Abstract
The asymmetric organocatalytic hydrogenation of benzoxazines, quinolines, quinoxalines and 3H-indoles in continuous-flow microreactors has been developed. Reaction monitoring was achieved by using an inline ReactIR flow cell, which allows fast and convenient optimization of reaction parameters. The reductions proceeded well, and the desired products were isolated in high yields and with excellent enantioselectivities.
Graphical Abstract
Introduction
In recent years, a growing interest in microreactor technology has been seen in the scientific community and the development of microfabricated reaction systems is actively pursued. Microreactor technology offers numerous advantages, including precise control of reaction variables, enhanced mixing quality, improved operational safety, reduced reagent consumption and ready scale-up of chemical processes. Due to the high surface-area-to-volume ratios of microstructured reactors, a high thermal rate and high portability of substrates can be achieved, which leads to improved product formation [1-42]. Furthermore, by incorporating inline analytical devices the progress of reactions can be monitored and analyzed in real time, allowing fast reaction screening and optimization [43-55].
Continuous flow microreactors have been applied to a number of standard transformations in organic synthesis [56-80]; however, examples regarding asymmetric reactions as well as organocatalytic reactions are scarce [81-96]. Herein, we present the first example of a continuous-flow organocatalytic asymmetric transfer hydrogenation performed in a microreactor. In this work a ReactIR flow cell was coupled with the microreactor and applied as an inline monitoring device for optimizing the reactions.
Results and Discussion
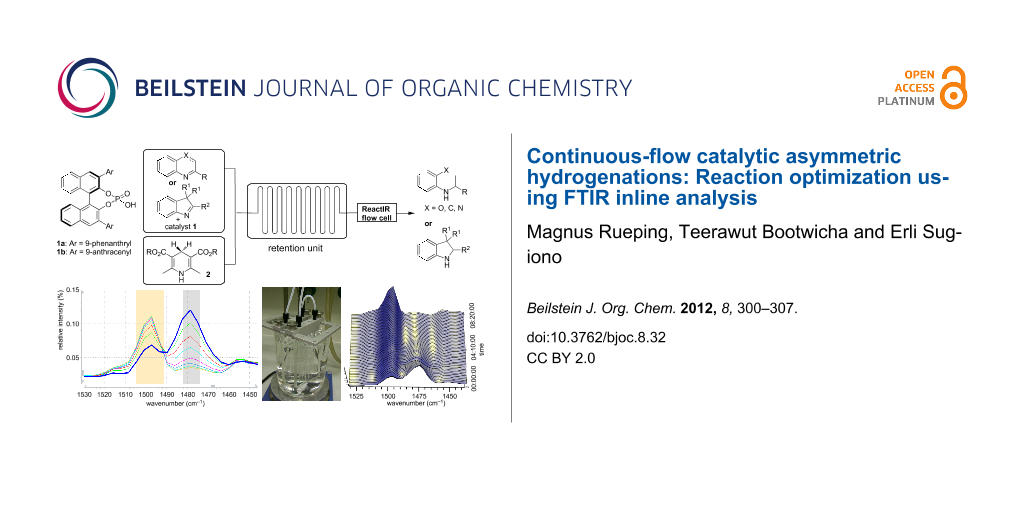
The continuous-flow microreactor system for the experiment was set up according to Scheme 1. The flow device was set up either with a single reactor, or with multiple reactors when a prolonged residence time was needed. The reagents were introduced separately, by using a syringe pump, through two inlets connected to Y-shaped connectors. The internal reaction temperature was monitored with an internal thermal sensor. The ReactIR 45m microflow cell equipped with a DiComp ATR (diamond-composite attenuated total reflection) probe was attached to the microreactor at the end of the reaction stream and was used as an inline analytical tool to determine the optimum reaction conditions. The IR spectra were recorded at predefined intervals and the raw data were analysed with iC-IR analysis software.
Scheme 1: Experimental setup for the asymmetric transfer hydrogenation.
Scheme 1: Experimental setup for the asymmetric transfer hydrogenation.
The first reaction examined the asymmetric organocatalytic transfer hydrogenation [97-101] of benzoxazine 3a in the presence of Hantzsch dihydropyridine 2a as hydrogen source and a catalytic amount of chiral Brønsted acid 1a (Scheme 2) [102].
Scheme 2: Asymmetric hydrogenation of benzoxazines.
Scheme 2: Asymmetric hydrogenation of benzoxazines.
Initial experiments were carried out at 0.1 mL min−1 flow rate in a commercial glass microreactor, which was attached to the ReactIR flow cell for in situ reaction monitoring. In order to control the reaction and to determine the use of educts and formation of product, reference spectra of the starting materials, solvents and reagents were recorded. Figure 1b and Figure 1c show real time IR spectra of the reaction mixtures after the subtraction of solvent in the spectral region of 1440 and 1530 cm−1. For direct inline analysis the signals at ![[Graphic 27]](/bjoc/content/inline/1860-5397-8-32-i29.png?max-width=637&scale=1.18182) = 1479 cm−1 and
= 1479 cm−1 and ![[Graphic 27]](/bjoc/content/inline/1860-5397-8-32-i29.png?max-width=637&scale=1.18182) = 1495 cm−1 were ideal as they could easily be assigned to benzoxazine 3a and dihydrobenzoxazine 4a. Thus, in continuous flow the substrate consumption and product formation could readily be determined.
= 1495 cm−1 were ideal as they could easily be assigned to benzoxazine 3a and dihydrobenzoxazine 4a. Thus, in continuous flow the substrate consumption and product formation could readily be determined.
Figure 1: In situ ReactIR monitoring: (a) Trend curve of product formation at different temperatures. (b) Reaction spectra showing the consumption of the substrate and the formation of product at different temperatures. (c) Three-dimensional time-resolved spectral data.
Figure 1: In situ ReactIR monitoring: (a) Trend curve of product formation at different temperatures. (b) Rea...
In order to find the optimal temperature for the asymmetric continuous-flow reduction, a temperature profile was recorded. The reaction temperature was initially 5 °C and was increased to 60 °C over a period of 8 h, while the conversion was monitored by inline IR-spectroscopy. Figure 1a shows the real-time plot of the peak intensity versus reaction time for the 1495 cm−1 absorption band at different temperatures. The trend-curve analysis by peak-height integration of this absorption band shows increased product formation with increasing temperature. By monitoring the signal change in this spectral region over the time of the reaction, the product formation (![[Graphic 27]](/bjoc/content/inline/1860-5397-8-32-i29.png?max-width=637&scale=1.18182) = 1495 cm−1) and substrate consumption (
= 1495 cm−1) and substrate consumption (![[Graphic 27]](/bjoc/content/inline/1860-5397-8-32-i29.png?max-width=637&scale=1.18182) = 1479 cm−1) can be determined in real time. Analysis of the spectra provided us with an optimal temperature of 60 °C for this reaction. In general the IR-flow-cell technology is a good tool for in situ monitoring and provides a fast read out of reaction progress as the intensity of substrate and product peaks can be directly related to the conversion. Thus, as exemplified above, applying the inline analysis to different reaction parameters provides a fast and convenient method for reaction optimization.
= 1479 cm−1) can be determined in real time. Analysis of the spectra provided us with an optimal temperature of 60 °C for this reaction. In general the IR-flow-cell technology is a good tool for in situ monitoring and provides a fast read out of reaction progress as the intensity of substrate and product peaks can be directly related to the conversion. Thus, as exemplified above, applying the inline analysis to different reaction parameters provides a fast and convenient method for reaction optimization.
By using the optimized reaction temperature and flow rate of 0.1 mL min−1, further experiments were conducted to examine the influence of the residence time on the conversion (Table 1). By performing the reaction with a residence time of 20 min, the product was isolated in 50% yield. With residence times of 40 min and 60 min, the product was isolated in 87% and 98% yields, respectively (Table 1).
Table 1: Optimization of the Brønsted acid catalyzed reduction of benzoxazines.a
|
|
||||
| Entry |
1a
[mol %] |
Residence time
[min] |
Flow rate
[mL min−1] |
Yield
[%]b |
|---|---|---|---|---|
| 1 | 2 | 20 | 0.1 | 50% |
| 2 | 2 | 40 | 0.1 | 87% |
| 3 | 2 | 60 | 0.1 | 98% |
aReaction conditions: 3a, 2a (1.2 equiv), 1a in CHCl3 (0.05 M) at 60 °C. bIsolated yields after column chromatography.
Having found the optimum reaction conditions, we next investigated the scope of the Brønsted acid catalyzed reduction of 3-aryl-substituted benzoxazines 3 (Table 2). In general, 3-aryl benzoxazines 3 bearing either electron-withdrawing or electron-donating groups can be reduced in a continuous fashion and the products 4 were isolated in good yields and with excellent enantioselectivities.
Table 2: Scope of the Brønsted acid catalyzed reduction of benzoxazines.a
|
|
|||
| Entry | Product 4 | Yield [%]b | ee [%]c |
|---|---|---|---|
| 1 |
4a |
98 | 98 |
| 2 |
4b |
96 | 97 |
| 3 |
4c |
98 | 98 |
| 4 |
4d |
81 | 97 |
| 5 |
4e |
85 | 99 |
aReaction conditions: 3, 2a (1.2 equiv), 2 mol % 1a in CHCl3 (0.05 M) at 60 °C, flow rate 0.1 mL min−1, residence time = 60 min. bIsolated yields after column chromatography. cDetermined by chiral HPLC analysis.
Encouraged by the results, we next studied the transfer hydrogenation of quinolines 5 [103-106]. The optimum reaction temperature was determined according to the experiment described above. The effects of catalyst loading and residence time on the conversion and the enantioselectivity are summarized in Table 3. Performing the reaction at 60 °C with 5 mol % of Brønsted acid 1a and residence time of 20 min afforded the desired product in 88% yield and 94% enantioselectivity (Table 3, entry 1). When the catalyst loading was reduced from 5 mol % to 2 mol %, a residence time of 40 min was found to be optimal to achieve comparable results (Table 3, entry 1 versus entry 2). A slight improvement of the conversion was observed by increasing the residence time to 60 min (Table 3, entry 3 versus entry 2). The catalyst loading can be decreased to 0.5 mol % without loss of reactivity and selectivity; the desired tetrahydroquinoline was isolated in 96% yield with 94% enantiomeric excess (Table 3, entry 5). A further decrease of catalyst loading to 0.1 mol % resulted in a significant drop in chemical yield, affording the product in lower yield while enantioselectivity was maintained (Table 3, entry 6).
Table 3: Optimization of the Brønsted acid catalyzed transfer hydrogenation of quinolines.a
|
|
|||||
| Entry |
1a
[mol %] |
t
[min] |
Flow rate
[mL min−1] |
Yield
[%]b |
ee
[%]c |
|---|---|---|---|---|---|
| 1 | 5 | 20 | 0.1 | 88 | 94 |
| 2 | 2 | 40 | 0.1 | 91 | 92 |
| 3 | 2 | 60 | 0.1 | 97 | 92 |
| 4 | 1 | 60 | 0.1 | 97 | 92 |
| 5 | 0.5 | 60 | 0.1 | 96 | 94 |
| 6 | 0.1 | 60 | 0.1 | 72 | 94 |
| 7d | 0.5 | 60 | batch | 67 | 94 |
aReaction conditions: 5a, 2a (2.4 equiv), 1a in CHCl3 (0,1 M) at 60 °C, flow rate 0.1 mL min−1. bIsolated yields after column chromatography. cDetermined by chiral HPLC analysis. dPerformed under batch conditions.
Although continuous-flow reactions provide many advantages, in certain cases it can be beneficial to conduct reactions under classical batch conditions. Therefore, we decided to carry out a direct comparison. Transferring the reaction conditions from continuous-flow to the batch showed a noticeable drop in conversion and the product was isolated only in 67% yield (Table 3, entry 5 vs entry 7). This observation is general, and typically lower reactivities were obtained. This can be explained by the better heat transfer in the microreactors as compared to the glass flask typically used in our batch reactions.
The scope and applicability of the method was then tested on various 2-substituted quinolines (Table 4). In general the asymmetric continuous-flow transfer hydrogenation of 2-substituted quinolines 5 proceeded well and afforded tetrahydroquinolines 6a–e with excellent yields and enantioselectivities (Table 4).
Table 4: Scope of the Brønsted acid catalyzed transfer hydrogenation of quinolines.a
|
|
|||
| Entry | Product 6 |
Yield
[%]b |
ee
[%]c |
|---|---|---|---|
| 1 |
6a |
96 | 94 |
| 2 |
6b |
91 | 96 |
| 3 |
6c |
94 | 99 |
| 4 |
6d |
91 | 99 |
| 5 |
6e |
97 | 96 |
aReaction conditions: 5, 2a (2.4 equiv), 5 mol % 1a in CHCl3 (0.1 M) at 60 °C, flow rate 0.1 mL min−1, residence time = 60 min. bIsolated yields after column chromatography. cDetermined by chiral HPLC analysis.
Having established a protocol for a general and highly enantioselective transfer hydrogenation of quinolines, we decided to extend its scope to the reduction of quinoxalines 7 (Table 5) [107]. The asymmetric reduction of quinoxalines is typically more difficult to achieve. Using the optimized conditions for the fast inline reaction, we found that the continuous-flow reduction could be performed using 10 mol % Brønsted acid 1b, a flow rate of 0.1 mL min−1 and 60 min residence time (Table 5).
Table 5: Scope of the Brønsted acid catalyzed transfer hydrogenation of quinoxalines.a
|
|
|||
| Entry | Product 8 |
Yield
[%]b |
ee
[%]c |
|---|---|---|---|
| 1 |
8a |
77 | 90 |
| 2 |
8b |
68 | 84 |
| 3 |
8c |
53 | 86 |
| 4 |
8d |
86 | 94 |
| 5 |
8e |
41 | 76 |
aReaction conditions: 7, 2a (2.4 equiv), 10 mol % 1b in CHCl3 (0.1 M) at 60 °C, flow rate 0.1 mL min−1, residence time = 60 min. bIsolated yields after column chromatography. cDetermined by chiral HPLC analysis.
To broaden the scope of the asymmetric hydrogenations in continuous flow further, the reduction of 3H-indoles 9 was studied (Table 6) [108]. Here the best reaction conditions turned out to be a temperature of 30 °C, a flow rate of 0.1 mL min−1, and a residence time of 20 min. The desired indolines 10 were isolated in good to high yields and with excellent enantioselectivities.
Table 6: Scope of the Brønsted acid catalyzed transfer hydrogenation of 3H-indoles.a
|
|
|||
| Entry | Product 10 |
Yield
[%]b |
ee
[%]c |
|---|---|---|---|
| 1 |
10a |
95d | 90 |
| 2 |
10b |
88d
98 |
98
98 |
| 3 |
10c |
60d
96 |
99
99 |
| 4 |
10d |
78d
95 |
99
99 |
| 5 |
10e |
94 | 97 |
aReaction conditions: 9, 2b (1.3 equiv), 5 mol % 1b in toluene/CHCl3 (2:1) (0.1 M) at 30 °C, flow rate 0.1 mL min−1, residence time = 20 min. bIsolated yields after column chromatography. cDetermined by chiral HPLC analysis. dRetention time: 10 min.
Conclusion
In conclusion, we have demonstrated the potential of a microreactor setup coupled with FTIR inline analysis for monitoring asymmetric continuous-flow hydrogenations of benzoxazines, quinolines, quinoxalines and 3H-indoles. Following a real-time continuous-flow optimization, the corresponding products were obtained in good yields and with excellent enantioselectivities. By applying the FTIR inline monitoring, reaction parameters can be screened rapidly in a single reaction setup, and the optimal reaction conditions can be obtained much faster as compared to the classical sequence of conducting the reaction followed by analysis. Further work will include automated integration and feedback optimization of reaction parameters.
References
-
Ehrfeld, W.; Hessel, V.; Löwe, H. Microreactors: New Technology for Modern Chemistry; Wiley-VCH: Weinheim, Germany, 2000.
Return to citation in text: [1] -
Wirth, T., Ed. Microreactors in Organic Synthesis and Catalysis;; Wiley-VCH: Weinheim, Germany, 2008.
Return to citation in text: [1] -
Jas, G.; Kirschning, A. Chem.–Eur. J. 2003, 9, 5708–5723. doi:10.1002/chem.200305212
Return to citation in text: [1] -
Kikutani, Y.; Kitamori, T. Macromol. Rapid Commun. 2004, 25, 158–168. doi:10.1002/marc.200300192
Return to citation in text: [1] -
Jähnisch, K.; Hessel, V.; Löwe, H.; Baerns, M. Angew. Chem., Int. Ed. 2004, 43, 406–446. doi:10.1002/anie.200300577
Return to citation in text: [1] -
Doku, G. N.; Verboom, W.; Reinhoudt, D. N.; van den Berg, A. Tetrahedron 2005, 61, 2733–2742. doi:10.1016/j.tet.2005.01.028
Return to citation in text: [1] -
Watts, P.; Haswell, S. J. Chem. Soc. Rev. 2005, 34, 235–246. doi:10.1039/b313866f
Return to citation in text: [1] -
Geyer, K.; Codée, J. D. C.; Seeberger, P. H. Chem.–Eur. J. 2006, 12, 8434–8442. doi:10.1002/chem.200600596
Return to citation in text: [1] -
deMello, A. J. Nature 2006, 442, 394–402. doi:10.1038/nature05062
Return to citation in text: [1] -
Song, H.; Chen, D. L.; Ismagilov, R. F. Angew. Chem., Int. Ed. 2006, 45, 7336–7356. doi:10.1002/anie.200601554
Return to citation in text: [1] -
Kobayashi, J.; Mori, Y.; Kobayashi, S. Chem.–Asian J. 2006, 1, 22–35. doi:10.1002/asia.200600058
Return to citation in text: [1] -
Mason, B. P.; Price, K. E.; Steinbacher, J. L.; Bogdan, A. R.; McQuade, D. T. Chem. Rev. 2007, 107, 2300–2318. doi:10.1021/cr050944c
Return to citation in text: [1] -
Watts, P.; Wiles, C. Chem. Commun. 2007, 443–467. doi:10.1039/b609428g
Return to citation in text: [1] -
Ahmed-Omer, B.; Brandt, J. C.; Wirth, T. Org. Biomol. Chem. 2007, 5, 733–740. doi:10.1039/b615072a
Return to citation in text: [1] -
Fukuyama, T.; Rahman, M. T.; Sato, M.; Ryu, I. Synlett 2008, 151–163. doi:10.1055/s-2007-1000884
Return to citation in text: [1] -
Yoshida, J.-i.; Nagaki, A.; Yamada, T. Chem.–Eur. J. 2008, 14, 7450–7459. doi:10.1002/chem.200800582
Return to citation in text: [1] -
Wiles, C.; Watts, P. Eur. J. Org. Chem. 2008, 1655–1671. doi:10.1002/ejoc.200701041
Return to citation in text: [1] -
Kirschning, A. Beilstein J. Org. Chem. 2009, 5, No. 15. doi:10.3762/bjoc.5.15
Return to citation in text: [1] -
Geyer, K.; Gustafsson, T.; Seeberger, P. H. Synlett 2009, 2382–2391. doi:10.1055/s-0029-1217828
Return to citation in text: [1] -
Nagaki, A.; Takabayashi, N.; Tomida, Y.; Yoshida, J.-i. Beilstein J. Org. Chem. 2009, 5, No. 16. doi:10.3762/bjoc.5.16
Return to citation in text: [1] -
Yamada, Y. M. A.; Torii, K.; Uozumi, Y. Beilstein J. Org. Chem. 2009, 5, No. 18. doi:10.3762/bjoc.5.18
Return to citation in text: [1] -
Brandt, J. C.; Wirth, T. Beilstein J. Org. Chem. 2009, 5, No. 30. doi:10.3762/bjoc.5.30
Return to citation in text: [1] -
Fukuyama, T.; Rahman, M. T.; Kamata, N.; Ryu, I. Beilstein J. Org. Chem. 2009, 5, No. 34. doi:10.3762/bjoc.5.34
Return to citation in text: [1] -
Tanaka, K.; Fukase, K. Beilstein J. Org. Chem. 2009, 5, No. 40. doi:10.3762/bjoc.5.40
Return to citation in text: [1] -
Kunz, U.; Turek, T. Beilstein J. Org. Chem. 2009, 5, No. 70. doi:10.3762/bjoc.5.70
Return to citation in text: [1] -
Marre, S.; Jensen, K. F. Chem. Soc. Rev. 2010, 39, 1183–1202. doi:10.1039/b821324k
Return to citation in text: [1] -
Yoshida, J-i.; Kim, H.; Nagaki, A. ChemSusChem 2011, 4, 331–340. doi:10.1002/cssc.201000271
Return to citation in text: [1] -
Wegner, J.; Ceylan, S.; Kirschning, A. Chem. Commun. 2011, 47, 4583–4592. doi:10.1039/c0cc05060a
Return to citation in text: [1] -
Min, K.-I.; Lee, T.-H.; Park, C. P.; Wu, Z.-Y.; Girault, H. H.; Ryu, I.; Fukuyama, T.; Mukai, Y.; Kim, D.-P. Angew. Chem., Int. Ed. 2010, 49, 7063–7067. doi:10.1002/anie.201002004
Return to citation in text: [1] -
McMullen, J. P.; Stone, M. T.; Buchwald, S. L.; Jensen, K. F. Angew. Chem., Int. Ed. 2010, 49, 7076–7080. doi:10.1002/anie.201002590
Return to citation in text: [1] -
McMullen, J. P.; Jensen, K. F. Annu. Rev. Anal. Chem. 2010, 3, 19–42. doi:10.1146/annurev.anchem.111808.073718
Return to citation in text: [1] -
Hartman, R. L.; McMullen, J. P.; Jensen, K. F. Angew. Chem., Int. Ed. 2011, 50, 7502–7519. doi:10.1002/anie.201004637
Return to citation in text: [1] -
Shvydkiv, O.; Nolan, K.; Oelgemöller, M. Beilstein J. Org. Chem. 2011, 7, 1055–1063. doi:10.3762/bjoc.7.121
Return to citation in text: [1] -
Nagaki, A.; Uesugi, Y.; Tomida, Y.; Yoshida, J.-i. Beilstein J. Org. Chem. 2011, 7, 1064–1069. doi:10.3762/bjoc.7.122
Return to citation in text: [1] -
Watts, K.; Gattrell, W.; Wirth, T. Beilstein J. Org. Chem. 2011, 7, 1108–1114. doi:10.3762/bjoc.7.127
Return to citation in text: [1] -
Roper, K. A.; Lange, H.; Polyzos, A.; Berry, M. B.; Baxendale, I. R.; Ley, S. V. Beilstein J. Org. Chem. 2011, 7, 1648–1655. doi:10.3762/bjoc.7.194
Return to citation in text: [1] -
Saito, K.; Ueoka, K.; Matsumoto, K.; Suga, S.; Nokami, T.; Yoshida, J-i. Angew. Chem., Int. Ed. 2011, 50, 5153–5156. doi:10.1002/anie.201100854
Return to citation in text: [1] -
Wiles, C.; Watts, P. Chem. Commun. 2011, 47, 6512–6535. doi:10.1039/c1cc00089f
Return to citation in text: [1] -
Yoshida, J-i.; Saito, K.; Nokami, T.; Nagaki, A. Synlett 2011, 1189–1194. doi:10.1055/s-0030-1259946
Return to citation in text: [1] -
Bogdan, A.; McQuade, D. T. Beilstein J. Org. Chem. 2009, 5, No. 17. doi:10.3762/bjoc.5.17
Return to citation in text: [1] -
Wiles, C.; Watts, P. Green Chem. 2012, 14, 38–54. doi:10.1039/c1gc16022b
Return to citation in text: [1] -
Wegner, J.; Ceylan, S.; Kirschning, A. Adv. Synth. Catal. 2012, 354, 17–57. doi:10.1002/adsc.201100584
Return to citation in text: [1] -
Carter, C. F.; Baxendale, I. R.; O’Brien, M.; Pavey, J. B. J.; Ley, S. V. Org. Biomol. Chem. 2009, 7, 4594–4597. doi:10.1039/b917289k
Return to citation in text: [1] -
Carter, C. F.; Lange, H.; Ley, S. V.; Baxendale, I. R.; Wittkamp, B.; Goode, J. G.; Gaunt, N. L. Org. Process Res. Dev. 2010, 14, 393–404. doi:10.1021/op900305v
Return to citation in text: [1] -
Qian, Z.; Baxendale, I. R.; Ley, S. V. Chem.–Eur. J. 2010, 16, 12342–12348. doi:10.1002/chem.201002147
Return to citation in text: [1] -
Carter, C. F.; Baxendale, I. R.; Pavey, J. B. J.; Ley, S. V. Org. Biomol. Chem. 2010, 8, 1588–1595. doi:10.1039/b924309g
Return to citation in text: [1] -
Leadbeater, N. E. Chem. Commun. 2010, 46, 6693–6695. doi:10.1039/c0cc01921f
Return to citation in text: [1] -
Malet-Sanz, L.; Madrzak, J.; Ley, S. V.; Baxendale, I. R. Org. Biomol. Chem. 2010, 8, 5324–5332. doi:10.1039/c0ob00450b
Return to citation in text: [1] -
McMullen, J. P.; Jensen, K. F. Org. Process Res. Dev. 2010, 14, 1169–1176. doi:10.1021/op100123e
Return to citation in text: [1] -
Foley, D. A.; Doecke, C. W.; Buser, J. Y.; Merritt, J. M.; Murphy, L.; Kissane, M.; Collins, S. G.; Maguire, A. R.; Kaerner, A. J. Org. Chem. 2011, 76, 9630–9640. doi:10.1021/jo201212p
Return to citation in text: [1] -
Smith, C. J.; Nikbin, N.; Ley, S. V.; Lange, H.; Baxendale, I. R. Org. Biomol. Chem. 2011, 9, 1938–1947. doi:10.1039/c0ob00815j
Return to citation in text: [1] -
Lange, H.; Carter, C. F.; Hopkin, M. D.; Burke, A.; Goode, J. G.; Baxendale, I. R.; Ley, S. V. Chem. Sci. 2011, 2, 765–769. doi:10.1039/c0sc00603c
Return to citation in text: [1] -
Koos, P.; Gross, U.; Polyzos, A.; O’Brien, M.; Baxendale, I. R.; Ley, S. V. Org. Biomol. Chem. 2011, 9, 6903–6908. doi:10.1039/c1ob06017a
Return to citation in text: [1] -
Keybl, J.; Jensen, K. F. Ind. Eng. Chem. Res. 2011, 50, 11013–11022. doi:10.1021/ie200936b
Return to citation in text: [1] -
Brodmann, T.; Koos, P.; Metzger, A.; Knochel, P.; Ley, S. V. Org. Process Res. Dev. 2011. doi:10.1021/op200275d
Return to citation in text: [1] -
Hook, B. D. A.; Dohle, W.; Hirst, P. R.; Pickworth, M.; Berry, M. B.; Booker-Milburn, K. I. J. Org. Chem. 2005, 70, 7558–7564. doi:10.1021/jo050705p
Return to citation in text: [1] -
Wiles, C.; Watts, P.; Haswell, S. J. Tetrahedron Lett. 2007, 48, 7362–7365. doi:10.1016/j.tetlet.2007.08.027
Return to citation in text: [1] -
Griffiths-Jones, C. M.; Hopkin, M. D.; Jönsson, D.; Ley, S. V.; Tapolczay, D. J.; Vickerstaffe, E.; Ladlow, M. J. Comb. Chem. 2007, 9, 422–430. doi:10.1021/cc060152b
Return to citation in text: [1] -
Mennecke, K.; Solodenko, W.; Kirschning, A. Synthesis 2008, 1589–1599. doi:10.1055/s-2008-1072579
Return to citation in text: [1] -
Mennecke, K.; Kirschning, A. Synthesis 2008, 3267–3272. doi:10.1055/s-2008-1067274
Return to citation in text: [1] -
Baxendale, I. R.; Ley, S. V.; Mansfield, A. C.; Smith, C. D. Angew. Chem., Int. Ed. 2009, 48, 4017–4021. doi:10.1002/anie.200900970
Return to citation in text: [1] -
Wang, N.; Matsumoto, T.; Ueno, M.; Miyamura, H.; Kobayashi, S. Angew. Chem., Int. Ed. 2009, 48, 4744–4746. doi:10.1002/anie.200900565
Return to citation in text: [1] -
Brasholz, M.; Macdonald, J. M.; Saubern, S.; Ryan, J. H.; Holmes, A. B. Chem.–Eur. J. 2010, 16, 11471–11480. doi:10.1002/chem.201001435
Return to citation in text: [1] -
Costantini, F.; Benetti, E. M.; Tiggelaar, R. M.; Gardeniers, H. J. G. E.; Reinhoudt, D. N.; Huskens, J.; Vancso, G. J.; Verboom, W. Chem.–Eur. J. 2010, 16, 12406–12411. doi:10.1002/chem.201000948
Return to citation in text: [1] -
Gutmann, B.; Roduit, J.-P.; Roberge, D.; Kappe, C. O. Angew. Chem., Int. Ed. 2010, 49, 7101–7105. doi:10.1002/anie.201003733
Return to citation in text: [1] -
Wahab, B.; Ellames, G.; Passey, S.; Watts, P. Tetrahedron 2010, 66, 3861–3865. doi:10.1016/j.tet.2010.03.005
Return to citation in text: [1] -
Fuse, S.; Tanabe, N.; Yoshida, M.; Yoshida, H.; Doi, T.; Takahashi, T. Chem. Commun. 2010, 46, 8722–8724. doi:10.1039/c0cc02239j
Return to citation in text: [1] -
Venturoni, F.; Nikbin, N.; Ley, S. V.; Baxendale, I. R. Org. Biomol. Chem. 2010, 8, 1798–1806. doi:10.1039/b925327k
Return to citation in text: [1] -
Webb, D.; Jamison, T. F. Chem. Sci. 2010, 1, 675–680. doi:10.1039/c0sc00381f
Return to citation in text: [1] -
Gutmann, B.; Roduit, J.-P.; Roberge, D.; Kappe, C. O. Chem.–Eur. J. 2011, 17, 13146–13150. doi:10.1002/chem.201102772
Return to citation in text: [1] -
Maurya, R. A.; Park, C. P.; Lee, J. H.; Kim, D.-P. Angew. Chem., Int. Ed. 2011, 50, 5952–5955. doi:10.1002/anie.201101977
Return to citation in text: [1] -
Sniady, A.; Bedore, M. W.; Jamison, T. F. Angew. Chem., Int. Ed. 2011, 50, 2155–2158. doi:10.1002/anie.201006440
Return to citation in text: [1] -
Li, P.; Buchwald, S. L. Angew. Chem., Int. Ed. 2011, 50, 6396–6400. doi:10.1002/anie.201102401
Return to citation in text: [1] -
Noël, T.; Maimone, T. J.; Buchwald, S. L. Angew. Chem., Int. Ed. 2011, 50, 8900–8903. doi:10.1002/anie.201104652
Return to citation in text: [1] -
Shu, W.; Pellegatti, L.; Oberli, M. A.; Buchwald, S. L. Angew. Chem., Int. Ed. 2011, 50, 10665–10669. doi:10.1002/anie.201105223
Return to citation in text: [1] -
O’Brien, A. G.; Lévesque, F.; Seeberger, P. H. Chem. Commun. 2011, 47, 2688–2690. doi:10.1039/c0cc04481d
Return to citation in text: [1] -
Noël, T.; Buchwald, S. L. Chem. Soc. Rev. 2011, 40, 5010–5029. doi:10.1039/c1cs15075h
Return to citation in text: [1] -
Kim, H.; Nagaki, A.; Yoshida, J.-i. Nat. Commun. 2011, 264. doi:10.1038/ncomms1264
Return to citation in text: [1] -
Browne, D. L.; Baumann, M.; Harji, B. H.; Baxendale, I. R.; Ley, S. V. Org. Lett. 2011, 13, 3312–3315. doi:10.1021/ol2010006
Return to citation in text: [1] -
Allian, A. D.; Richter, S. M.; Kallemeyn, J. M.; Robbins, T. A.; Kishore, V. Org. Process Res. Dev. 2011, 15, 91–97. doi:10.1021/op100249z
Return to citation in text: [1] -
Wiles, C.; Wattts, P.; Haswell, S. J.; Pombo-Villar, E. Lab Chip 2004, 4, 171–173. doi:10.1039/b400280f
Return to citation in text: [1] -
Jönsson, C.; Lundgren, S.; Haswell, S. J.; Moberg, C. Tetrahedron 2004, 60, 10515–10520. doi:10.1016/j.tet.2004.08.080
Return to citation in text: [1] -
de Bellefon, C.; Lamouille, T.; Pestre, N.; Bornette, F.; Pennemann, H.; Neumann, F.; Hessel, V. Catal. Today 2005, 110, 179–187. doi:10.1016/j.cattod.2005.09.002
Return to citation in text: [1] -
Hamberg, A.; Lundgren, S.; Wingstrand, E.; Moberg, C.; Hult, K. Chem.–Eur. J. 2007, 13, 4334–4341. doi:10.1002/chem.200601638
Return to citation in text: [1] -
Sakeda, K.; Wakabayashi, K.; Matsushita, Y.; Ichimura, T.; Suzuki, T.; Wada, T.; Inoue, Y. J. Photochem. Photobiol., A 2007, 192, 166–171. doi:10.1016/j.jphotochem.2007.05.019
Return to citation in text: [1] -
Mak, X. Y.; Laurino, P.; Seeberger, P. H. Beilstein J. Org. Chem. 2009, 5, No. 19. doi:10.3762/bjoc.5.19
Return to citation in text: [1] -
Shi, L.; Wang, X.; Sandoval, C. A.; Wang, Z.; Li, H.; Wu, J.; Yu, L.; Ding, K. Chem.–Eur. J. 2009, 15, 9855–9867. doi:10.1002/chem.200900899
Return to citation in text: [1] -
Rolland, J.; Cambeiro, X. C.; Rodríguez-Escrich, C.; Pericàs, M. A. Beilstein J. Org. Chem. 2009, 5, No. 56. doi:10.3762/bjoc.5.56
Return to citation in text: [1] -
Alza, E.; Rodríguez-Escrich, C.; Sayalero, S.; Bastero, A.; Pericàs, M. A. Chem.–Eur. J. 2009, 15, 10167–10172. doi:10.1002/chem.200901310
Return to citation in text: [1] -
Tomida, Y.; Nagaki, A.; Yoshida, J.-i. J. Am. Chem. Soc. 2011, 133, 3744–3747. doi:10.1021/ja110898s
Return to citation in text: [1] -
Carter, C. F.; Lange, H.; Sakai, D.; Baxendale, I. R.; Ley, S. V. Chem.–Eur. J. 2011, 17, 3398–3405. doi:10.1002/chem.201003148
Return to citation in text: [1] -
Massi, A.; Cavazzzini, A.; Del Zoppo, L.; Pandoli, O.; Costa, V.; Pasti, L.; Giovannini, P. P. Tetrahedron Lett. 2011, 52, 619–622. doi:10.1016/j.tetlet.2010.11.157
Return to citation in text: [1] -
Takeda, K.; Oohara, T.; Shimada, N.; Nambu, H.; Hashimoto, S. Chem.–Eur. J. 2011, 17, 13992–13998. doi:10.1002/chem.201102733
Return to citation in text: [1] -
Fritzsche, S.; Ohla, S.; Glaser, P.; Giera, D. S.; Sickert, M.; Schneider, C.; Belder, D. Angew. Chem., Int. Ed. 2011, 50, 9467–9470. doi:10.1002/anie.201102331
Return to citation in text: [1] -
Cambeiro, X. C.; Martín-Rapún, R.; Miranda, P. O.; Sayalero, S.; Alza, E.; Llanes, P.; Pericàs, M. A. Beilstein J. Org. Chem. 2011, 7, 1486–1493. doi:10.3762/bjoc.7.172
Return to citation in text: [1] -
Ayats, C.; Henseler, A. H.; Pericàs, M. A. ChemSusChem 2012, 5, 320–325. doi:10.1002/cssc.201100570
Return to citation in text: [1] -
Rueping, M.; Sugiono, E.; Azap, C.; Theissmann, T.; Bolte, M. Org. Lett. 2005, 7, 3781–3783. doi:10.1021/ol0515964
Return to citation in text: [1] -
Rueping, M.; Antonchick, A. P. Angew. Chem., Int. Ed. 2007, 46, 4562–4565. doi:10.1002/anie.200701158
Return to citation in text: [1] -
Rueping, M.; Sugiono, E.; Schoepke, F. R. Synlett 2010, 852–865. doi:10.1055/s-0029-1219528
Return to citation in text: [1] -
Rueping, M.; Merino, E.; Koenigs, R. M. Adv. Synth. Catal. 2010, 352, 2629–2634. doi:10.1002/adsc.201000547
Return to citation in text: [1] -
Rueping, M.; Dufour, J.; Schoepke, F. R. Green Chem. 2011, 13, 1084–1105. doi:10.1039/c1gc15027h
Return to citation in text: [1] -
Rueping, M.; Antonchick, A. P.; Theissmann, T. Angew. Chem., Int. Ed. 2006, 45, 6751–6755. doi:10.1002/anie.200601832
Return to citation in text: [1] -
Rueping, M.; Antonchick, A. P.; Theissmann, T. Angew. Chem., Int. Ed. 2006, 45, 3683–3686. doi:10.1002/anie.200600191
Return to citation in text: [1] -
Rueping, M.; Theissmann, T.; Raja, S.; Bats, J. W. Adv. Synth. Catal. 2008, 350, 1001–1006. doi:10.1002/adsc.200800020
Return to citation in text: [1] -
Rueping, M.; Stoeckel, M.; Sugiono, E.; Theissmann, T. Tetrahedron 2010, 66, 6565–6568. doi:10.1016/j.tet.2010.04.091
Return to citation in text: [1] -
Rueping, M.; Theissmann, T.; Stoeckel, M.; Antonchick, A. P. Org. Biomol. Chem. 2011, 9, 6844–6850. doi:10.1039/c1ob05870c
Return to citation in text: [1] -
Rueping, M.; Tato, F.; Schoepke, F. R. Chem.–Eur. J. 2010, 16, 2688–2691. doi:10.1002/chem.200902907
Return to citation in text: [1] -
Rueping, M.; Brinkmann, C.; Antonchick, A. P.; Atodiresei, I. Org. Lett. 2010, 12, 4604–4607. doi:10.1021/ol1019234
Return to citation in text: [1]
| 1. | Ehrfeld, W.; Hessel, V.; Löwe, H. Microreactors: New Technology for Modern Chemistry; Wiley-VCH: Weinheim, Germany, 2000. |
| 2. | Wirth, T., Ed. Microreactors in Organic Synthesis and Catalysis;; Wiley-VCH: Weinheim, Germany, 2008. |
| 3. | Jas, G.; Kirschning, A. Chem.–Eur. J. 2003, 9, 5708–5723. doi:10.1002/chem.200305212 |
| 4. | Kikutani, Y.; Kitamori, T. Macromol. Rapid Commun. 2004, 25, 158–168. doi:10.1002/marc.200300192 |
| 5. | Jähnisch, K.; Hessel, V.; Löwe, H.; Baerns, M. Angew. Chem., Int. Ed. 2004, 43, 406–446. doi:10.1002/anie.200300577 |
| 6. | Doku, G. N.; Verboom, W.; Reinhoudt, D. N.; van den Berg, A. Tetrahedron 2005, 61, 2733–2742. doi:10.1016/j.tet.2005.01.028 |
| 7. | Watts, P.; Haswell, S. J. Chem. Soc. Rev. 2005, 34, 235–246. doi:10.1039/b313866f |
| 8. | Geyer, K.; Codée, J. D. C.; Seeberger, P. H. Chem.–Eur. J. 2006, 12, 8434–8442. doi:10.1002/chem.200600596 |
| 9. | deMello, A. J. Nature 2006, 442, 394–402. doi:10.1038/nature05062 |
| 10. | Song, H.; Chen, D. L.; Ismagilov, R. F. Angew. Chem., Int. Ed. 2006, 45, 7336–7356. doi:10.1002/anie.200601554 |
| 11. | Kobayashi, J.; Mori, Y.; Kobayashi, S. Chem.–Asian J. 2006, 1, 22–35. doi:10.1002/asia.200600058 |
| 12. | Mason, B. P.; Price, K. E.; Steinbacher, J. L.; Bogdan, A. R.; McQuade, D. T. Chem. Rev. 2007, 107, 2300–2318. doi:10.1021/cr050944c |
| 13. | Watts, P.; Wiles, C. Chem. Commun. 2007, 443–467. doi:10.1039/b609428g |
| 14. | Ahmed-Omer, B.; Brandt, J. C.; Wirth, T. Org. Biomol. Chem. 2007, 5, 733–740. doi:10.1039/b615072a |
| 15. | Fukuyama, T.; Rahman, M. T.; Sato, M.; Ryu, I. Synlett 2008, 151–163. doi:10.1055/s-2007-1000884 |
| 16. | Yoshida, J.-i.; Nagaki, A.; Yamada, T. Chem.–Eur. J. 2008, 14, 7450–7459. doi:10.1002/chem.200800582 |
| 17. | Wiles, C.; Watts, P. Eur. J. Org. Chem. 2008, 1655–1671. doi:10.1002/ejoc.200701041 |
| 18. | Kirschning, A. Beilstein J. Org. Chem. 2009, 5, No. 15. doi:10.3762/bjoc.5.15 |
| 19. | Geyer, K.; Gustafsson, T.; Seeberger, P. H. Synlett 2009, 2382–2391. doi:10.1055/s-0029-1217828 |
| 20. | Nagaki, A.; Takabayashi, N.; Tomida, Y.; Yoshida, J.-i. Beilstein J. Org. Chem. 2009, 5, No. 16. doi:10.3762/bjoc.5.16 |
| 21. | Yamada, Y. M. A.; Torii, K.; Uozumi, Y. Beilstein J. Org. Chem. 2009, 5, No. 18. doi:10.3762/bjoc.5.18 |
| 22. | Brandt, J. C.; Wirth, T. Beilstein J. Org. Chem. 2009, 5, No. 30. doi:10.3762/bjoc.5.30 |
| 23. | Fukuyama, T.; Rahman, M. T.; Kamata, N.; Ryu, I. Beilstein J. Org. Chem. 2009, 5, No. 34. doi:10.3762/bjoc.5.34 |
| 24. | Tanaka, K.; Fukase, K. Beilstein J. Org. Chem. 2009, 5, No. 40. doi:10.3762/bjoc.5.40 |
| 25. | Kunz, U.; Turek, T. Beilstein J. Org. Chem. 2009, 5, No. 70. doi:10.3762/bjoc.5.70 |
| 26. | Marre, S.; Jensen, K. F. Chem. Soc. Rev. 2010, 39, 1183–1202. doi:10.1039/b821324k |
| 27. | Yoshida, J-i.; Kim, H.; Nagaki, A. ChemSusChem 2011, 4, 331–340. doi:10.1002/cssc.201000271 |
| 28. | Wegner, J.; Ceylan, S.; Kirschning, A. Chem. Commun. 2011, 47, 4583–4592. doi:10.1039/c0cc05060a |
| 29. | Min, K.-I.; Lee, T.-H.; Park, C. P.; Wu, Z.-Y.; Girault, H. H.; Ryu, I.; Fukuyama, T.; Mukai, Y.; Kim, D.-P. Angew. Chem., Int. Ed. 2010, 49, 7063–7067. doi:10.1002/anie.201002004 |
| 30. | McMullen, J. P.; Stone, M. T.; Buchwald, S. L.; Jensen, K. F. Angew. Chem., Int. Ed. 2010, 49, 7076–7080. doi:10.1002/anie.201002590 |
| 31. | McMullen, J. P.; Jensen, K. F. Annu. Rev. Anal. Chem. 2010, 3, 19–42. doi:10.1146/annurev.anchem.111808.073718 |
| 32. | Hartman, R. L.; McMullen, J. P.; Jensen, K. F. Angew. Chem., Int. Ed. 2011, 50, 7502–7519. doi:10.1002/anie.201004637 |
| 33. | Shvydkiv, O.; Nolan, K.; Oelgemöller, M. Beilstein J. Org. Chem. 2011, 7, 1055–1063. doi:10.3762/bjoc.7.121 |
| 34. | Nagaki, A.; Uesugi, Y.; Tomida, Y.; Yoshida, J.-i. Beilstein J. Org. Chem. 2011, 7, 1064–1069. doi:10.3762/bjoc.7.122 |
| 35. | Watts, K.; Gattrell, W.; Wirth, T. Beilstein J. Org. Chem. 2011, 7, 1108–1114. doi:10.3762/bjoc.7.127 |
| 36. | Roper, K. A.; Lange, H.; Polyzos, A.; Berry, M. B.; Baxendale, I. R.; Ley, S. V. Beilstein J. Org. Chem. 2011, 7, 1648–1655. doi:10.3762/bjoc.7.194 |
| 37. | Saito, K.; Ueoka, K.; Matsumoto, K.; Suga, S.; Nokami, T.; Yoshida, J-i. Angew. Chem., Int. Ed. 2011, 50, 5153–5156. doi:10.1002/anie.201100854 |
| 38. | Wiles, C.; Watts, P. Chem. Commun. 2011, 47, 6512–6535. doi:10.1039/c1cc00089f |
| 39. | Yoshida, J-i.; Saito, K.; Nokami, T.; Nagaki, A. Synlett 2011, 1189–1194. doi:10.1055/s-0030-1259946 |
| 40. | Bogdan, A.; McQuade, D. T. Beilstein J. Org. Chem. 2009, 5, No. 17. doi:10.3762/bjoc.5.17 |
| 41. | Wiles, C.; Watts, P. Green Chem. 2012, 14, 38–54. doi:10.1039/c1gc16022b |
| 42. | Wegner, J.; Ceylan, S.; Kirschning, A. Adv. Synth. Catal. 2012, 354, 17–57. doi:10.1002/adsc.201100584 |
| 97. | Rueping, M.; Sugiono, E.; Azap, C.; Theissmann, T.; Bolte, M. Org. Lett. 2005, 7, 3781–3783. doi:10.1021/ol0515964 |
| 98. | Rueping, M.; Antonchick, A. P. Angew. Chem., Int. Ed. 2007, 46, 4562–4565. doi:10.1002/anie.200701158 |
| 99. | Rueping, M.; Sugiono, E.; Schoepke, F. R. Synlett 2010, 852–865. doi:10.1055/s-0029-1219528 |
| 100. | Rueping, M.; Merino, E.; Koenigs, R. M. Adv. Synth. Catal. 2010, 352, 2629–2634. doi:10.1002/adsc.201000547 |
| 101. | Rueping, M.; Dufour, J.; Schoepke, F. R. Green Chem. 2011, 13, 1084–1105. doi:10.1039/c1gc15027h |
| 81. | Wiles, C.; Wattts, P.; Haswell, S. J.; Pombo-Villar, E. Lab Chip 2004, 4, 171–173. doi:10.1039/b400280f |
| 82. | Jönsson, C.; Lundgren, S.; Haswell, S. J.; Moberg, C. Tetrahedron 2004, 60, 10515–10520. doi:10.1016/j.tet.2004.08.080 |
| 83. | de Bellefon, C.; Lamouille, T.; Pestre, N.; Bornette, F.; Pennemann, H.; Neumann, F.; Hessel, V. Catal. Today 2005, 110, 179–187. doi:10.1016/j.cattod.2005.09.002 |
| 84. | Hamberg, A.; Lundgren, S.; Wingstrand, E.; Moberg, C.; Hult, K. Chem.–Eur. J. 2007, 13, 4334–4341. doi:10.1002/chem.200601638 |
| 85. | Sakeda, K.; Wakabayashi, K.; Matsushita, Y.; Ichimura, T.; Suzuki, T.; Wada, T.; Inoue, Y. J. Photochem. Photobiol., A 2007, 192, 166–171. doi:10.1016/j.jphotochem.2007.05.019 |
| 86. | Mak, X. Y.; Laurino, P.; Seeberger, P. H. Beilstein J. Org. Chem. 2009, 5, No. 19. doi:10.3762/bjoc.5.19 |
| 87. | Shi, L.; Wang, X.; Sandoval, C. A.; Wang, Z.; Li, H.; Wu, J.; Yu, L.; Ding, K. Chem.–Eur. J. 2009, 15, 9855–9867. doi:10.1002/chem.200900899 |
| 88. | Rolland, J.; Cambeiro, X. C.; Rodríguez-Escrich, C.; Pericàs, M. A. Beilstein J. Org. Chem. 2009, 5, No. 56. doi:10.3762/bjoc.5.56 |
| 89. | Alza, E.; Rodríguez-Escrich, C.; Sayalero, S.; Bastero, A.; Pericàs, M. A. Chem.–Eur. J. 2009, 15, 10167–10172. doi:10.1002/chem.200901310 |
| 90. | Tomida, Y.; Nagaki, A.; Yoshida, J.-i. J. Am. Chem. Soc. 2011, 133, 3744–3747. doi:10.1021/ja110898s |
| 91. | Carter, C. F.; Lange, H.; Sakai, D.; Baxendale, I. R.; Ley, S. V. Chem.–Eur. J. 2011, 17, 3398–3405. doi:10.1002/chem.201003148 |
| 92. | Massi, A.; Cavazzzini, A.; Del Zoppo, L.; Pandoli, O.; Costa, V.; Pasti, L.; Giovannini, P. P. Tetrahedron Lett. 2011, 52, 619–622. doi:10.1016/j.tetlet.2010.11.157 |
| 93. | Takeda, K.; Oohara, T.; Shimada, N.; Nambu, H.; Hashimoto, S. Chem.–Eur. J. 2011, 17, 13992–13998. doi:10.1002/chem.201102733 |
| 94. | Fritzsche, S.; Ohla, S.; Glaser, P.; Giera, D. S.; Sickert, M.; Schneider, C.; Belder, D. Angew. Chem., Int. Ed. 2011, 50, 9467–9470. doi:10.1002/anie.201102331 |
| 95. | Cambeiro, X. C.; Martín-Rapún, R.; Miranda, P. O.; Sayalero, S.; Alza, E.; Llanes, P.; Pericàs, M. A. Beilstein J. Org. Chem. 2011, 7, 1486–1493. doi:10.3762/bjoc.7.172 |
| 96. | Ayats, C.; Henseler, A. H.; Pericàs, M. A. ChemSusChem 2012, 5, 320–325. doi:10.1002/cssc.201100570 |
| 56. | Hook, B. D. A.; Dohle, W.; Hirst, P. R.; Pickworth, M.; Berry, M. B.; Booker-Milburn, K. I. J. Org. Chem. 2005, 70, 7558–7564. doi:10.1021/jo050705p |
| 57. | Wiles, C.; Watts, P.; Haswell, S. J. Tetrahedron Lett. 2007, 48, 7362–7365. doi:10.1016/j.tetlet.2007.08.027 |
| 58. | Griffiths-Jones, C. M.; Hopkin, M. D.; Jönsson, D.; Ley, S. V.; Tapolczay, D. J.; Vickerstaffe, E.; Ladlow, M. J. Comb. Chem. 2007, 9, 422–430. doi:10.1021/cc060152b |
| 59. | Mennecke, K.; Solodenko, W.; Kirschning, A. Synthesis 2008, 1589–1599. doi:10.1055/s-2008-1072579 |
| 60. | Mennecke, K.; Kirschning, A. Synthesis 2008, 3267–3272. doi:10.1055/s-2008-1067274 |
| 61. | Baxendale, I. R.; Ley, S. V.; Mansfield, A. C.; Smith, C. D. Angew. Chem., Int. Ed. 2009, 48, 4017–4021. doi:10.1002/anie.200900970 |
| 62. | Wang, N.; Matsumoto, T.; Ueno, M.; Miyamura, H.; Kobayashi, S. Angew. Chem., Int. Ed. 2009, 48, 4744–4746. doi:10.1002/anie.200900565 |
| 63. | Brasholz, M.; Macdonald, J. M.; Saubern, S.; Ryan, J. H.; Holmes, A. B. Chem.–Eur. J. 2010, 16, 11471–11480. doi:10.1002/chem.201001435 |
| 64. | Costantini, F.; Benetti, E. M.; Tiggelaar, R. M.; Gardeniers, H. J. G. E.; Reinhoudt, D. N.; Huskens, J.; Vancso, G. J.; Verboom, W. Chem.–Eur. J. 2010, 16, 12406–12411. doi:10.1002/chem.201000948 |
| 65. | Gutmann, B.; Roduit, J.-P.; Roberge, D.; Kappe, C. O. Angew. Chem., Int. Ed. 2010, 49, 7101–7105. doi:10.1002/anie.201003733 |
| 66. | Wahab, B.; Ellames, G.; Passey, S.; Watts, P. Tetrahedron 2010, 66, 3861–3865. doi:10.1016/j.tet.2010.03.005 |
| 67. | Fuse, S.; Tanabe, N.; Yoshida, M.; Yoshida, H.; Doi, T.; Takahashi, T. Chem. Commun. 2010, 46, 8722–8724. doi:10.1039/c0cc02239j |
| 68. | Venturoni, F.; Nikbin, N.; Ley, S. V.; Baxendale, I. R. Org. Biomol. Chem. 2010, 8, 1798–1806. doi:10.1039/b925327k |
| 69. | Webb, D.; Jamison, T. F. Chem. Sci. 2010, 1, 675–680. doi:10.1039/c0sc00381f |
| 70. | Gutmann, B.; Roduit, J.-P.; Roberge, D.; Kappe, C. O. Chem.–Eur. J. 2011, 17, 13146–13150. doi:10.1002/chem.201102772 |
| 71. | Maurya, R. A.; Park, C. P.; Lee, J. H.; Kim, D.-P. Angew. Chem., Int. Ed. 2011, 50, 5952–5955. doi:10.1002/anie.201101977 |
| 72. | Sniady, A.; Bedore, M. W.; Jamison, T. F. Angew. Chem., Int. Ed. 2011, 50, 2155–2158. doi:10.1002/anie.201006440 |
| 73. | Li, P.; Buchwald, S. L. Angew. Chem., Int. Ed. 2011, 50, 6396–6400. doi:10.1002/anie.201102401 |
| 74. | Noël, T.; Maimone, T. J.; Buchwald, S. L. Angew. Chem., Int. Ed. 2011, 50, 8900–8903. doi:10.1002/anie.201104652 |
| 75. | Shu, W.; Pellegatti, L.; Oberli, M. A.; Buchwald, S. L. Angew. Chem., Int. Ed. 2011, 50, 10665–10669. doi:10.1002/anie.201105223 |
| 76. | O’Brien, A. G.; Lévesque, F.; Seeberger, P. H. Chem. Commun. 2011, 47, 2688–2690. doi:10.1039/c0cc04481d |
| 77. | Noël, T.; Buchwald, S. L. Chem. Soc. Rev. 2011, 40, 5010–5029. doi:10.1039/c1cs15075h |
| 78. | Kim, H.; Nagaki, A.; Yoshida, J.-i. Nat. Commun. 2011, 264. doi:10.1038/ncomms1264 |
| 79. | Browne, D. L.; Baumann, M.; Harji, B. H.; Baxendale, I. R.; Ley, S. V. Org. Lett. 2011, 13, 3312–3315. doi:10.1021/ol2010006 |
| 80. | Allian, A. D.; Richter, S. M.; Kallemeyn, J. M.; Robbins, T. A.; Kishore, V. Org. Process Res. Dev. 2011, 15, 91–97. doi:10.1021/op100249z |
| 43. | Carter, C. F.; Baxendale, I. R.; O’Brien, M.; Pavey, J. B. J.; Ley, S. V. Org. Biomol. Chem. 2009, 7, 4594–4597. doi:10.1039/b917289k |
| 44. | Carter, C. F.; Lange, H.; Ley, S. V.; Baxendale, I. R.; Wittkamp, B.; Goode, J. G.; Gaunt, N. L. Org. Process Res. Dev. 2010, 14, 393–404. doi:10.1021/op900305v |
| 45. | Qian, Z.; Baxendale, I. R.; Ley, S. V. Chem.–Eur. J. 2010, 16, 12342–12348. doi:10.1002/chem.201002147 |
| 46. | Carter, C. F.; Baxendale, I. R.; Pavey, J. B. J.; Ley, S. V. Org. Biomol. Chem. 2010, 8, 1588–1595. doi:10.1039/b924309g |
| 47. | Leadbeater, N. E. Chem. Commun. 2010, 46, 6693–6695. doi:10.1039/c0cc01921f |
| 48. | Malet-Sanz, L.; Madrzak, J.; Ley, S. V.; Baxendale, I. R. Org. Biomol. Chem. 2010, 8, 5324–5332. doi:10.1039/c0ob00450b |
| 49. | McMullen, J. P.; Jensen, K. F. Org. Process Res. Dev. 2010, 14, 1169–1176. doi:10.1021/op100123e |
| 50. | Foley, D. A.; Doecke, C. W.; Buser, J. Y.; Merritt, J. M.; Murphy, L.; Kissane, M.; Collins, S. G.; Maguire, A. R.; Kaerner, A. J. Org. Chem. 2011, 76, 9630–9640. doi:10.1021/jo201212p |
| 51. | Smith, C. J.; Nikbin, N.; Ley, S. V.; Lange, H.; Baxendale, I. R. Org. Biomol. Chem. 2011, 9, 1938–1947. doi:10.1039/c0ob00815j |
| 52. | Lange, H.; Carter, C. F.; Hopkin, M. D.; Burke, A.; Goode, J. G.; Baxendale, I. R.; Ley, S. V. Chem. Sci. 2011, 2, 765–769. doi:10.1039/c0sc00603c |
| 53. | Koos, P.; Gross, U.; Polyzos, A.; O’Brien, M.; Baxendale, I. R.; Ley, S. V. Org. Biomol. Chem. 2011, 9, 6903–6908. doi:10.1039/c1ob06017a |
| 54. | Keybl, J.; Jensen, K. F. Ind. Eng. Chem. Res. 2011, 50, 11013–11022. doi:10.1021/ie200936b |
| 55. | Brodmann, T.; Koos, P.; Metzger, A.; Knochel, P.; Ley, S. V. Org. Process Res. Dev. 2011. doi:10.1021/op200275d |
| 108. | Rueping, M.; Brinkmann, C.; Antonchick, A. P.; Atodiresei, I. Org. Lett. 2010, 12, 4604–4607. doi:10.1021/ol1019234 |
| 107. | Rueping, M.; Tato, F.; Schoepke, F. R. Chem.–Eur. J. 2010, 16, 2688–2691. doi:10.1002/chem.200902907 |
| 103. | Rueping, M.; Antonchick, A. P.; Theissmann, T. Angew. Chem., Int. Ed. 2006, 45, 3683–3686. doi:10.1002/anie.200600191 |
| 104. | Rueping, M.; Theissmann, T.; Raja, S.; Bats, J. W. Adv. Synth. Catal. 2008, 350, 1001–1006. doi:10.1002/adsc.200800020 |
| 105. | Rueping, M.; Stoeckel, M.; Sugiono, E.; Theissmann, T. Tetrahedron 2010, 66, 6565–6568. doi:10.1016/j.tet.2010.04.091 |
| 106. | Rueping, M.; Theissmann, T.; Stoeckel, M.; Antonchick, A. P. Org. Biomol. Chem. 2011, 9, 6844–6850. doi:10.1039/c1ob05870c |
| 102. | Rueping, M.; Antonchick, A. P.; Theissmann, T. Angew. Chem., Int. Ed. 2006, 45, 6751–6755. doi:10.1002/anie.200601832 |
© 2012 Rueping et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)