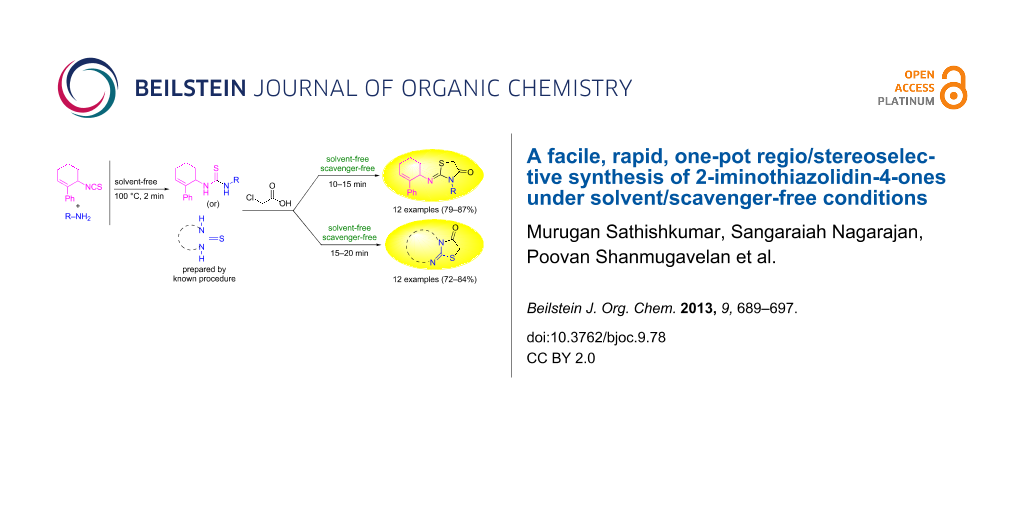
Abstract
A rapid and efficient one pot solvent/scavenger-free protocol for the synthesis of 2-iminothiazolidin-4-ones has been developed. Interestingly, the regio/stereoselective synthesis affords the regioisomeric (Z)-3-alkyl/aryl-2-(2-phenylcyclohex-2-enylimino)thiazolidin-4-one as the sole product in good yield. The selectivities observed have been rationalized based on the relative magnitude of the allylic strains developed during the course of the reaction. This is the first report wherein the impact of allylic strains in directing the regiocyclization has been noted.
Graphical Abstract
Introduction
Thiazolidin-4-one derivatives are well known for their bioactivities such as antidiabetic [1], anticancer [2], calcium-channel blocker [3,4], platelet activating factor (PAF) antagonist [5] and anti-HIV [6] activity. In addition, 2-iminothiazolidin-4-ones exhibit remarkable hypnotic [7,8], antitubercular [9], cardiovascular [10] and cyclooxygenase (COX) inhibitory [11] activities (Figure 1).
Figure 1: Medicinally relevant 2-iminothiazolidin-4-ones.
Figure 1: Medicinally relevant 2-iminothiazolidin-4-ones.
A common strategy involved in the prevailing synthetic protocols for 2-iminothiazolidin-4-ones [12-14] is the cyclization of thioureas with α-halocarboxylic acids [15] or acyl halides [16,17] or carboxylic esters [18]. These protocols are generally solution phase methods using organic solvents and acid scavengers. In the present scenario, such protocols may not be recommended by the principles of green chemistry. Consequently, the search for simple and efficient environmentally friendly methodologies for the synthesis of 2-iminothiazolidin-4-ones is worth attempting.
In this regard, and in continuation of our recent reports on the solvent-free synthesis of amides [19,20], thioamides [21], cyclic imides [22], thiazolidin-4-ones [23], spirothiazolidin-4-ones [24], 1,2,3-triazoles [25], 1,2,3-triazolylchalcones [26], and 1,2,3-triazolyldihydropyrimidine-2-thiones [27], we herein present a one-pot solvent/scavenger-free synthetic protocol for 2-iminothiazolidin-4-ones. This environmentally benign method avoids toxic organic solvents and acid scavengers, the details of which are presented below.
Results and Discussion
At the outset, optimization of the one-pot reaction was attempted by varying the solvents and using triethylamine as the acid scavenger (Table 1). The reaction was also attempted under solvent-free conditions. The latter was more promising in the sense that the reaction was very rapid affording the product 4f in 15–20 min (Table 1, entries 7 and 8) compared to 2–6 h (Table 1, entries 1–6) in solvents. The structure of the product 4f was assigned as (Z)-2-(2-phenylcyclohex-2-enylimino)-3-p-tolylthiazolidin-4-one based on the single-crystal XRD data [28,29] of its analogues (4c and 4j).
Table 1: Optimization of solution-phase and solvent-free synthesis of 2-iminothiazolidin-4-one 4f.
|
|
||||
| Entry | Solvent | Temperature | Time | Yield (%)a |
|---|---|---|---|---|
| 1 | ethanol | reflux | 4 h | 52 |
| 2 | acetonitrile | reflux | 2 h | 66 |
| 3 | dioxane | reflux | 4 h | 46 |
| 4 | THF | reflux | 3 h | 51 |
| 5 | acetonitrile/ethanol (1:1) | reflux | 3 h | 49 |
| 6 | DCM | reflux | 6 h | 41 |
| 7 | solvent-free | 80 °C | 20 min | 41 |
| 8 | solvent-free | 100 °C | 15 min | 54 |
aYield of isolated product.
Though the rate of reaction rate could be accelerated, the yield of 2-iminothiazolidin-4-one 4f was not good (41–66%) under both solution-phase and solvent-free conditions. Hence, as an attempt to optimize the yield, the solvent-free protocol was screened with and without the acid scavenger. Hereto, the yield of the product was poor (Table 2). Thus, the screening indicated that the scavengers had no positive, but rather an impeding effect. To develop an insight in this regard, a plausible mechanism of the reaction in the presence of the acid scavenger was proposed (Scheme 1).
From the mechanism, it can be envisaged that the acid scavenger may neutralize the HCl (that is generated during the course of the reaction) or the iminium ions by deprotonation. Also, another possibility is that the use of base as the scavenger may lead to the acid–base reaction resulting in the formation of the carboxylate anion of one the starting materials viz. chloroacetic acid or the thiourea-chloroacetic acid coupled product. This may retard the direct amine–carboxylic acid coupling, thus decreasing the yield of the product.
In view of the above perception, the solvent-free protocol was screened with one equivalent or in the absence of acid scavenger and varying equivalents of chloroacetic acid at 100 °C (Table 2). The optimum conditions were found to be with 3 equivalents of chloroacetic acid in the absence of acid scavenger affording a good yield of 2-iminothiazolidin-4-one 4f (Table 2, entry 6).
Table 2: Screening of base and equivalents of chloroacetic acid in the solvent-free synthesis of 2-iminothiazolidin-4-one 4f.
|
|
||||
| Entry | Base | Base (equiv) | Chloroacetic acid (equiv) | Yield (%)a |
|---|---|---|---|---|
| 1 | Et3N | 1 | 1 | 41 |
| 2 | K2CO3 | 1 | 1 | 25 |
| 3 | K2CO3 | 1 | 2 | 35 |
| 4 | NaOH | 1 | 2 | 24 |
| 5 | pyridine | 1 | 2 | 57 |
| 6 | — | — | 3 | 82 |
aYield of the isolated product.
In this context, it is pertinent to mention that while the prevailing solution-phase protocol [30-32] uses an acid scavenger, such as sodium hydroxide, triethylamine, pyridine or sodium acetate, the solvent-free methodology involved in the present investigation does not require any acid scavenger.
The scope of the new synthetic protocol was proved through the synthesis of a library of 2-iminothiazolidin-4-ones (Table 3). However, its limitations were realized when the synthesis of ortho-tolyl/1-napthyl analogues and the para-substituted (NO2 and COOH) phenyl analogues failed. Apparently, the reason for this can be attributed to the retardation of the nucleophilic attack of the amines on the isothiocyanate due to the steric effect (Figure 2) in the former, and decrease of the nucleophilicity of the amines by the electron-withdrawing group in the latter, thus not affording the expected thiourea.
Table 3: Solvent/scavenger-free synthesis of 2-iminothiazolidin-4-ones 4a–n.
|
|
|||
| Entry | Amine | Product | Yield (%)a |
|---|---|---|---|
| 1 |
|
4a |
79 |
| 2 |
|
4b |
84 |
| 3 |
|
4c |
87 |
| 4 |
|
4d |
84 |
| 5 |
|
4e |
80 |
| 6 |
|
4f |
82 |
| 7 |
|
4g |
80 |
| 8 |
|
4h |
83 |
| 9 |
|
4i |
85 |
| 10 |
|
4j |
81 |
| 11 |
|
4k |
— |
| 12 |
|
4l |
— |
| 13 |
|
4m |
trace |
| 14 |
|
4n |
— |
aYield of isolated product.
Figure 2: Retardation of the nucleophilic attack of amines on the isothiocyanate due to the steric effect.
Figure 2: Retardation of the nucleophilic attack of amines on the isothiocyanate due to the steric effect.
Having established the new protocol for the synthesis of 2-iminothiazolidin-4-ones, the method was extended to the rapid synthesis of a library of thiazolidinone derivatives (Table 4).
Table 4: Solvent/scavenger-free synthesis of thiazolidinone derivatives.
aYield of isolated product, bregioisomeric mixtures obtained.
Further, it is pertinent to mention here the interesting regio/stereoselectivity noted in the synthesis. Though the formation of the four regio/stereoisomeric 2-iminothiazolidin-4-ones 4a, 4b, 5a and 5b is possible, it is novel to note that only one of them, viz. 4b, is formed exclusively (Figure 3).
Figure 3: Possible regio/stereoisomeric products.
Figure 3: Possible regio/stereoisomeric products.
The high regio/stereoselectivity of the reaction can be rationalized based on the relative magnitudes of allylic strains (A1,2 and A1,3) developed during the course of the regiocyclization (Scheme 2).
Scheme 2: Regioselective cyclization in 2-iminothiazolidin-4-one synthesis directed by allylic strains.
Scheme 2: Regioselective cyclization in 2-iminothiazolidin-4-one synthesis directed by allylic strains.
In this context, it is relevant to recall the literature reports on the factors directing the regioselectivity in the synthesis of 2-iminothiazolidin-4-ones. Only a couple of reports in this regard are available in the literature. While one of these reports suggests that the pKa [17] of amines directs the regioselectivity, another investigation indicates that the chelating effect [18] of the substituent directs the regiochemical outcome. In both the reports, two regioisomeric 2-iminothiazolidin-4-ones are obtained. Thus, the present investigation affording a single regioisomeric product exclusively is the first report wherein the allylic strains are noted to direct the high regioselectivity.
Finally, the stereoselective formation of the (Z)-stereoisomer is also explicable based on allylic strain, which is summarized in Figure 4.
Figure 4: Stereoselectivity of the reaction directed by A1,3 strain.
Figure 4: Stereoselectivity of the reaction directed by A1,3 strain.
Conclusion
In conclusion, a new solvent/scavenger-free synthetic protocol for 2-iminothiazolidin-4-ones has been reported. Unlike the prevailing solution-phase protocols employing organic solvents and acid scavengers, the present study avoids solvents and scavengers. The rate of the reaction is prominently enhanced under solvent-free conditions compared to that in the solution phase. Apparently, the intimacy of the highly polar reactants in the fused state in the absence of solvent may be responsible for the rate enhancement.
Supporting Information
| Supporting Information File 1: Experimental procedures and product characterization for compounds (4a–j). | ||
| Format: PDF | Size: 2.9 MB | Download |
References
-
Ueno, H.; Oe, T.; Snehiro, I.; Nakamura, S. Preparation of 5-[2-naphthylmethyl(or methylene)]-thiazolidine-2,4-diones, 2-thioxy-thiazolidine-4-ones and 1H-tetrazoles for reducing blood sugar and blood lipid levels. U.S. Patent 5,594,016, Jan 14, 1997.
Return to citation in text: [1] -
Ebeid, M. Y.; Fathallah, O. A.; El-Zaher, M. I.; Kamel, M. M.; Abdon, W. A.; Anwar, M. M. Bull. Fac. Pharm. (Cairo Univ.) 1996, 34, 125–135.
Return to citation in text: [1] -
Hara, A.; Suzuki, T.; Hashizume, H.; Shishido, N.; Nakamura, M.; Ushikubi, F.; Abiko, Y. Eur. J. Pharmacol. 1999, 385, 81–88. doi:10.1016/S0014-2999(99)00708-6
Return to citation in text: [1] -
Kato, T.; Ozaki, T.; Tamura, K.; Suzuki, Y.; Akima, M.; Ohi, N. J. Med. Chem. 1999, 42, 3134–3146. doi:10.1021/jm9900927
Return to citation in text: [1] -
Tanabe, Y.; Suzukamo, G.; Komuro, Y.; Imanishi, N.; Morooka, S.; Enomoto, M.; Kojima, A.; Sanemitsu, Y.; Mizutani, M. Tetrahedron Lett. 1991, 32, 379–382. doi:10.1016/S0040-4039(00)92633-9
Return to citation in text: [1] -
Rawal, R. K.; Prabhakar, Y. S.; Katti, S. B.; De Clercq, E. Bioorg. Med. Chem. 2005, 13, 6771–6776. doi:10.1016/j.bmc.2005.07.063
Return to citation in text: [1] -
Chaudhari, S. K.; Verma, M.; Chaturvedi, A. K.; Parmar, S. S. J. Pharm. Sci. 1975, 64, 614–617. doi:10.1002/jps.2600640408
Return to citation in text: [1] -
Chaudhary, M.; Parmar, S. S.; Chaudhary, S. K.; Chaturvedi, A. K.; Rama Sastry, B. V. J. Pharm. Sci. 1976, 65, 443–446. doi:10.1002/jps.2600650336
Return to citation in text: [1] -
Turkevich, N. M.; Ladnaya, L. Y.; Pleshnev, I. V.; Grom, O. M. Khim. Issled. Farm. 1970, 64.
Chem. Abstr. 1972, 76, 34154.
Return to citation in text: [1] -
Nagar, S.; Singh, H. H.; Sinha, J. N.; Parmar, S. S. J. Med. Chem. 1973, 16, 178–180. doi:10.1021/jm00260a027
Return to citation in text: [1] -
Ottaná, R.; Mazzon, E.; Dugo, L.; Monforte, F.; Maccari, R.; Sautebin, L.; De Luca, G.; Vigorita, M. G.; Alcaro, S.; Ortuso, F.; Caputi, A. P.; Cuzzocrea, S. Eur. J. Pharmacol. 2002, 448, 71–80. doi:10.1016/S0014-2999(02)01888-5
Return to citation in text: [1] -
Blanchet, J.; Zhu, J. Tetrahedron Lett. 2004, 45, 4449–4452. doi:10.1016/j.tetlet.2004.04.055
Return to citation in text: [1] -
Sedlák, M.; Hanusek, J.; Macháček, V.; Hejtmánková, L. J. Heterocycl. Chem. 2002, 39, 1105–1107. doi:10.1002/jhet.5570390543
Return to citation in text: [1] -
Alizadeh, A.; Noaparast, Z.; Sabahno, H.; Zohreh, N. Helv. Chim. Acta 2010, 93, 1401–1406. doi:10.1002/hlca.200900402
Return to citation in text: [1] -
Kasmi-Mir, S.; Djafri, A.; Paquin, L.; Hamelin, J.; Rahmouni, M. Molecules 2006, 11, 597–602. doi:10.3390/11080597
Return to citation in text: [1] -
Saeed, A.; Abbas, N.; Flörke, U. J. Braz. Chem. Soc. 2007, 18, 559–565. doi:10.1590/S0103-50532007000300010
Return to citation in text: [1] [2] [3] -
Yella, R.; Ghosh, H.; Patel, B. K. Green Chem. 2008, 10, 1307–1312. doi:10.1039/B807775D
Return to citation in text: [1] [2] [3] [4] [5] -
St. Laurent, D. R.; Gao, Q.; Wu, D.; Serrano-Wu, M. H. Tetrahedron Lett. 2004, 45, 1907–1910. doi:10.1016/j.tetlet.2004.01.001
Return to citation in text: [1] [2] -
Sathishkumar, M.; Shanmugavelan, P.; Nagarajan, S.; Maheswari, M.; Dinesh, M.; Ponnuswamy, A. Tetrahedron Lett. 2011, 52, 2830–2833. doi:10.1016/j.tetlet.2011.03.069
Return to citation in text: [1] -
Nagarajan, S.; Ran, P.; Shanmugavelan, P.; Sathishkumar, M.; Ponnusamy, A.; Nahm, K. S.; Gnanakumar, G. New J. Chem. 2012, 36, 1312–1319. doi:10.1039/c2nj40119c
Return to citation in text: [1] -
Nagarajan, S.; Shanmugavelan, P.; Sathishkumar, M.; Priyadharshini, N.; Sudakar, P.; Ponnuswamy, A. Synth. Commun. 2013, 43, 668–680. doi:10.1080/00397911.2011.606043
Return to citation in text: [1] -
Sathishkumar, M.; Palanikumar, K.; Mariappan, A.; Archana, S.; Ponnuswamy, A. J. Iran. Chem. Soc. 2012, 9, 681–685. doi:10.1007/s13738-012-0090-7
Return to citation in text: [1] -
Shanmugavelan, P.; Sathishkumar, M.; Nagarajan, S.; Ponnuswamy, A. J. Heterocycl. Chem., in press.
Return to citation in text: [1] -
Ponnuswamy, A.; Shanmugavelan, P.; Nagarajan, S.; Sathishkumar, M. Helv. Chim. Acta 2012, 95, 922–928. doi:10.1002/hlca.201100441
Return to citation in text: [1] -
Shanmugavelan, P.; Nagarajan, S.; Sathishkumar, M.; Ponnuswamy, A.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2011, 21, 7273–7276. doi:10.1016/j.bmcl.2011.10.048
Return to citation in text: [1] -
Shanmugavelan, P.; Sathishkumar, M.; Nagarajan, S.; Ponnuswamy, A. J. Chem. Sci. 2012, 124, 941–950. doi:10.1007/s12039-012-0281-x
Return to citation in text: [1] -
Nagarajan, S.; Sathishkumar, M.; Shanmugavelan, P.; Ranganathan, R.; Ponnuswamy, A.; Venkatesan, R.; Shanmugaiah, V. Eur. J. Med. Chem. 2012, 58, 464–469. doi:10.1016/j.ejmech.2012.10.029
Return to citation in text: [1] -
Ooi, C. W.; Fun, H.-K.; Quah, C. K.; Sathishkumar, M.; Ponnuswamy, A. Acta Crystallogr., Sect. E 2012, 68, o2563–2564. doi:10.1107/S1600536812033211
Return to citation in text: [1] -
Ooi, C. W.; Fun, H.-K.; Quah, C. K.; Sathishkumar, M.; Ponnuswamy, A. Acta Crystallogr., Sect. E 2012, 68, o1994. doi:10.1107/S1600536812024646
Return to citation in text: [1] -
Mamaghani, M.; Loghmanifar, A.; Taati, M. R. Ultrason. Sonochem. 2011, 18, 45–48. doi:10.1016/j.ultsonch.2010.05.009
Return to citation in text: [1] -
Ottanà, R.; Maccari, R.; Barreca, M. L.; Bruno, G.; Rotondo, A.; Chiricosta, G.; Di Paola, R.; Sautebin, L.; Cuzzocrea, S.; Vigorita, M. G. Bioorg. Med. Chem. 2005, 13, 4243–4252. doi:10.1016/j.bmc.2005.04.058
Return to citation in text: [1] -
Mavrova, A. T.; Anichina, K. K.; Vuchev, D. I.; Tsenov, J. A.; Kondeva, M. S.; Micheva, M. K. Bioorg. Med. Chem. 2005, 13, 5550–5559. doi:10.1016/j.bmc.2005.06.046
Return to citation in text: [1] [2] -
Kulakov, I. V. Russ. J. Org. Chem. 2009, 45, 1262–1263. doi:10.1134/S1070428009080296
Return to citation in text: [1] [2] [3] [4] -
Feng, Y.; Ding, X.; Chen, T.; Chen, L.; Liu, F.; Jia, X.; Luo, X.; Shen, X.; Chen, K.; Jiang, H.; Wang, H.; Liu, H.; Liu, D. J. Med. Chem. 2010, 53, 3465–3479. doi:10.1021/jm901004c
Return to citation in text: [1] [2]
| 16. | Saeed, A.; Abbas, N.; Flörke, U. J. Braz. Chem. Soc. 2007, 18, 559–565. doi:10.1590/S0103-50532007000300010 |
| 33. | Kulakov, I. V. Russ. J. Org. Chem. 2009, 45, 1262–1263. doi:10.1134/S1070428009080296 |
| 33. | Kulakov, I. V. Russ. J. Org. Chem. 2009, 45, 1262–1263. doi:10.1134/S1070428009080296 |
| 1. | Ueno, H.; Oe, T.; Snehiro, I.; Nakamura, S. Preparation of 5-[2-naphthylmethyl(or methylene)]-thiazolidine-2,4-diones, 2-thioxy-thiazolidine-4-ones and 1H-tetrazoles for reducing blood sugar and blood lipid levels. U.S. Patent 5,594,016, Jan 14, 1997. |
| 6. | Rawal, R. K.; Prabhakar, Y. S.; Katti, S. B.; De Clercq, E. Bioorg. Med. Chem. 2005, 13, 6771–6776. doi:10.1016/j.bmc.2005.07.063 |
| 21. | Nagarajan, S.; Shanmugavelan, P.; Sathishkumar, M.; Priyadharshini, N.; Sudakar, P.; Ponnuswamy, A. Synth. Commun. 2013, 43, 668–680. doi:10.1080/00397911.2011.606043 |
| 18. | St. Laurent, D. R.; Gao, Q.; Wu, D.; Serrano-Wu, M. H. Tetrahedron Lett. 2004, 45, 1907–1910. doi:10.1016/j.tetlet.2004.01.001 |
| 5. | Tanabe, Y.; Suzukamo, G.; Komuro, Y.; Imanishi, N.; Morooka, S.; Enomoto, M.; Kojima, A.; Sanemitsu, Y.; Mizutani, M. Tetrahedron Lett. 1991, 32, 379–382. doi:10.1016/S0040-4039(00)92633-9 |
| 22. | Sathishkumar, M.; Palanikumar, K.; Mariappan, A.; Archana, S.; Ponnuswamy, A. J. Iran. Chem. Soc. 2012, 9, 681–685. doi:10.1007/s13738-012-0090-7 |
| 3. | Hara, A.; Suzuki, T.; Hashizume, H.; Shishido, N.; Nakamura, M.; Ushikubi, F.; Abiko, Y. Eur. J. Pharmacol. 1999, 385, 81–88. doi:10.1016/S0014-2999(99)00708-6 |
| 4. | Kato, T.; Ozaki, T.; Tamura, K.; Suzuki, Y.; Akima, M.; Ohi, N. J. Med. Chem. 1999, 42, 3134–3146. doi:10.1021/jm9900927 |
| 18. | St. Laurent, D. R.; Gao, Q.; Wu, D.; Serrano-Wu, M. H. Tetrahedron Lett. 2004, 45, 1907–1910. doi:10.1016/j.tetlet.2004.01.001 |
| 32. | Mavrova, A. T.; Anichina, K. K.; Vuchev, D. I.; Tsenov, J. A.; Kondeva, M. S.; Micheva, M. K. Bioorg. Med. Chem. 2005, 13, 5550–5559. doi:10.1016/j.bmc.2005.06.046 |
| 2. | Ebeid, M. Y.; Fathallah, O. A.; El-Zaher, M. I.; Kamel, M. M.; Abdon, W. A.; Anwar, M. M. Bull. Fac. Pharm. (Cairo Univ.) 1996, 34, 125–135. |
| 19. | Sathishkumar, M.; Shanmugavelan, P.; Nagarajan, S.; Maheswari, M.; Dinesh, M.; Ponnuswamy, A. Tetrahedron Lett. 2011, 52, 2830–2833. doi:10.1016/j.tetlet.2011.03.069 |
| 20. | Nagarajan, S.; Ran, P.; Shanmugavelan, P.; Sathishkumar, M.; Ponnusamy, A.; Nahm, K. S.; Gnanakumar, G. New J. Chem. 2012, 36, 1312–1319. doi:10.1039/c2nj40119c |
| 17. | Yella, R.; Ghosh, H.; Patel, B. K. Green Chem. 2008, 10, 1307–1312. doi:10.1039/B807775D |
| 11. | Ottaná, R.; Mazzon, E.; Dugo, L.; Monforte, F.; Maccari, R.; Sautebin, L.; De Luca, G.; Vigorita, M. G.; Alcaro, S.; Ortuso, F.; Caputi, A. P.; Cuzzocrea, S. Eur. J. Pharmacol. 2002, 448, 71–80. doi:10.1016/S0014-2999(02)01888-5 |
| 15. | Kasmi-Mir, S.; Djafri, A.; Paquin, L.; Hamelin, J.; Rahmouni, M. Molecules 2006, 11, 597–602. doi:10.3390/11080597 |
| 34. | Feng, Y.; Ding, X.; Chen, T.; Chen, L.; Liu, F.; Jia, X.; Luo, X.; Shen, X.; Chen, K.; Jiang, H.; Wang, H.; Liu, H.; Liu, D. J. Med. Chem. 2010, 53, 3465–3479. doi:10.1021/jm901004c |
| 10. | Nagar, S.; Singh, H. H.; Sinha, J. N.; Parmar, S. S. J. Med. Chem. 1973, 16, 178–180. doi:10.1021/jm00260a027 |
| 16. | Saeed, A.; Abbas, N.; Flörke, U. J. Braz. Chem. Soc. 2007, 18, 559–565. doi:10.1590/S0103-50532007000300010 |
| 17. | Yella, R.; Ghosh, H.; Patel, B. K. Green Chem. 2008, 10, 1307–1312. doi:10.1039/B807775D |
| 34. | Feng, Y.; Ding, X.; Chen, T.; Chen, L.; Liu, F.; Jia, X.; Luo, X.; Shen, X.; Chen, K.; Jiang, H.; Wang, H.; Liu, H.; Liu, D. J. Med. Chem. 2010, 53, 3465–3479. doi:10.1021/jm901004c |
| 9. |
Turkevich, N. M.; Ladnaya, L. Y.; Pleshnev, I. V.; Grom, O. M. Khim. Issled. Farm. 1970, 64.
Chem. Abstr. 1972, 76, 34154. |
| 33. | Kulakov, I. V. Russ. J. Org. Chem. 2009, 45, 1262–1263. doi:10.1134/S1070428009080296 |
| 7. | Chaudhari, S. K.; Verma, M.; Chaturvedi, A. K.; Parmar, S. S. J. Pharm. Sci. 1975, 64, 614–617. doi:10.1002/jps.2600640408 |
| 8. | Chaudhary, M.; Parmar, S. S.; Chaudhary, S. K.; Chaturvedi, A. K.; Rama Sastry, B. V. J. Pharm. Sci. 1976, 65, 443–446. doi:10.1002/jps.2600650336 |
| 12. | Blanchet, J.; Zhu, J. Tetrahedron Lett. 2004, 45, 4449–4452. doi:10.1016/j.tetlet.2004.04.055 |
| 13. | Sedlák, M.; Hanusek, J.; Macháček, V.; Hejtmánková, L. J. Heterocycl. Chem. 2002, 39, 1105–1107. doi:10.1002/jhet.5570390543 |
| 14. | Alizadeh, A.; Noaparast, Z.; Sabahno, H.; Zohreh, N. Helv. Chim. Acta 2010, 93, 1401–1406. doi:10.1002/hlca.200900402 |
| 33. | Kulakov, I. V. Russ. J. Org. Chem. 2009, 45, 1262–1263. doi:10.1134/S1070428009080296 |
| 25. | Shanmugavelan, P.; Nagarajan, S.; Sathishkumar, M.; Ponnuswamy, A.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2011, 21, 7273–7276. doi:10.1016/j.bmcl.2011.10.048 |
| 23. | Shanmugavelan, P.; Sathishkumar, M.; Nagarajan, S.; Ponnuswamy, A. J. Heterocycl. Chem., in press. |
| 24. | Ponnuswamy, A.; Shanmugavelan, P.; Nagarajan, S.; Sathishkumar, M. Helv. Chim. Acta 2012, 95, 922–928. doi:10.1002/hlca.201100441 |
| 17. | Yella, R.; Ghosh, H.; Patel, B. K. Green Chem. 2008, 10, 1307–1312. doi:10.1039/B807775D |
| 16. | Saeed, A.; Abbas, N.; Flörke, U. J. Braz. Chem. Soc. 2007, 18, 559–565. doi:10.1590/S0103-50532007000300010 |
| 17. | Yella, R.; Ghosh, H.; Patel, B. K. Green Chem. 2008, 10, 1307–1312. doi:10.1039/B807775D |
| 17. | Yella, R.; Ghosh, H.; Patel, B. K. Green Chem. 2008, 10, 1307–1312. doi:10.1039/B807775D |
| 28. | Ooi, C. W.; Fun, H.-K.; Quah, C. K.; Sathishkumar, M.; Ponnuswamy, A. Acta Crystallogr., Sect. E 2012, 68, o2563–2564. doi:10.1107/S1600536812033211 |
| 29. | Ooi, C. W.; Fun, H.-K.; Quah, C. K.; Sathishkumar, M.; Ponnuswamy, A. Acta Crystallogr., Sect. E 2012, 68, o1994. doi:10.1107/S1600536812024646 |
| 30. | Mamaghani, M.; Loghmanifar, A.; Taati, M. R. Ultrason. Sonochem. 2011, 18, 45–48. doi:10.1016/j.ultsonch.2010.05.009 |
| 31. | Ottanà, R.; Maccari, R.; Barreca, M. L.; Bruno, G.; Rotondo, A.; Chiricosta, G.; Di Paola, R.; Sautebin, L.; Cuzzocrea, S.; Vigorita, M. G. Bioorg. Med. Chem. 2005, 13, 4243–4252. doi:10.1016/j.bmc.2005.04.058 |
| 32. | Mavrova, A. T.; Anichina, K. K.; Vuchev, D. I.; Tsenov, J. A.; Kondeva, M. S.; Micheva, M. K. Bioorg. Med. Chem. 2005, 13, 5550–5559. doi:10.1016/j.bmc.2005.06.046 |
| 26. | Shanmugavelan, P.; Sathishkumar, M.; Nagarajan, S.; Ponnuswamy, A. J. Chem. Sci. 2012, 124, 941–950. doi:10.1007/s12039-012-0281-x |
| 27. | Nagarajan, S.; Sathishkumar, M.; Shanmugavelan, P.; Ranganathan, R.; Ponnuswamy, A.; Venkatesan, R.; Shanmugaiah, V. Eur. J. Med. Chem. 2012, 58, 464–469. doi:10.1016/j.ejmech.2012.10.029 |
© 2013 Sathishkumar et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)