Abstract
A new selaginellin named selaginellin O (1), along with three other known selaginellins (2–4) were isolated from Selaginella tamariscina (Beauv.) Spring. On the basis of spectroscopic analysis, the structure of selaginellin O was demonstrated to be 4-[(4’-hydroxy-4-formyl-3-((4-hydroxyphenyl)ethynyl)biphenyl-2-yl)(4-hydroxyphenyl)methylene]cyclohexa-2,5-dien-1-one. Compound 1, 2 and 3 exhibited appreciable cytotoxic activity against cultured HeLa cells (human cervical carcinoma cells), as well as significant antioxidant activity.
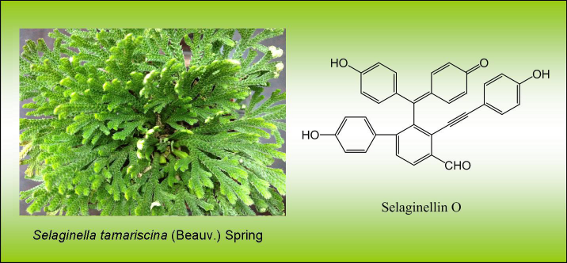
Graphical Abstract
Introduction
There are about 700 species of the genus Selaginella (family selaginellacea) widely found in the world, with more than 50 species being found in China [1]. Twenty of them are widely used in Chinese folk medicine, most frequently employed for the treatment of cancer, cardiovascular problems, hepatitis, gastritis, hematuria, diabetes, and skin diseases [2]. Selaginella tamariscina (Beauv.) Spring is one of the two qualified species listed in Chinese Pharmacopoeia that has long been used as a traditional Chinese medicine for promoting blood circulation [3]. Phytochemical and pharmacological studies on genus Selaginella led to identifications of numerous bioactive compounds, including biflavonoids, alkaloids, and lignans, with broad biological activities, including antivirus, antifungal, antibacterial, cytotoxic, and anti-inflammatory properties [4-20]. In the past five years, more than 10 selaginellins (novel pigments with a unique para-quinone methide and alkynylphenol carbon skeleton) have been isolated from several Selaginella species in China [13-20]. Selaginellin derivatives have been hitherto found only in genus Selaginella. In the course of our phytochemical investigations on Selaginella tamariscina (Beauv.) Spring, four selaginellin derivatives (Figure 1), namely selaginellin M (2) [20], selaginellin (3) [13], selaginellin A (4) [14], and a new analogue selaginellin O (1), were isolated from the entire plant. Herein, we report the isolation and structural elucidation of these selaginellin derivatives, as well as the evaluation of their bioactivities.
Figure 1: Structures of selaginellins from S. tamariscina.
Figure 1: Structures of selaginellins from S. tamariscina.
Results and Discussion
Selaginellin O (1), was obtained as a red powder, with the molecular formula C34H22O5, deduced from HRMS–ESI on the basis of the quasi-molecular ion peak at m/z 511.1543 [M + H]+ (calcd 511.1540). The IR spectrum indicated absorption bands for hydroxyl (3378 cm−1), formyl (2829, 2817, 1726 cm−1), alkynyl (2198 cm−1), unsaturated carboxyl (1680 cm−1) and aromatic ring (1570 and 1524 cm−1).
The assignment of all 1H and 13C NMR data (shown in Table 1) was confirmed by 2D NMR techniques. The NMR spectra of 1 showed the typical signals of a formyl group (δH 10.73 and δC 190.9), an alkynyl band (δC 82.4, 101.0), three phenolic hydroxyl (δH 8.94 and 8.59), and five aromatic rings, including one AB-spin system (δH 7.52 and 8.05, each 1H, d, J = 8.0 Hz) for the ortho-tetrasubstituted A-ring, three AA’BB’ systems (δH 7.22 and 6.78, each 2H, d, J = 8.4 Hz), (δH 6.87 and 6.71, each 2H, d, J = 8.4 Hz) and (δH 6.96 and 6.70, each 2H, d, J = 8.4 Hz) for the respective para-substituted B-, D- and E-ring, and one ABMN system (δH 7.61, 7.42, 6.41 and 6.35, each 1H, d, J = 10.0 Hz) for the C-ring. The above structural features suggested 1 was a selaginellin with a formyl group. Key evidence for the structure of 1 obtained from the HMBC experiment further confirmed this suggestion (Figure 2). The HMBC correlations H16/C-26 and H-26/C-15 concluded the substitution of the formyl group at C-15 of the A-ring. The alkynyl group was connected to the B-ring based on correlations between H-29,33 and C-28. The linkage between the C-ring and the B-ring was located at C-7 demonstrated by the HMBC cross-peaks of H-3,5/C-7 and H-8,12/C-7. The E-ring was connected to the A-ring at C-18 due to HMBC correlations H-20,24/C-18 and H-17/C-25. Hence, C-19 in the A-ring was the position left for C-7. Consequently, the structure of compound 1 was characterized as 4-[(4’-hydroxy-4-formyl-3-((4-hydroxyphenyl)ethynyl)biphenyl-2-yl)(4-hydroxyphenyl)methylene]cyclohexa-2,5-dien-1-one, named as selaginellin O.
Table 1: 1H and 13C NMR data and key HMBC correlations for compound 1.a
| Position | δH | δC | (DEPT) | HMBC (H→C) |
|---|---|---|---|---|
| 1 | – | 185.6 | (qC) | – |
| 2 | 6.35 d (10.0) | 128.4 | (CH) | C-4 |
| 3 | 7.42 d (9.6) | 139.2 | (CH) | C-1,7 |
| 4 | – | 131.4 | (qC) | – |
| 5 | 7.61 d (9.6) | 138.1 | (CH) | C-1,7 |
| 6 | 6.41 d (10.0) | 128.5 | (CH) | C-4 |
| 7 | – | 156.3 | (qC) | – |
| 8 | 6.87 d (8.4) | 133.0 | (CH) | C-7,10 |
| 9 | 6.71 d (8.4) | 114.9 | (CH) | C-13 |
| 10 | – | 158.8 | (qC) | – |
| 11 | 6.71 d (8.4) | 114.9 | (CH) | C-13 |
| 12 | 6.87 d (8.4) | 133.0 | (CH) | C-7,10 |
| 13 | – | 131.4 | (qC) | – |
| 14 | – | 127.7 | (qC) | – |
| 15 | – | 134.2 | (qC) | – |
| 16 | 8.05 d (8.0) | 127.5 | (CH) | C-14,18,26 |
| 17 | 7.52 d (8.0) | 130.1 | (CH) | C-15,19,25 |
| 18 | – | 148.1 | (qC) | – |
| 19 | – | 142.3 | (qC) | – |
| 20 | 6.96 d (8.4) | 129.8 | (CH) | C-18, 22 |
| 21 | 6.70 d (8.4) | 114.9 | (CH) | C-25 |
| 22 | – | 157.4 | (qC) | – |
| 23 | 6.70 d (8.4) | 114.9 | (CH) | C-25 |
| 24 | 6.96 d (8.4) | 129.8 | (CH) | C-18, 22 |
| 25 | – | 130.8 | (qC) | – |
| 26 | 10.73 s | 190.9 | (CH) | C-14, 16 |
| 27 | – | 82.4 | (qC) | – |
| 28 | – | 101.0 | (qC) | – |
| 29 | 7.22 d (8.4) | 133.5 | (CH) | C-28, 31 |
| 30 | 6.78 d (8.4) | 115.6 | (CH) | C-34 |
| 31 | – | 158.7 | (qC) | – |
| 32 | 6.78 d (8.4) | 115.6 | (CH) | C-34 |
| 33 | 7.22 d (8.4) | 133.5 | (CH) | C-28, 31 |
| 34 | – | 112.5 | (qC) | – |
| 10-OH | 8.94 (br)b | C-9, 11 | ||
| 22-OH | 8.59 (br) | C-21, 23 | ||
| 31-OH | 8.94 (br)b | C-30, 32 | ||
a400 MHz, acetone-d6, δ in parts per million, J in hertz. bOverlapping signals.
Figure 2: Key HMBC correlations of compound 1.
Figure 2: Key HMBC correlations of compound 1.
The chemical structures of selaginellin M (2), selaginellin (3) and selaginellin A (4) were identified by spectral analysis by 1D and 2D NMR spectroscopy and high-resolution MS. Their 1H and 13C NMR data were assigned in Table 2.
Table 2: 1H and 13C NMR data for compound 2, 3 and 4.a
| 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|
| Position | δH | δC | δH | δC | δH | δC |
| 1 | – | 185.7 | – | 173.1 | – | 185.9 |
| 2 | 6.32 dd (2.0, 10.0) | 128.1 | 6.54 d (8.8) | 121.4 | 6.35 m | 128.2 |
| 3 | 7.34 dd (2.4, 10.0) | 139.5 | 7.16 d (9.2) | 136.5 | 7.48 d (8.0) | 139.5 |
| 4 | – | 129.7 | – | 130.2 | – | 128.4 |
| 5 | 7.52 dd (2.4, 10.0) | 138.1 | 7.16 d (9.2) | 136.5 | 7.59 d (8.0) | 138.0 |
| 6 | 6.36 dd (2.0, 10.0) | 130.2 | 6.54 d (8.8) | 121.4 | 6.35 m | 128.2 |
| 7 | – | 158.1 | – | 160.1 | – | 158.0 |
| 8 | 6.88 d (9.2) | 132.7 | 7.16 d (9.2) | 136.5 | 6.75 d (8.8) | 133.0 |
| 9 | 6.66 d (8.8) | 113.2 | 6.54 d (8.8) | 121.4 | 6.64 m | 114.7 |
| 10 | – | 160.8 | – | 173.1 | – | 158.8 |
| 11 | 6.66 d (8.8) | 113.2 | 6.54 d (8.8) | 121.4 | 6.64 m | 114.7 |
| 12 | 6.88 d (9.2) | 132.7 | 7.16 d (9.2) | 136.5 | 6.75 d (8.8) | 133.0 |
| 13 | – | 131.7 | – | 130.2 | – | 133.5 |
| 14 | – | 121.5 | – | 121.7 | – | 124.6 |
| 15 | – | 142.7 | – | 142.5 | 7.67 d (8.0) | 130.2 |
| 16 | 7.79 d (8.0) | 126.8 | 7.79 d (8.0) | 127.0 | 7.59 t (7.6) | 129.1 |
| 17 | 7.38 d (8.0) | 129.7 | 7.37 d (8.0) | 129.7 | 7.48 d (8.0) | 130.0 |
| 18 | – | 140.9 | – | 141.3 | – | 143.0 |
| 19 | – | 141.0 | – | 140.9 | – | 141.1 |
| 20 | 6.91 d (8.4) | 130.2 | 6.87 d (8.4) | 129.9 | 6.89 d (8.4) | 129.8 |
| 21 | 6.80 d (9.2) | 114.7 | 6.65 d (8.4) | 114.8 | 6.64 m | 114.7 |
| 22 | – | 156.7 | – | 156.8 | – | 156.8 |
| 23 | 6.80 d (9.2) | 114.7 | 6.65 d (8.4) | 114.8 | 6.64 m | 114.7 |
| 24 | 6.91 d (8.4) | 130.2 | 6.87 d (8.4) | 129.9 | 6.89 d (8.4) | 129.8 |
| 25 | – | 131.7 | – | 131.6 | – | 131.7 |
| 26 | 5.01 s | 62.2 | 5.02 s | 62.2 | ||
| 27 | – | 83.8 | – | 83.8 | – | 86.6 |
| 28 | – | 98.8 | – | 99.0 | – | 93.5 |
| 29 | 7.11 d (8.4) | 133.0 | 7.09 d (8.4) | 133.0 | 7.09 d (8.8) | 133.1 |
| 30 | 6.76 d (8.8) | 115.5 | 6.75 d (8.4) | 115.6 | 6.74 d (8.4) | 115.5 |
| 31 | – | 157.8 | – | 158.4 | – | 158.0 |
| 32 | 6.76 d (8.8) | 115.5 | 6.75 d (8.4) | 115.6 | 6.74 d (8.4) | 115.5 |
| 33 | 7.11 d (8.4) | 133.0 | 7.09 d (8.4) | 133.0 | 7.09 d (8.8) | 133.1 |
| 34 | – | 113.6 | – | 113.3 | – | 113.6 |
| -OMe | 3.79 s | 54.8 | ||||
a400 MHz, acetone-d6, δ in parts per million, J in hertz.
Several species of the genus Selaginella have long been used in traditional medicine as anticancer agents, but only limited literature information on the cytotoxic activity of their constituents is available, which encourages us to investigate the cytotoxic effect of the seleginellins [4,5,7]. The cytotoxic activities of the three selaginellins: selaginellin O (1), selaginellin M (2) and selaginellin (3) were evaluated by using human cervical carcinoma (HeLa) cells. It is noticeable that all of these selaginellins exhibited appreciable cytotoxic activity (Supporting Information File 1; Table S1). Selaginellin O (1), the new selaginellin with a formyl group, showed the highest inhibitory activity, with an IC50 value of 26.4 μM. Consistent with earlier literature [20], the considerable inhibition of the expression of HeLa cells was observed for the known compounds selaginellin M (2) and selaginellin (3), with IC50 equal to 28.5 and 33.1 μM, respectively.
Over the past few decades, considerable biochemical, physiological and pharmacological evidence has accumulated to support the hypothesis that free-radical-mediated oxidative processes are implicated in various human diseases. The role of free radicals in ageing, in cancer, and in cardiovascular, neurodegenerative and other diseases is more and more widely accepted [21,22]. Antioxidants are attracting increasing scientific and clinical attention.
Natural phenolic compounds (flavonoids, lignans, phenolic acids, tocopherols, polyphenols and tannins) are the main class of antioxidants and are known to reduce the rate of oxidation by H-transfer (from their phenol groups) to the radicals [23]. There are multiple phenol groups on the large conjugated aromatic skeleton of selaginallins, and this potential antioxidant structural feature motivated the study on their antioxidant activity. Accordingly, the antioxidant capacity of selaginellin O (1), selaginellin M (2) and selaginellin (3) were estimated with the widely used ABTS radical-scavenging assay and FRAP assay. In both assays, all the selaginellins tested displayed significant antioxidant activity, with more potent antioxidant capacity than the reference compound Trolox (Supporting Information File 1; Table S2). Likewise, the new compound, selaginellin O (1), exhibited the highest antioxidant activity, and a general increase in antioxidant activity was observed compared with its reduced form selaginellin (3). This may be correlated with the electron-accepting and delocalization effects of the formyl group, which favor the ionization of ArOH to the phenoxide anion ArO−, benefitting from both H-transfer and stabilization of the important mediator ArO−. The above two main factors of the antioxidant contribute to the potent antioxidant properties of selaginallin O.
The current pharmacological evidence shows that antioxidant treatment may significantly inhibit atherosclerosis, which indicates that selaginallins may be partly related to the herbal use of S. tamariscina for promoting blood circulation and eliminating blood stasis [24].
Despite its preliminary character, this study is the first to report the antioxidant activity of the selaginellins. These promising biological results and the structural specificity of the selaginellins stimulated us to search for more potent and selective selaginellin analogues and their biogenetic precursors. Additional controlled studies are needed to investigate the efficacy and safety of selaginellins as antioxidant and anticancer agents.
Experimental
General experimental procedures. IR spectra were measured on a Perkin Elmer Spectrum 100 FT-IR Spectrometer. All NMR spectra data were recorded on a Bruker 400 MHz AVANCE III FT-NMR spectrometer operating at 400 MHz for 1H and 100 MHz for 13C NMR by using TMS as the internal standard. HRMS–ESI spectrum was obtained on an Agilent 1200-6520 QTOF. UV spectra were recorded on a Perkin Elmer LAMBDA 750 UV/Vis/NIR spectrophotometer. The melting point was measured by Büchi B-540 Melting Point Apparatus. Optical rotations were measured with a WZZ-3 automatic Polarimeter. Column chromatography was performed over silica gel (200–300 mesh). Thin-layer chromatography (TLC) was conducted on precoated silica gel plates GF254 (Qingdao Marine Chemical Factory). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), cisplatin, and 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ) were from Aladdin Reagent Co. Ltd. 2,2’-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were obtained from Sigma.
Plant material. The herb of Selaginella tamariscina was purchased from Tongtai Medicine, Harbin Co. Ltd., China. The material was authenticated by Prof. Zhenyue Wang, Heilongjiang University of Chinese Medicine. A voucher specimen (No. 200705JB) was deposited in the Lab of Applied Organic Chemistry, Harbin Institute of Technology.
Extraction and isolation. Dried whole herb of S. tamariscina (5.0 kg) was pulverized and extracted with methanol (3 × 5.0 L) at room temperature. The combined methanolic extract was concentrated in vacuum giving a dark residue (605 g), which was partitioned into five fractions, petroleum ether (80 g), Et2O (41 g), EtOAc (112 g), Me2CO (96 g) and MeOH (221 g), by silica-gel column chromatography. The ethyl acetate fraction (112 g) was chromatographed on silica gel (chloroform/methanol 1:0→0:1) to afford fractions 1–20. Fraction 15 (600 mg) was resubjected to silica gel CC with gradient petrol ether/acetone (10:1→0:1) to give amentoflavone (328.4 mg) and some red powder, which was then further purified on PTLC (petrol ether/acetone 4:1) to afford selaginellin M (2) (1.83 mg) and selaginellin O (1) (2.37 mg). Selaginellin (3) (83.0 mg) and selaginellin A (4) (0.7 mg) were obtained from fraction 16 (152 mg) by CC (silica gel; petrol ether/ethyl acetate 10:1, 5:1, 2:1, 1:1 and 1:5), followed by PTLC (chloroform/methanol 15:1).
Selaginellin O (1): Red powder; UV (MeOH) λmax (log ε): 298 (3.15), 415 (1.8) nm; IR (NaCl) νmax: 3378, 2198, 2829, 2817, 1726, 1680, 1567 and 1524 cm−1; 1H and 13C NMR (DEPT) data were shown in Table 1; HRMS–ESI (m/z): [M + H]+ calcd for C34H23O5+, 511.1540; found, 511.1543.
Selaginellin M (2): Red powder; UV (MeOH) λmax (log ε): 297 (3.15), 431 (1.05); IR (NaCl) νmax: 3420, 2196, 1708, 1545 and 1535 cm−1; 1H and 13C NMR (DEPT) data were shown in Table 2; HRMS–ESI (m/z): [M + H]+ calcd for C35H27O5+, 527.1853; found, 527.1868.
Selaginellin (3): Red crystals (MeOH); IR (NaCl) νmax: 3387, 2195, 1689, 1595 and 1531 cm−1; 1H and 13C NMR data were shown in Table 2; HRMS–ESI (m/z): [M + H]+ calcd for C34H25O5+, 513.1697; found, 513.1702.
Selaginellin A (4): Red powder; IR (NaCl) νmax: 3404, 2205, 1656, 1575 and 1531 cm−1; 1H and 13C NMR data were shown in Table 2; HRMS–ESI (m/z): [M + H]+ calcd for C33H23O4+, 483.1591; found, 483.1587.
Cytotoxicity assay. Due to insufficient material, selaginellin O (1), selaginellin M (2) and selaginellin (3), were evaluated for their cytotoxic activity against cultured human cervical carcinoma HeLa cells by using the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric method [25]. The anticancer agent cisplatin was used as a positive control. The cytotoxicity data were expressed as IC50 (half inhibition concentration) values.
ABTS radical scavenging assay. The assay was performed according to the established protocol [26]. The ABTS•+ radical was generated by mixing 7 mM aqueous ABTS solution with 2.45 mM potassium persulfate solution (final concentration) followed by incubation in the dark at room temperature for 16 h. The resultant ABTS•+ solution was diluted with ethanol to give an absorbance of 0.70 ± 0.02 at 734 nm and equilibrated at 30 °C. After the addition of 2.85 mL of diluted ABTS•+ solution to 0.15 mL of different concentrations of samples in ethanol, the absorbance reading was taken at 30 °C, exactly 6 min after the initial mixing. The percentage inhibition of absorbance at 734 nm was calculated and plotted as a function of the concentration of samples and of Trolox as standard. The antioxidant activities are expressed as the IC50 value.
FRAP (Ferric reducing antioxidant power) assay. This assay was carried out following the procedure described previously with modifications [27]. FRAP reagent was prepared fresh by mixing 10 mM TPTZ, 20 mM FeCl3 and 300 mM acetate buffer (pH 3.6) in a 1:1:10 (v/v/v) ratio and warmed to 37 °C before use. A 0.2 mL amount of the sample including Trolox as a reference compound in methanol was added to 1.8 mL freshly prepared FRAP reagent and then incubated at 37 °C for 8 min. Absorbance of the resulting FeII-TPTZ (ferrous-tripyridyltriazine) complex was measured at 595 nm. Antioxidant power was expressed as micromolar FeII-TPTZ equivalents, calculated from a calibration curve prepared with various concentrations of FeSO4.
Supporting Information
| Supporting Information File 1: Spectroscopic data and other relevant information for compounds 1–4. | ||
| Format: PDF | Size: 1.3 MB | Download |
References
-
Editorial Committee of FRPS. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1978; Vol. 6(3), pp 100–104.
Return to citation in text: [1] -
Dai, Z.; Wang, G. L.; Hou, Q. Y.; Ni, L.; Wei, F.; Lin, R. C. Chin. Trad. Herb. Drugs 2001, 32, 784–785.
Return to citation in text: [1] -
Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing House: Beijing, China, 2010; Vol. 1, pp 210–211.
Return to citation in text: [1] -
Silva, G. L.; Chai, H.; Gupta, M. P.; Farnsworth, N. R.; Cordell, G. A.; Pezzuto, J. M.; Beecher, C. W. W.; Kinghorn, A. D. Phytochemistry 1995, 40, 129–134. doi:10.1016/0031-9422(95)00212-P
Return to citation in text: [1] [2] -
Chen, J.-J.; Duh, C.-Y.; Chen, J.-F. Planta Med. 2005, 71, 659–665. doi:10.1055/s-2005-871273
Return to citation in text: [1] [2] -
Lee, C.-W.; Choi, H.-J.; Kim, H.-S.; Kim, D.-H.; Chang, I.-S.; Moon, H. T.; Lee, S.-Y.; Oh, W. K.; Woo, E.-R. Bioorg. Med. Chem. 2008, 16, 732–738. doi:10.1016/j.bmc.2007.10.036
Return to citation in text: [1] -
Cao, Y.; Tan, N.-H.; Chen, J.-J.; Zeng, G.-Z.; Ma, Y.-B.; Wu, Y.-P.; Yan, H.; Yang, J.; Lu, L.-F.; Wang, Q. Fitoterapia 2010, 81, 253–258. doi:10.1016/j.fitote.2009.09.007
Return to citation in text: [1] [2] -
Wang, Y. Z.; Chen, H.; Zheng, X. K.; Feng, W. S. Chin. Chem. Lett. 2007, 18, 1224–1226. doi:10.1016/j.cclet.2007.08.016
Return to citation in text: [1] -
Feng, W.-s.; Li, K.-k.; Zheng, X.-k. Acta Pharm. Sinica B 2011, 1, 36–39. doi:10.1016/j.apsb.2011.04.001
Return to citation in text: [1] -
Chao, L. R.; Seguin, E.; Tillequin, F.; Koch, M. J. Nat. Prod. 1987, 50, 422–426. doi:10.1021/np50051a013
Return to citation in text: [1] -
Wang, Y.-H.; Long, C.-L.; Yang, F.-M.; Wang, X.; Sun, Q.-Y.; Wang, H.-S.; Shi, Y.-N.; Tang, G.-H. J. Nat. Prod. 2009, 72, 1151–1154. doi:10.1021/np9001515
Return to citation in text: [1] -
Zheng, X.; Du, J.; Xu, Y.; Zhu, B.; Liao, D. Fitoterapia 2007, 78, 598–599. doi:10.1016/j.fitote.2007.04.008
Return to citation in text: [1] -
Zhang, L.-P.; Liang, Y.-M.; Wei, X.-C.; Cheng, D.-L. J. Org. Chem. 2007, 72, 3921–3924. doi:10.1021/jo0701177
Return to citation in text: [1] [2] [3] -
Cheng, X.-L.; Ma, S.-C.; Yu, J.-D.; Yang, S.-Y.; Xiao, X.-Y.; Hu, J.-Y.; Lu, Y.; Shaw, P.-C.; But, P. P.-H.; Lin, R.-C. Chem. Pharm. Bull. 2008, 56, 982–984. doi:10.1248/cpb.56.982
Return to citation in text: [1] [2] [3] -
Tan, G.-S.; Xu, K.-P.; Li, F.-S.; Wang, C.-J.; Li, T.-Y.; Hu, C.-P.; Shen, J.; Zhou, Y.-J.; Li, Y. J. J. Asian. Nat. Prod. Res. 2009, 11, 1001–1004. doi:10.1080/10286020903207043
Return to citation in text: [1] [2] -
Cao, Y.; Chen, J.-J.; Tan, N.-H.; Wu, Y.-P.; Yang, J.; Wang, Q. Magn. Reson. Chem. 2010, 48, 656–659. doi:10.1002/mrc.2623
Return to citation in text: [1] [2] -
Cao, Y.; Chen, J.-J.; Tan, N.-H.; Oberer, L.; Wagner, T.; Wu, Y.-P.; Zeng, G.-Z.; Yan, H.; Wang, Q. Bioorg. Med. Chem. Lett. 2010, 20, 2456–2460. doi:10.1016/j.bmcl.2010.03.016
Return to citation in text: [1] [2] -
Xu, K.-P.; Zou, H.; Tan, Q.; Li, F.-S.; Liu, J.-F.; Xiang, H.-L.; Zou, Z.-X.; Long, H.-P.; Li, Y.-J.; Tan, G.-S. J. Asian. Nat. Prod. Res. 2011, 13, 93–96. doi:10.1080/10286020.2010.536535
Return to citation in text: [1] [2] -
Xu, K.-P.; Zou, H.; Li, F.-S.; Xiang, H.-L.; Zou, Z.-X.; Long, H.-P.; Li, J.; Luo, Y.-J.; Li, Y.-J.; Tan, G.-S. J. Asian. Nat. Prod. Res. 2011, 13, 356–360. doi:10.1080/10286020.2011.558840
Return to citation in text: [1] [2] -
Zhang, G.-g.; Jing, Y.; Zhang, H.-m.; Ma, E.-l.; Guan, J.; Xue, F.-n.; Liu, H.-x.; Sun, X.-y. Planta Med. 2012, 78, 390–392. doi:10.1055/s-0031-1298175
Return to citation in text: [1] [2] [3] [4] -
Poon, H. F.; Calabrese, V.; Scapagnini, G.; Butterfield, D. A. Clin. Geriatr. Med. 2004, 20, 329–359. doi:10.1016/j.cger.2004.02.005
Return to citation in text: [1] -
Abrescia, P.; Golino, P. Expert. Rev. Cardiovasc. Ther. 2005, 3, 159–171. doi:10.1586/14779072.3.1.159
Return to citation in text: [1] -
Rahman, K. Clin. Interv. Aging 2007, 2, 219–236.
Return to citation in text: [1] -
Foti, M. C. J. Pharm. Pharmacol. 2007, 59, 1673–1685. doi:10.1211/jpp.59.12.0010
Return to citation in text: [1] -
Pagé, M.; Bejaoui, N.; Cinq-Mars, B.; Lemieux, P. Int. J. Immunopharmacol. 1988, 10, 785–793. doi:10.1016/0192-0561(88)90001-X
Return to citation in text: [1] -
Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Free Radical Biol. Med. 1999, 26, 1231–1237. doi:10.1016/S0891-5849(98)00315-3
Return to citation in text: [1] -
Benzie, I. F. F.; Strain, J. J. Anal. Biochem. 1996, 239, 70–76. doi:10.1006/abio.1996.0292
Return to citation in text: [1]
| 1. | Editorial Committee of FRPS. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1978; Vol. 6(3), pp 100–104. |
| 13. | Zhang, L.-P.; Liang, Y.-M.; Wei, X.-C.; Cheng, D.-L. J. Org. Chem. 2007, 72, 3921–3924. doi:10.1021/jo0701177 |
| 14. | Cheng, X.-L.; Ma, S.-C.; Yu, J.-D.; Yang, S.-Y.; Xiao, X.-Y.; Hu, J.-Y.; Lu, Y.; Shaw, P.-C.; But, P. P.-H.; Lin, R.-C. Chem. Pharm. Bull. 2008, 56, 982–984. doi:10.1248/cpb.56.982 |
| 15. | Tan, G.-S.; Xu, K.-P.; Li, F.-S.; Wang, C.-J.; Li, T.-Y.; Hu, C.-P.; Shen, J.; Zhou, Y.-J.; Li, Y. J. J. Asian. Nat. Prod. Res. 2009, 11, 1001–1004. doi:10.1080/10286020903207043 |
| 16. | Cao, Y.; Chen, J.-J.; Tan, N.-H.; Wu, Y.-P.; Yang, J.; Wang, Q. Magn. Reson. Chem. 2010, 48, 656–659. doi:10.1002/mrc.2623 |
| 17. | Cao, Y.; Chen, J.-J.; Tan, N.-H.; Oberer, L.; Wagner, T.; Wu, Y.-P.; Zeng, G.-Z.; Yan, H.; Wang, Q. Bioorg. Med. Chem. Lett. 2010, 20, 2456–2460. doi:10.1016/j.bmcl.2010.03.016 |
| 18. | Xu, K.-P.; Zou, H.; Tan, Q.; Li, F.-S.; Liu, J.-F.; Xiang, H.-L.; Zou, Z.-X.; Long, H.-P.; Li, Y.-J.; Tan, G.-S. J. Asian. Nat. Prod. Res. 2011, 13, 93–96. doi:10.1080/10286020.2010.536535 |
| 19. | Xu, K.-P.; Zou, H.; Li, F.-S.; Xiang, H.-L.; Zou, Z.-X.; Long, H.-P.; Li, J.; Luo, Y.-J.; Li, Y.-J.; Tan, G.-S. J. Asian. Nat. Prod. Res. 2011, 13, 356–360. doi:10.1080/10286020.2011.558840 |
| 20. | Zhang, G.-g.; Jing, Y.; Zhang, H.-m.; Ma, E.-l.; Guan, J.; Xue, F.-n.; Liu, H.-x.; Sun, X.-y. Planta Med. 2012, 78, 390–392. doi:10.1055/s-0031-1298175 |
| 26. | Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Free Radical Biol. Med. 1999, 26, 1231–1237. doi:10.1016/S0891-5849(98)00315-3 |
| 4. | Silva, G. L.; Chai, H.; Gupta, M. P.; Farnsworth, N. R.; Cordell, G. A.; Pezzuto, J. M.; Beecher, C. W. W.; Kinghorn, A. D. Phytochemistry 1995, 40, 129–134. doi:10.1016/0031-9422(95)00212-P |
| 5. | Chen, J.-J.; Duh, C.-Y.; Chen, J.-F. Planta Med. 2005, 71, 659–665. doi:10.1055/s-2005-871273 |
| 6. | Lee, C.-W.; Choi, H.-J.; Kim, H.-S.; Kim, D.-H.; Chang, I.-S.; Moon, H. T.; Lee, S.-Y.; Oh, W. K.; Woo, E.-R. Bioorg. Med. Chem. 2008, 16, 732–738. doi:10.1016/j.bmc.2007.10.036 |
| 7. | Cao, Y.; Tan, N.-H.; Chen, J.-J.; Zeng, G.-Z.; Ma, Y.-B.; Wu, Y.-P.; Yan, H.; Yang, J.; Lu, L.-F.; Wang, Q. Fitoterapia 2010, 81, 253–258. doi:10.1016/j.fitote.2009.09.007 |
| 8. | Wang, Y. Z.; Chen, H.; Zheng, X. K.; Feng, W. S. Chin. Chem. Lett. 2007, 18, 1224–1226. doi:10.1016/j.cclet.2007.08.016 |
| 9. | Feng, W.-s.; Li, K.-k.; Zheng, X.-k. Acta Pharm. Sinica B 2011, 1, 36–39. doi:10.1016/j.apsb.2011.04.001 |
| 10. | Chao, L. R.; Seguin, E.; Tillequin, F.; Koch, M. J. Nat. Prod. 1987, 50, 422–426. doi:10.1021/np50051a013 |
| 11. | Wang, Y.-H.; Long, C.-L.; Yang, F.-M.; Wang, X.; Sun, Q.-Y.; Wang, H.-S.; Shi, Y.-N.; Tang, G.-H. J. Nat. Prod. 2009, 72, 1151–1154. doi:10.1021/np9001515 |
| 12. | Zheng, X.; Du, J.; Xu, Y.; Zhu, B.; Liao, D. Fitoterapia 2007, 78, 598–599. doi:10.1016/j.fitote.2007.04.008 |
| 13. | Zhang, L.-P.; Liang, Y.-M.; Wei, X.-C.; Cheng, D.-L. J. Org. Chem. 2007, 72, 3921–3924. doi:10.1021/jo0701177 |
| 14. | Cheng, X.-L.; Ma, S.-C.; Yu, J.-D.; Yang, S.-Y.; Xiao, X.-Y.; Hu, J.-Y.; Lu, Y.; Shaw, P.-C.; But, P. P.-H.; Lin, R.-C. Chem. Pharm. Bull. 2008, 56, 982–984. doi:10.1248/cpb.56.982 |
| 15. | Tan, G.-S.; Xu, K.-P.; Li, F.-S.; Wang, C.-J.; Li, T.-Y.; Hu, C.-P.; Shen, J.; Zhou, Y.-J.; Li, Y. J. J. Asian. Nat. Prod. Res. 2009, 11, 1001–1004. doi:10.1080/10286020903207043 |
| 16. | Cao, Y.; Chen, J.-J.; Tan, N.-H.; Wu, Y.-P.; Yang, J.; Wang, Q. Magn. Reson. Chem. 2010, 48, 656–659. doi:10.1002/mrc.2623 |
| 17. | Cao, Y.; Chen, J.-J.; Tan, N.-H.; Oberer, L.; Wagner, T.; Wu, Y.-P.; Zeng, G.-Z.; Yan, H.; Wang, Q. Bioorg. Med. Chem. Lett. 2010, 20, 2456–2460. doi:10.1016/j.bmcl.2010.03.016 |
| 18. | Xu, K.-P.; Zou, H.; Tan, Q.; Li, F.-S.; Liu, J.-F.; Xiang, H.-L.; Zou, Z.-X.; Long, H.-P.; Li, Y.-J.; Tan, G.-S. J. Asian. Nat. Prod. Res. 2011, 13, 93–96. doi:10.1080/10286020.2010.536535 |
| 19. | Xu, K.-P.; Zou, H.; Li, F.-S.; Xiang, H.-L.; Zou, Z.-X.; Long, H.-P.; Li, J.; Luo, Y.-J.; Li, Y.-J.; Tan, G.-S. J. Asian. Nat. Prod. Res. 2011, 13, 356–360. doi:10.1080/10286020.2011.558840 |
| 20. | Zhang, G.-g.; Jing, Y.; Zhang, H.-m.; Ma, E.-l.; Guan, J.; Xue, F.-n.; Liu, H.-x.; Sun, X.-y. Planta Med. 2012, 78, 390–392. doi:10.1055/s-0031-1298175 |
| 27. | Benzie, I. F. F.; Strain, J. J. Anal. Biochem. 1996, 239, 70–76. doi:10.1006/abio.1996.0292 |
| 3. | Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing House: Beijing, China, 2010; Vol. 1, pp 210–211. |
| 24. | Foti, M. C. J. Pharm. Pharmacol. 2007, 59, 1673–1685. doi:10.1211/jpp.59.12.0010 |
| 2. | Dai, Z.; Wang, G. L.; Hou, Q. Y.; Ni, L.; Wei, F.; Lin, R. C. Chin. Trad. Herb. Drugs 2001, 32, 784–785. |
| 25. | Pagé, M.; Bejaoui, N.; Cinq-Mars, B.; Lemieux, P. Int. J. Immunopharmacol. 1988, 10, 785–793. doi:10.1016/0192-0561(88)90001-X |
| 4. | Silva, G. L.; Chai, H.; Gupta, M. P.; Farnsworth, N. R.; Cordell, G. A.; Pezzuto, J. M.; Beecher, C. W. W.; Kinghorn, A. D. Phytochemistry 1995, 40, 129–134. doi:10.1016/0031-9422(95)00212-P |
| 5. | Chen, J.-J.; Duh, C.-Y.; Chen, J.-F. Planta Med. 2005, 71, 659–665. doi:10.1055/s-2005-871273 |
| 7. | Cao, Y.; Tan, N.-H.; Chen, J.-J.; Zeng, G.-Z.; Ma, Y.-B.; Wu, Y.-P.; Yan, H.; Yang, J.; Lu, L.-F.; Wang, Q. Fitoterapia 2010, 81, 253–258. doi:10.1016/j.fitote.2009.09.007 |
| 21. | Poon, H. F.; Calabrese, V.; Scapagnini, G.; Butterfield, D. A. Clin. Geriatr. Med. 2004, 20, 329–359. doi:10.1016/j.cger.2004.02.005 |
| 22. | Abrescia, P.; Golino, P. Expert. Rev. Cardiovasc. Ther. 2005, 3, 159–171. doi:10.1586/14779072.3.1.159 |
| 14. | Cheng, X.-L.; Ma, S.-C.; Yu, J.-D.; Yang, S.-Y.; Xiao, X.-Y.; Hu, J.-Y.; Lu, Y.; Shaw, P.-C.; But, P. P.-H.; Lin, R.-C. Chem. Pharm. Bull. 2008, 56, 982–984. doi:10.1248/cpb.56.982 |
| 13. | Zhang, L.-P.; Liang, Y.-M.; Wei, X.-C.; Cheng, D.-L. J. Org. Chem. 2007, 72, 3921–3924. doi:10.1021/jo0701177 |
| 20. | Zhang, G.-g.; Jing, Y.; Zhang, H.-m.; Ma, E.-l.; Guan, J.; Xue, F.-n.; Liu, H.-x.; Sun, X.-y. Planta Med. 2012, 78, 390–392. doi:10.1055/s-0031-1298175 |
| 20. | Zhang, G.-g.; Jing, Y.; Zhang, H.-m.; Ma, E.-l.; Guan, J.; Xue, F.-n.; Liu, H.-x.; Sun, X.-y. Planta Med. 2012, 78, 390–392. doi:10.1055/s-0031-1298175 |
© 2012 Yang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)