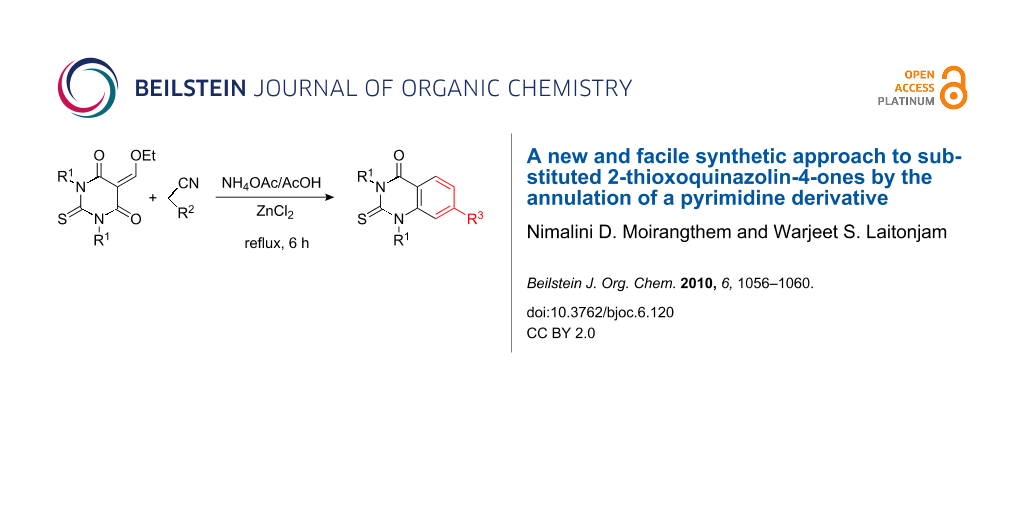
Abstract
A new and facile synthesis of 2-thioxoquinazolin-4-ones by introducing a benzenoid system in the pyrimidine moiety by reacting ethoxymethylene derivatives of 1,3-diarylthiobarbituric acids (DTBA) with active methylene compounds, such as malononitrile and ethyl cyanoacetate, in presence of ZnCl2 has been developed.
Graphical Abstract
Introduction
Quinazolines and derivatives are of much interest due to their biological activities [1,2]. Additionally, quinazolines are interesting targets for new method development due to their importance in numerous therapeutic areas. Recently, antitumor [3] and anti-HIV activities [4,5] of quinazolines have been described. A large number of quinazoline derivatives, which contain the 4-oxo-2-thioxo-1,2,3,4-tetrahydropyrimidine structural moiety in their heterocyclic rings, possess a wide range of biological activities [6-8]. There are a number of synthetic methods available for the preparation of quinazolines [9]. The most common synthetic route involves the amidation of 2-aminobenzoic acid or its derivatives, i.e., 2-aminobenzonitrile, 2-aminobenzoates, and 2-arylnitrilium salts, followed by oxidative ring closure [10-13]. Other synthetic pathways include the cyclization of anthranilamides with aldehydes [14], and with ketones or acid chlorides under acidic or basic conditions [15-17]. However, most of the methods involve multistep processes and time-consuming experimental procedures, and give poor yields or use toxic reagents. Moreover, very few methods are reported for the synthesis of 2-thioxoquinazolin-4-ones, as most of the methods reported are for quinazolin-2,4(1H,3H)-diones. Recently, Saeed et al. [18] reported the base catalyzed intramolecular nucleophilic cyclization of substituted thioureas in the presence of DMF to afford 2-thioxoquinazolin-4-ones. The preparation of 2-thioxoquinazolin-4-one libraries by solid-phase synthesis has been reported [19-21].
There are two approaches for the solution-phase parallel synthesis of 2-thioxoquinazolin-4-ones [22]. The first approach is based on the reaction of methyl anthranilates with isothiocyanates in refluxing pyridine or DMF. The second approach involves briefly heating 2-(methylcarboxy)-benzeneisothiocyanates in isopropyl alcohol with a wide variety of primary aliphatic or aromatic amines and their derivatives. Thus, most of the methods for the preparation of such compounds start with the benzene ring in place followed by construction of the pyrimidine ring. We have developed a new facile and convenient synthetic approach to 2-thioxoquinazolin-4-ones by constructing the benzene ring onto an existing pyrimidine moiety.
As a part of our synthetic strategy, 1,3-diarylthiobarbituric acids (DTBA) were used as precursors for the synthesis of various fused heterocyclic compounds. In recent years, we have reported one-pot cyclizations of DTBA with hydrazine [23,24], hydroxylamine [25], guanidine [26], etc. In addition, one-pot cyclizations of DTBA-derived arylidenes have also been reported. Recently, we reported the synthesis of fused heterocycles from ethoxymethylene derivatives of DTBA [27]. In continuation of our work on the synthesis of fused heterocycles [28,29], we herein report full details of the work and studies related to the synthesis of 2-thioxoquinazolin-4-ones from the reaction of ethoxymethylene derivatives of DTBA and active methylene compounds, such as, malononitrile and ethylcyanoacetate.
Results and Discussion
DTBA are among the simplest synthetic intermediates and can be easily prepared in a one-pot reaction by treating 1,3-diaryl thioureas with malonic acid in the presence of acetyl chloride. DTBA undergoes condensation with ethyl orthoformate to give the condensation products, 5-ethoxymethylene-1,3-diaryl-2-thiobarbituric acids 1. These condensation products possess three electrophilic centers and can undergo cyclocondensation with various nucleophiles to give a number of fused heterocyclic systems that contain a pyrimidine ring. Thus, treatment of 1 with malononitrile in presence of NH4OAc with ZnCl2 as catalyst in refluxing acetic acid gives the corresponding 2-thioxoquinazolin-4-ones 2 in 78–85% overall yields (Scheme 1).
Scheme 1: Synthesis of 2, reagents and conditions: (i) CH2(CN)2, NH4OAc/AcOH, reflux, ZnCl2 (ii) H+/H2O.
Scheme 1: Synthesis of 2, reagents and conditions: (i) CH2(CN)2, NH4OAc/AcOH, reflux, ZnCl2 (ii) H+/H2O.
During the optimization of the cyclization of ethoxymethylene derivatives of DTBA with malononitrile, the choice of the base proved to be an important parameter. The use of NEt3 (TEA) or piperidine (in DCM or ethanol) resulted in the formation of complex mixtures (Table 1, entries 1–4). Screening at different temperatures demonstrates that some of the catalysts failed to react at room temperature (rt) and also even after heating under reflux (Table 1, entries 5–8). The reaction failed with both ZnCl2 and FeCl3 in MeOH solution at rt. Similarly, no reaction was observed with NaOCH3 and MeOH at rt. On the other hand, with NaOCH3 as base in MeOH and ZnCl2 as catalyst at rt, a relatively low yield of 2a was obtained (Table 1, entry 9). However, when this reaction was repeated in refluxing solvent the yield was increased.
Table 1: Effect of base and solvent on the yield for the synthesis of 2a.
| Entry | Conditions | Yieldc (%) |
|---|---|---|
| 1 | NEt3 in DCM | no product |
| 2 | NEt3 in EtOH | no product |
| 3 | piperidine in DCM | no product |
| 4 | piperidine in EtOH | no product |
| 5 | ZnCl2 in MeOHa,b | no product |
| 6 | FeCl3 in MeOHa,b | no product |
| 7 | AlCl3 in MeOH a,b | no product |
| 8 | NaOCH3 in MeOHa | no product |
| 9 | NaOCH3 in MeOHb | 24 |
| 10 | NaOCH3 in MeOH, ZnCl2b | 35 |
| 11 | NH4OAc in AcOHb | 67 |
| 12 | NH4OAc in AcOHb | 85 |
aReactions were carried out at room temperature. bReactions were carried out at reflux. cIsolated yield.
In contrast, the use of NH4OAc in refluxing acetic acid resulted in a clean cyclization to give the desired product. Dehydration and decarboxylation induced by the higher temperature and the acid produces the required quinazoline. To obtain the optimal conditions, a variety of catalysts were also investigated to detect the catalytic activities of different metal ions and acetate in the production of 2a (Table 2). It was found that NH4OAc/AcOH in ZnCl2 was the most effective (Table 2, entries 1 and 7–13); CuCl2 and HgCl2 also promoted the reactions, but the yields were poor, 22% and 12%, respectively (Table 2, entries 2 and 3). Other catalysts, including FeCl3, AlCl3 etc. failed to afford any 2a (Table 2, entries 4–6). We further found that the best yield of 2a was obtained when 5 equiv of ZnCl2 was used (Table 2, entry 13). The excessive amount of ZnCl2 for the annulation is probably due to the chelating effect of zinc ion. Thus, the NH4OAc/AcOH combination in ZnCl2 was found to be the best and gave the highest yield of 2b (85%) after refluxing for 6 h.
Table 2: Effect of catalysts in the yield for synthesis of 2aa.
| Entry | Conditions | Yieldb (%) |
|---|---|---|
| 1 | ZnCl2 (1 equiv)a | 30 |
| 2 | CuCl2 (1 equiv)a | 22 |
| 3 | HgCl2 (1 equiv)a | 12 |
| 4 | FeCl3 (1 equiv)a | 0 |
| 5 | AlCl3 (1 equiv)a | 0 |
| 6 | SnCl2 (1 equiv)a | 0 |
| 7 | ZnCl2 (0.5 equiv)a | 18 |
| 8 | ZnCl2 (2 equiv)a | 45 |
| 9 | ZnCl2 (5 equiv, 2 h)a | 62 |
| 10 | ZnCl2 (5 equiv, rt, 4 h) | 28 |
| 11 | ZnCl2 (5 equiv, rt, 6 h) | 38 |
| 12 | ZnCl2 (5 equiv, 4 h)a | 74 |
| 13 | ZnCl2 (5 equiv, 6 h)a | 85 |
aReactions were carried out with NH4OAc and AcOH at reflux. bIsolated yield.
After optimizing the conditions, various DTBAs were used to react with malononitrile and the results are listed in Table 3. On the basis of the above noted results, a possible reaction mechanism is shown in Scheme 2. The reaction of the 5-ethoxymethylene-1,3-diaryl-2-thiobarbituric acids with malononitrile gave intermediate A, which undergoes intramolecular cyclization to form the intermediate B, and then acid hydrolysis of B afforded 2. Further evidence is that the reaction of 5-ethoxymethylene-1,3-diaryl-2-thiobarbituric acids with malononitrile under the standard conditions. This reaction only gave quinazolines and no other products were detected. In addition, this proposed mechanism was also confirmed from the literature [30,31].
Table 3: Synthesis of 7-amino-2,3-dihydro-2-thioxo-1,3-diarylquinazolin-4(1H)-onesa.
| Product | R | Yield (%)b |
|---|---|---|
| 2a | 2-CH3C6H4 | 83 |
| 2b | 4-ClC6H4 | 85 |
| 2c | 2-OCH3C6H4 | 78 |
aReactions were carried out with NH4OAc and AcOH at reflux. bIsolated yield.
Scheme 2: Synthesis of 2, reagents and conditions: (i) CH2(CN)2, NH4OAc/AcOH, reflux, ZnCl2 (ii) H+/H2O.
Scheme 2: Synthesis of 2, reagents and conditions: (i) CH2(CN)2, NH4OAc/AcOH, reflux, ZnCl2 (ii) H+/H2O.
The reaction of 5-ethoxymethylene-1,3-diaryl-2-thiobarbituric acids 1 with ethylcyanoacetate in presence of ammonium acetate and acetic acid with ZnCl2 as a catalyst afforded 7-hydroxy-2,3-dihydro-2-thioxo-1,3-diarylquinazolin-4(1H)-ones 3 in 76–87% overall yields (Scheme 3) [32,33] and these results are listed in Table 4.
Scheme 3: Synthesis of 3, reagents and conditions: (i) NC-CH2-CO2Et, NH4OAc/AcOH, reflux, ZnCl2 (ii) H3O+.
Scheme 3: Synthesis of 3, reagents and conditions: (i) NC-CH2-CO2Et, NH4OAc/AcOH, reflux, ZnCl2 (ii) H3O+.
Conclusion
The cyclocondensation of ethoxymethylene thiobarbituric acids with active methylene compounds under the above noted catalytic system, resulted in a new method for the formation of quinazoline derivatives. Thus, the reaction of 5-ethoxymethylenepyrimidine-4,6-diones 1 with malononitrile and ethyl cyanoacetate gave 7-amino-2,3-dihydro-2-thioxo-1,3-diarylquinazolin-4(1H)-ones 2 and 7-hydroxy-2,3-dihydro-2-thioxo-1,3-diarylquinozolin-4(1H)-ones 3, respectively. This new procedure avoids the use of toxic reagents which are traditionally used for the preparation of quinazolines.
Supporting Information
| Supporting Information File 1: Experimental part. | ||
| Format: PDF | Size: 142.1 KB | Download |
| Supporting Information File 2:
IR and NMR spectra.
Supporting Information feature copies of IR and 1H NMR spectra of 7-amino-2,3-dihydro-2-thioxo-1,3-di(2-methoxyphenyl)quinazolin-4(1H)-one (2c) and 1H and 13C NMR spectra of 7-hydroxy-2,3-dihydro-2-thioxo-1,3-di(2-methylphenyl)quinazolin-4(1H)-one (3a). |
||
| Format: PDF | Size: 436.1 KB | Download |
References
-
Connolly, D. J.; Cusack, D.; O’Sullivian, T. P.; Guiry, P. J. Tetrahedron 2005, 61, 10153–10202. doi:10.1016/j.tet.2005.07.010
Return to citation in text: [1] -
Mhaske, S. B.; Argade, N. P. Tetrahedron 2006, 62, 9787–9826. doi:10.1016/j.tet.2006.07.098
Return to citation in text: [1] -
Xia, Y.; Yang, Z.-Y.; Hour, M.-J.; Kuo, S.-C.; Xia, P.; Bastow, K. F.; Nakanishi, Y.; Nampoothiri, P.; Hackl, T.; Hamel, E.; Lee, K.-H. Bioorg. Med. Chem. Lett. 2001, 11, 1193–1196. doi:10.1016/S0960-894X(01)00190-1
Return to citation in text: [1] -
De Clercq, E. Curr. Med. Chem. 2001, 8, 1543–1572.
Return to citation in text: [1] -
Corbett, J. W. Curr. Med. Chem. - Anti-Infect. Agents 2002, 1, 119–140.
Return to citation in text: [1] -
LeMahieu, R. A.; Carson, M.; Welton, A. F.; Baruth, H. W.; Yaremko, B. J. Med. Chem. 1983, 26, 107–110. doi:10.1021/jm00355a022
Return to citation in text: [1] -
Vandenberk, J.; Kennis, L.; Van der Aa, M.; Van Heertum, A. U.S. Patent 4, 522, 945, June 11, 1985.
Return to citation in text: [1] -
Sohda, T.; Makino, H.; Baba, A. Eur. Patent EP0567107, Oct 27, 1993.
Return to citation in text: [1] -
Jiarong, L.; Xian, C.; Daxin, S.; Shuling, M.; Qing, L.; Qi, Z.; Jianhong, T. Org. Lett. 2009, 11, 1193–1196. doi:10.1021/ol900093h
Return to citation in text: [1] -
Li, Z.; Huang, H.; Sun, H.; Jiang, H.; Liu, H. J. Comb. Chem. 2008, 10, 484–486. doi:10.1021/cc800040z
Return to citation in text: [1] -
Patil, Y. P.; Tambade, P. J.; Parghi, K. D.; Jayaram, R. V.; Bhanage, B. M. Catal. Lett 2009, 133, 201–208. doi:10.1007/s10562-009-0126-5
Return to citation in text: [1] -
Couture, A.; Cornet, H.; Grandclaudon, P. Synthesis 1991, 1009–1010. doi:10.1055/s-1991-26632
Return to citation in text: [1] -
Kotsuki, H.; Sakai, H.; Morimoto, H.; Suenaga, H. Synlett 1999, 1993–1995. doi:10.1055/s-1999-2998
Return to citation in text: [1] -
Abdel-Jalil, R. L.; Voelter, W.; Saeed, M. Tetrahedron Lett. 2004, 45, 3475–3476. doi:10.1016/j.tetlet.2004.03.003
Return to citation in text: [1] -
Feldman, J. R.; Wagner, E. C. J. Org. Chem. 1942, 7, 31–47. doi:10.1021/jo01195a006
Return to citation in text: [1] -
Yale, H. L. J. Heterocycl. Chem. 1977, 14, 1357–1359. doi:10.1002/jhet.5570140812
Return to citation in text: [1] -
Mhaske, S. B.; Argade, N. P. J. Org. Chem. 2004, 69, 4563–4566. doi:10.1021/jo040153v
Return to citation in text: [1] -
Saeed, A.; Shaheen, U.; Bolte, M. J. Chin. Chem. Soc. 2010, 57, 82–88.
Return to citation in text: [1] -
Makino, S.; Suzuki, N.; Nakanishi, E.; Tsuji, T. Tetrahedron Lett. 2000, 41, 8333–8337. doi:10.1016/S0040-4039(00)01442-8
Return to citation in text: [1] -
Makino, S.; Nakanishi, E.; Tsuji, T. Tetrahedron Lett. 2001, 42, 1749–1752. doi:10.1016/S0040-4039(01)00008-9
Return to citation in text: [1] -
Makino, S.; Nakanishi, E.; Tsuji, T. Bull. Korean Chem. Soc. 2003, 24, 389–392. doi:10.5012/bkcs.2003.24.3.389
Return to citation in text: [1] -
Ivachtchenko, A. V.; Kovalenko, S. M.; Drushlyak, O. G. J. Comb. Chem. 2003, 5, 775–788. doi:10.1021/cc020097g
Return to citation in text: [1] -
Devi, N. A.; Laitonjam, W. S. Indian J. Chem. 1994, 33B, 1091–1092.
Return to citation in text: [1] -
Devi, N. A.; Laitonjam, W. S. Indian J. Heterocycl. Chem. 1995, 5, 139–140.
Return to citation in text: [1] -
Devi, N. A.; Laitonjam, W. S. Indian J. Chem. 1996, 35B, 478–479.
Return to citation in text: [1] -
Devi, N. A.; Khuman, C. K.; Singh, R. K. T.; Laitonjam, W. S. Indian J. Heterocycl. Chem. 1998, 7, 193–196.
Return to citation in text: [1] -
Thokchom, H. S.; Devi, N. A.; Laitonjam, W. S. Can. J. Chem. 2005, 83, 1056–1062. doi:10.1139/v05-054
Return to citation in text: [1] -
Laitonjam, W. S.; Rajkumar, T. S.; Chingakham, B. S. Steroids 2002, 67, 203–209. doi:10.1016/S0039-128X(01)00146-5
Return to citation in text: [1] -
Tombisana, R. K.; Laitonjam, W. S. Indian J. Chem. 1999, 38B, 847–849.
Return to citation in text: [1] -
Green, B.; Khaidem, I. S.; Crane, R. I.; Newaz, S. S. Tetrahedron 1976, 32, 2997–3001. doi:10.1016/0040-4020(76)80158-5
Return to citation in text: [1] -
Khaidem, I. S.; Sagolsem, L. S.; Laishram, R. S.; Khan, M. Z. R. Indian J. Chem. 1996, 35B, 911–914.
Return to citation in text: [1] -
Ghosh, A. K.; Bilcer, G.; Schiltz, G. Synthesis 2001, 2203–2229. doi:10.1055/s-2001-18434
Return to citation in text: [1] -
List, B.; Castello, C. Synlett 2001, 1687–1689. doi:10.1055/s-2001-18095
Return to citation in text: [1]
| 30. | Green, B.; Khaidem, I. S.; Crane, R. I.; Newaz, S. S. Tetrahedron 1976, 32, 2997–3001. doi:10.1016/0040-4020(76)80158-5 |
| 31. | Khaidem, I. S.; Sagolsem, L. S.; Laishram, R. S.; Khan, M. Z. R. Indian J. Chem. 1996, 35B, 911–914. |
| 32. | Ghosh, A. K.; Bilcer, G.; Schiltz, G. Synthesis 2001, 2203–2229. doi:10.1055/s-2001-18434 |
| 33. | List, B.; Castello, C. Synlett 2001, 1687–1689. doi:10.1055/s-2001-18095 |
| 1. | Connolly, D. J.; Cusack, D.; O’Sullivian, T. P.; Guiry, P. J. Tetrahedron 2005, 61, 10153–10202. doi:10.1016/j.tet.2005.07.010 |
| 2. | Mhaske, S. B.; Argade, N. P. Tetrahedron 2006, 62, 9787–9826. doi:10.1016/j.tet.2006.07.098 |
| 9. | Jiarong, L.; Xian, C.; Daxin, S.; Shuling, M.; Qing, L.; Qi, Z.; Jianhong, T. Org. Lett. 2009, 11, 1193–1196. doi:10.1021/ol900093h |
| 27. | Thokchom, H. S.; Devi, N. A.; Laitonjam, W. S. Can. J. Chem. 2005, 83, 1056–1062. doi:10.1139/v05-054 |
| 6. | LeMahieu, R. A.; Carson, M.; Welton, A. F.; Baruth, H. W.; Yaremko, B. J. Med. Chem. 1983, 26, 107–110. doi:10.1021/jm00355a022 |
| 7. | Vandenberk, J.; Kennis, L.; Van der Aa, M.; Van Heertum, A. U.S. Patent 4, 522, 945, June 11, 1985. |
| 8. | Sohda, T.; Makino, H.; Baba, A. Eur. Patent EP0567107, Oct 27, 1993. |
| 28. | Laitonjam, W. S.; Rajkumar, T. S.; Chingakham, B. S. Steroids 2002, 67, 203–209. doi:10.1016/S0039-128X(01)00146-5 |
| 29. | Tombisana, R. K.; Laitonjam, W. S. Indian J. Chem. 1999, 38B, 847–849. |
| 4. | De Clercq, E. Curr. Med. Chem. 2001, 8, 1543–1572. |
| 5. | Corbett, J. W. Curr. Med. Chem. - Anti-Infect. Agents 2002, 1, 119–140. |
| 3. | Xia, Y.; Yang, Z.-Y.; Hour, M.-J.; Kuo, S.-C.; Xia, P.; Bastow, K. F.; Nakanishi, Y.; Nampoothiri, P.; Hackl, T.; Hamel, E.; Lee, K.-H. Bioorg. Med. Chem. Lett. 2001, 11, 1193–1196. doi:10.1016/S0960-894X(01)00190-1 |
| 26. | Devi, N. A.; Khuman, C. K.; Singh, R. K. T.; Laitonjam, W. S. Indian J. Heterocycl. Chem. 1998, 7, 193–196. |
| 22. | Ivachtchenko, A. V.; Kovalenko, S. M.; Drushlyak, O. G. J. Comb. Chem. 2003, 5, 775–788. doi:10.1021/cc020097g |
| 15. | Feldman, J. R.; Wagner, E. C. J. Org. Chem. 1942, 7, 31–47. doi:10.1021/jo01195a006 |
| 16. | Yale, H. L. J. Heterocycl. Chem. 1977, 14, 1357–1359. doi:10.1002/jhet.5570140812 |
| 17. | Mhaske, S. B.; Argade, N. P. J. Org. Chem. 2004, 69, 4563–4566. doi:10.1021/jo040153v |
| 23. | Devi, N. A.; Laitonjam, W. S. Indian J. Chem. 1994, 33B, 1091–1092. |
| 24. | Devi, N. A.; Laitonjam, W. S. Indian J. Heterocycl. Chem. 1995, 5, 139–140. |
| 14. | Abdel-Jalil, R. L.; Voelter, W.; Saeed, M. Tetrahedron Lett. 2004, 45, 3475–3476. doi:10.1016/j.tetlet.2004.03.003 |
| 10. | Li, Z.; Huang, H.; Sun, H.; Jiang, H.; Liu, H. J. Comb. Chem. 2008, 10, 484–486. doi:10.1021/cc800040z |
| 11. | Patil, Y. P.; Tambade, P. J.; Parghi, K. D.; Jayaram, R. V.; Bhanage, B. M. Catal. Lett 2009, 133, 201–208. doi:10.1007/s10562-009-0126-5 |
| 12. | Couture, A.; Cornet, H.; Grandclaudon, P. Synthesis 1991, 1009–1010. doi:10.1055/s-1991-26632 |
| 13. | Kotsuki, H.; Sakai, H.; Morimoto, H.; Suenaga, H. Synlett 1999, 1993–1995. doi:10.1055/s-1999-2998 |
| 19. | Makino, S.; Suzuki, N.; Nakanishi, E.; Tsuji, T. Tetrahedron Lett. 2000, 41, 8333–8337. doi:10.1016/S0040-4039(00)01442-8 |
| 20. | Makino, S.; Nakanishi, E.; Tsuji, T. Tetrahedron Lett. 2001, 42, 1749–1752. doi:10.1016/S0040-4039(01)00008-9 |
| 21. | Makino, S.; Nakanishi, E.; Tsuji, T. Bull. Korean Chem. Soc. 2003, 24, 389–392. doi:10.5012/bkcs.2003.24.3.389 |
© 2010 Moirangthem and Laitonjam; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)