added dropwise to a stirred solution of the appropriate 2-(dialkylamino)anthracene-1,4-dione 4 (1.00 mmol) and N-ethyldiisopropylamine (0.86 mL, 5.00 mmol) in tetrahydrofurane (65 mL) under argon at −30 °C. The reaction mixture was stirred for 1 h at room temperature and then heated under reflux for 2 h and filtered, the solvent was evaporated under reduced pressure, and the residue was separated by column chromatography (Silica gel Merck 60, hexane to hexane/CH2Cl2 mixtures).
General procedure for the synthesis of 3-methyl-2-thioxo-2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-dione (11) and 3-methyl-2-thioxo-2,3-dihydroanthra[2,3-d][1,3]thiazole-4,11-dione (10с) from 3-methyl-2,3-dihydronaphtho[2,3-d][1,3]thiazole-4,9-dione 1c and 3-methyl-2,3-dihydroanthra[2,3-d][1,3]thiazole-4,11-dione (2c): Sulfur monochloride (0.40 mL, 5.00 mmol) was added dropwise to a stirred solution of 1,4-diazabicyclooctane (1.12 g, 10.00 mmol) in chlorobenzene (80 mL) at −30 °C. The reaction mixture was stirred for 1 h at room temperature, and a solution of the appropriate quinonothiazole 1c or 2c (1.00 mmol) in chlorobenzene (40 mL) was added at −30 °C. The reaction mixture was stirred for 0.5 h at −20 °C, and triethylamine (1.4 mL, 10 mmol) was added at this temperature. The reaction mixture was stirred for 0.5 h at 115 °C and filtered, the solvent was evaporated under reduced pressure, and the residue was separated by column chromatography (Silica gel Merck 60 pretreated with triethylamine, hexane to hexane/CH2Cl2 mixtures).
General procedure for the synthesis of 5-methyl-2,3,4,5-tetrahydro-1H-benzo[b]carbazole-6,11-dione (15) and 5-methyl-2,3,4,5-tetrahydro-1H-naphtho[2,3-b]carbazole-6,13-dione (16): A solution of spiro compounds 1d or 2d (1 mmol) and triethylamine (0.17 mL, 1.20 mmol) in chlorobenzene (20 mL) was heated under reflux for 12 h. The solvent was evaporated under reduced pressure, and the residue was crystallized from hexane/CH2Cl2 mixture.
Supporting Information
| Supporting Information File 1: Spectroscopic and analytical data. | ||
| Format: PDF | Size: 195.7 KB | Download |
Acknowledgments
We gratefully acknowledge the financial support from the Russian Foundation for Basic Research (Grant No. 05-03-32032-a) and from the Presidium of the Russian Academy of Sciences (Programme No. 8). High-resolution mass spectra were recorded in the Department of Structural Studies of Zelinsky Institute of Organic Chemistry, Moscow. We also thank Professor F. S. Sirovski for helpful discussions.
References
-
Jacobs, J.; Tehrani, K. A.; De Kimpe, N. Tetrahedron 2011, 67, 9459–9471. doi:10.1016/j.tet.2011.10.017
And references cited therein.
Return to citation in text: [1] -
Nishikawa, Y.; Carr, B. I.; Wang, M.; Kar, S.; Finn, F.; Dowd, P.; Zheng, Z. B.; Kerns, J.; Naganathan, S. J. Biol. Chem. 1995, 270, 28304–28310. doi:10.1074/jbc.270.47.28304
Return to citation in text: [1] -
Gomez-Monterrey, I.; Campiglia, P.; Grieco, P.; Diurno, M. V.; Bolognese, A.; La Colla, P.; Novellino, E. Bioorg. Med. Chem. 2003, 11, 3769–3775. doi:10.1016/S0968-0896(03)00310-9
Return to citation in text: [1] -
Nakamori, T.; Sato, Y.; Kasai, T. Nippon Kagaku Kaishi 1982, 98–104. doi:10.1246/nikkashi.1982.98
Return to citation in text: [1] -
Yoshida, K.; Kenji, N.; Tetsuda, T.; Suganuma, H. Naphthothiazoledione derivative and agricultural and horticultural fungicide containing said derivative as active component. Jpn. Pat. Appl. JP 62185080 A, Aug 13, 1987.
Chem. Abstr. 1988, 108, 112434.
Return to citation in text: [1] -
Tandon, V. K.; Chhor, R. B.; Singh, R. V.; Rai, S.; Yadav, D. B. Bioorg. Med. Chem. Lett. 2004, 14, 1079–1083. doi:10.1016/j.bmcl.2004.01.002
Return to citation in text: [1] -
Tandon, V. K.; Yadav, D. B.; Singh, R. V.; Chaturvedi, A. K.; Shukla, P. K. Bioorg. Med. Chem. Lett. 2005, 15, 5324–5328. doi:10.1016/j.bmcl.2005.08.032
Return to citation in text: [1] -
Rao, V. R.; Sharma, V. M.; Rao, T. V. P. Collect. Czech. Chem. Commun. 1993, 58, 1191–1194. doi:10.1135/cccc19931191
Return to citation in text: [1] -
Konstantinova, L. S.; Rakitin, O. A. Mendeleev Commun. 2009, 19, 55–61. doi:10.1016/j.mencom.2009.03.001
Return to citation in text: [1] -
Shi, S.; Katz, T. J.; Yang, B. V.; Liu, L. J. Org. Chem. 1995, 60, 1285–1297. doi:10.1021/jo00110a036
Return to citation in text: [1] -
Yu, M.; Malinakova, H. C.; Stagliano, K. W. J. Org. Chem. 2006, 71, 6648–6651. doi:10.1021/jo060825c
Return to citation in text: [1] -
Rees, C. W.; White, A. J. P.; Williams, D. J.; Rakitin, O. A.; Marcos, C. F.; Polo, C.; Torroba, T. J. Org. Chem. 1998, 63, 2189–2196. doi:10.1021/jo971864e
Return to citation in text: [1] -
Konstantinova, L. S.; Rakitin, O. A.; Rees, C. W.; Souvorova, L. I.; Torroba, T. J. Chem. Soc., Perkin Trans. 1 1999, 1023–1028. doi:10.1039/A809808E
Return to citation in text: [1] [2] -
Rakitin, O. A.; Konstantinova, L. S. Adv. Heterocycl. Chem. 2008, 96, 175–229. doi:10.1016/S0065-2725(07)00004-9
And references cited therein.
Return to citation in text: [1] [2] -
Konstantinova, L. S.; Lysov, K. A.; Amelichev, S. A.; Obruchnikova, N. V.; Rakitin, O. A. Tetrahedron 2009, 65, 2178–2183. doi:10.1016/j.tet.2009.01.069
Return to citation in text: [1] [2] -
Konstantinova, L. S.; Rakitin, O. A.; Rees, C. W.; Amelichev, S. A. Mendeleev Commun. 2004, 14, 91–92. doi:10.1070/MC2004v014n03ABEH001912
Return to citation in text: [1] -
Baker, K.; Fierz-David, H. E. Helv. Chim. Acta 1950, 33, 2011–2018. doi:10.1002/hlca.19500330706
Return to citation in text: [1] -
Konstantinova, L. S.; Rakitin, O. A.; Rees, C. W. Mendeleev Commun. 2001, 11, 167–168. doi:10.1070/MC2001v011n05ABEH001504
Return to citation in text: [1] -
Konstantinova, L. S.; Berezin, A. A.; Rakitin, O. A. Russ. Chem. Bull. 2007, 56, 1178–1183. doi:10.1007/s11172-007-0179-9
Return to citation in text: [1] [2] -
Wu, Y.-L.; Chuang, C.-P.; Lin, P. Y. Tetrahedron 2001, 57, 5543–5549. doi:10.1016/S0040-4020(01)00480-X
Return to citation in text: [1] -
Kukharev, B. F.; Stankevich, V. K.; Kukhareva, V. A. Chem. Heterocycl. Comp. 1986, 22, 437–439. doi:10.1007/BF00542786
Return to citation in text: [1] -
Kukharev, B. F. Russ. Chem. Bull. 1997, 46, 1482–1483. doi:10.1007/BF02505694
Return to citation in text: [1] -
Belyakov, P. A.; Kadentsev, V. I.; Chizhov, A. O.; Kolotyrkina, N. G.; Shashkov, A. S.; Ananikov, V. P. Mendeleev Commun. 2010, 20, 125–131. doi:10.1016/j.mencom.2010.05.001
Return to citation in text: [1] -
Stasevych, M. V.; Plotnikov, M. Yu.; Platonov, M. O.; Sabat, S. I.; Musyanovych, R. Ya.; Novikov, V. P. Heteroatom Chem. 2005, 16, 205–211. doi:10.1002/hc.20112
Return to citation in text: [1] -
Hua, D. H.; Tamura, M.; Huang, X.; Stephany, H. A.; Helfrich, B. A.; Perchellet, E. M.; Sperfslage, B. J.; Perchellet, J.-P.; Jiang, S.; Kyle, D. E.; Chiang, P. K. J. Org. Chem. 2002, 67, 2907–2912. doi:10.1021/jo010958s
Return to citation in text: [1]
| 20. | Wu, Y.-L.; Chuang, C.-P.; Lin, P. Y. Tetrahedron 2001, 57, 5543–5549. doi:10.1016/S0040-4020(01)00480-X |
| 14. |
Rakitin, O. A.; Konstantinova, L. S. Adv. Heterocycl. Chem. 2008, 96, 175–229. doi:10.1016/S0065-2725(07)00004-9
And references cited therein. |
| 13. | Konstantinova, L. S.; Rakitin, O. A.; Rees, C. W.; Souvorova, L. I.; Torroba, T. J. Chem. Soc., Perkin Trans. 1 1999, 1023–1028. doi:10.1039/A809808E |
| 1. |
Jacobs, J.; Tehrani, K. A.; De Kimpe, N. Tetrahedron 2011, 67, 9459–9471. doi:10.1016/j.tet.2011.10.017
And references cited therein. |
| 6. | Tandon, V. K.; Chhor, R. B.; Singh, R. V.; Rai, S.; Yadav, D. B. Bioorg. Med. Chem. Lett. 2004, 14, 1079–1083. doi:10.1016/j.bmcl.2004.01.002 |
| 7. | Tandon, V. K.; Yadav, D. B.; Singh, R. V.; Chaturvedi, A. K.; Shukla, P. K. Bioorg. Med. Chem. Lett. 2005, 15, 5324–5328. doi:10.1016/j.bmcl.2005.08.032 |
| 18. | Konstantinova, L. S.; Rakitin, O. A.; Rees, C. W. Mendeleev Commun. 2001, 11, 167–168. doi:10.1070/MC2001v011n05ABEH001504 |
| 19. | Konstantinova, L. S.; Berezin, A. A.; Rakitin, O. A. Russ. Chem. Bull. 2007, 56, 1178–1183. doi:10.1007/s11172-007-0179-9 |
| 4. | Nakamori, T.; Sato, Y.; Kasai, T. Nippon Kagaku Kaishi 1982, 98–104. doi:10.1246/nikkashi.1982.98 |
| 5. |
Yoshida, K.; Kenji, N.; Tetsuda, T.; Suganuma, H. Naphthothiazoledione derivative and agricultural and horticultural fungicide containing said derivative as active component. Jpn. Pat. Appl. JP 62185080 A, Aug 13, 1987.
Chem. Abstr. 1988, 108, 112434. |
| 19. | Konstantinova, L. S.; Berezin, A. A.; Rakitin, O. A. Russ. Chem. Bull. 2007, 56, 1178–1183. doi:10.1007/s11172-007-0179-9 |
| 3. | Gomez-Monterrey, I.; Campiglia, P.; Grieco, P.; Diurno, M. V.; Bolognese, A.; La Colla, P.; Novellino, E. Bioorg. Med. Chem. 2003, 11, 3769–3775. doi:10.1016/S0968-0896(03)00310-9 |
| 15. | Konstantinova, L. S.; Lysov, K. A.; Amelichev, S. A.; Obruchnikova, N. V.; Rakitin, O. A. Tetrahedron 2009, 65, 2178–2183. doi:10.1016/j.tet.2009.01.069 |
| 2. | Nishikawa, Y.; Carr, B. I.; Wang, M.; Kar, S.; Finn, F.; Dowd, P.; Zheng, Z. B.; Kerns, J.; Naganathan, S. J. Biol. Chem. 1995, 270, 28304–28310. doi:10.1074/jbc.270.47.28304 |
| 17. | Baker, K.; Fierz-David, H. E. Helv. Chim. Acta 1950, 33, 2011–2018. doi:10.1002/hlca.19500330706 |
| 12. | Rees, C. W.; White, A. J. P.; Williams, D. J.; Rakitin, O. A.; Marcos, C. F.; Polo, C.; Torroba, T. J. Org. Chem. 1998, 63, 2189–2196. doi:10.1021/jo971864e |
| 14. |
Rakitin, O. A.; Konstantinova, L. S. Adv. Heterocycl. Chem. 2008, 96, 175–229. doi:10.1016/S0065-2725(07)00004-9
And references cited therein. |
| 15. | Konstantinova, L. S.; Lysov, K. A.; Amelichev, S. A.; Obruchnikova, N. V.; Rakitin, O. A. Tetrahedron 2009, 65, 2178–2183. doi:10.1016/j.tet.2009.01.069 |
| 24. | Stasevych, M. V.; Plotnikov, M. Yu.; Platonov, M. O.; Sabat, S. I.; Musyanovych, R. Ya.; Novikov, V. P. Heteroatom Chem. 2005, 16, 205–211. doi:10.1002/hc.20112 |
| 10. | Shi, S.; Katz, T. J.; Yang, B. V.; Liu, L. J. Org. Chem. 1995, 60, 1285–1297. doi:10.1021/jo00110a036 |
| 11. | Yu, M.; Malinakova, H. C.; Stagliano, K. W. J. Org. Chem. 2006, 71, 6648–6651. doi:10.1021/jo060825c |
| 16. | Konstantinova, L. S.; Rakitin, O. A.; Rees, C. W.; Amelichev, S. A. Mendeleev Commun. 2004, 14, 91–92. doi:10.1070/MC2004v014n03ABEH001912 |
| 25. | Hua, D. H.; Tamura, M.; Huang, X.; Stephany, H. A.; Helfrich, B. A.; Perchellet, E. M.; Sperfslage, B. J.; Perchellet, J.-P.; Jiang, S.; Kyle, D. E.; Chiang, P. K. J. Org. Chem. 2002, 67, 2907–2912. doi:10.1021/jo010958s |
| 9. | Konstantinova, L. S.; Rakitin, O. A. Mendeleev Commun. 2009, 19, 55–61. doi:10.1016/j.mencom.2009.03.001 |
| 21. | Kukharev, B. F.; Stankevich, V. K.; Kukhareva, V. A. Chem. Heterocycl. Comp. 1986, 22, 437–439. doi:10.1007/BF00542786 |
| 22. | Kukharev, B. F. Russ. Chem. Bull. 1997, 46, 1482–1483. doi:10.1007/BF02505694 |
| 8. | Rao, V. R.; Sharma, V. M.; Rao, T. V. P. Collect. Czech. Chem. Commun. 1993, 58, 1191–1194. doi:10.1135/cccc19931191 |
| 13. | Konstantinova, L. S.; Rakitin, O. A.; Rees, C. W.; Souvorova, L. I.; Torroba, T. J. Chem. Soc., Perkin Trans. 1 1999, 1023–1028. doi:10.1039/A809808E |
| 23. | Belyakov, P. A.; Kadentsev, V. I.; Chizhov, A. O.; Kolotyrkina, N. G.; Shashkov, A. S.; Ananikov, V. P. Mendeleev Commun. 2010, 20, 125–131. doi:10.1016/j.mencom.2010.05.001 |
© 2013 Konstantinova et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)