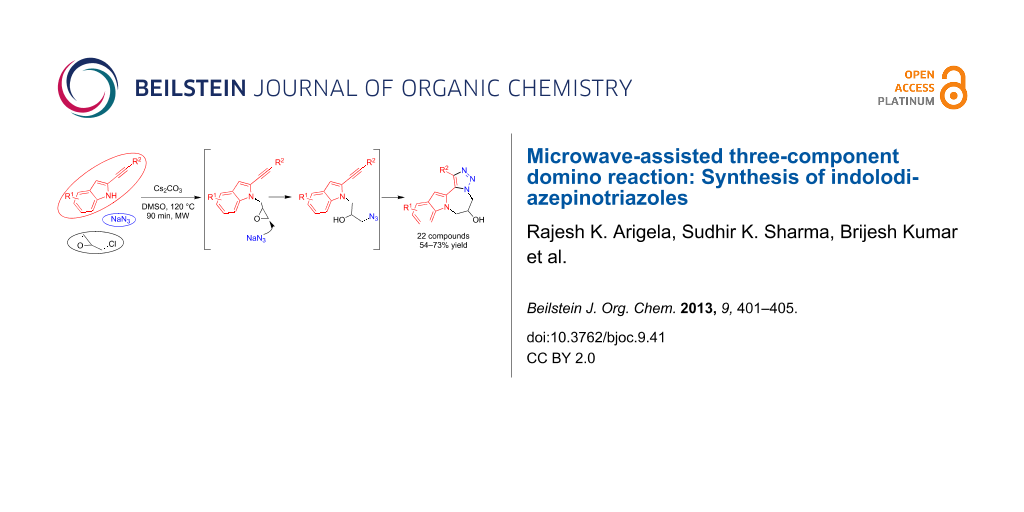
Abstract
A microwave-assisted three-component protocol involving N-1 alkylation of 2-alkynylindoles with epichlorohydrin, ring opening of the epoxide with sodium azide, and an intramolecular Huisgen azide–internal alkyne 1,3-dipolar cycloaddition domino sequence has been described. The efficacy of the methodology has been demonstrated by treating various 2-alkynylindoles (aromatic/aliphatic) with epichlorohydrin and sodium azide furnishing annulated tetracyclic indolodiazepinotriazoles in satisfactory yields.
Graphical Abstract
Introduction
The intermolecular Huisgen azide–alkyne 1,3-dipolar cycloaddition reaction [1-6] for the synthesis of 1,2,3-triazoles in both aqueous [7-10] and organic solvents under either metal-catalyzed [11-13] or metal-free conditions [14-16] has received increasing attention in drug discovery processes [17,18]. The ease of reaction in the intermolecular format has been successfully demonstrated by using both organic/inorganic azides as well as alkynes/diynes [19-21]. In contrast to its employment in an intermolecular format, intramolecular azide–alkyne 1,3-dipolar cycloaddition reactions have been also applied by us and others with the view to synthesize triazole-annulated polyheterocycles. Although these cyclizations have been successfully carried out in either one-pot [22-24] or multistep format [25-28], reports involving their application in a three-component domino format are scarce [29,30]. In our laboratory, we had been employing functionalized indoles for the synthesis of annulated indole-based polyheterocycles either in a multicomponent or in a one-pot format [31-35]. In this continuation, we next directed our efforts to the development of a three-component domino strategy for the synthesis of indole-based polyheterocycles by incorporating the intramolecular azide–alkyne 1,3-dipolar cycloaddition reaction as one of the domino steps. Here we propose a strategy where N-1 of 2-alkynylindole [36,37] can be first functionalized with epoxide by reacting 2-alkynylindole with epichlorohydrin. This can then be followed by ring opening of the oxirane by azide to furnish a bis-functionalized indole intermediate having azide and alkyne groups in close proximity. Such an intermediate may then undergo annulation following an intramolecular 1,3-dipolar cycloaddition pathway and in turn lead to the sequential formation of 7- or 5-membered diazepine and triazole rings in a single step. In this communication, we report a versatile microwave-assisted three component domino reaction to furnish annulated tetracyclic indolodiazepinotriazoles in good yields.
Results and Discussion
We commenced our studies with the development of a one-pot three-component strategy involving the condensation of the 2-(4-methylphenylethynyl)-1H-indole (1a) with epichlorohydrin (2) and sodium azide (3, Scheme 1, Table 1). Initially, a mixture of 1a, 2 and 3 was allowed to react both in the absence and presence of Cs2CO3 in toluene at rt. The reactants under both the conditions remained unchanged even after prolonged stirring for 15 h (Table 1, entries 1–3) and at higher temperature (110 °C).
Scheme 1: One-pot three-component domino reaction furnishing indole derivatives (4a and 5a) and indolodiazepinotriazole 6a.
Scheme 1: One-pot three-component domino reaction furnishing indole derivatives (4a and 5a) and indolodiazepi...
Table 1: Optimization of reaction conditions for the synthesis of 6a in a three-component domino format.
| Entry | Base | Solvent | Temp (°C) | Time | Yield (%)a of 4a/5a/6a |
|---|---|---|---|---|---|
| 1 | – | toluene | rt | 15 h | NR |
| 2 | Cs2CO3 | toluene | rt | 15 h | NR |
| 3 | Cs2CO3 | toluene | 110 | 15 h | NR |
| 4 | Cs2CO3 | CH3CN | 90 | 15 h | 65/–/– |
| 5 | Cs2CO3 | DMF | rt | 15 h | NR |
| 6 | Cs2CO3 | DMF | 120 | 1 h | 77/–/– |
| 7 | Cs2CO3 | DMF | 120 | 4 h | 40/30/– |
| 8 | Cs2CO3 | DMF | 120 | 15 h | –/15/50 |
| 9 | Cs2CO3 | DMF | 120 | 18 h | –/–/60 |
| 10 | Cs2CO3 | DMSO | 120 | 1 h | 82/–/– |
| 11 | Cs2CO3 | DMSO | 120 | 4 h | 42/40/– |
| 12 | Cs2CO3 | DMSO | 120 | 10 h | –/20/52 |
| 13 | Cs2CO3 | DMSO | 120 | 15 h | –/–/64 |
| 14 | Cs2CO3 | DMSO | 120 MW | 10 min | 80/–/– |
| 15 | Cs2CO3 | DMSO | 120 MW | 30 min | 20/45/10b |
| 16 | Cs2CO3 | DMSO | 120 MW | 1 h | –/18/42 |
| 17 | Cs2CO3 | DMSO | 120 MW | 1.5 h | –/–/71 |
| 18 | Cs2CO3 | DMF | 120 MW | 1.5 h | –/–/64 |
| 19 | Cs2CO3 | CH3CN | 90 MW | 1.5 h | 80/–/– |
| 20 | Cs2CO3 | CH3OH | 90 MW | 1.5 h | NR |
| 21 | K2CO3 | DMSO | 120 MW | 1.5 h | –/10/54b |
| 22 | Na2CO3 | DMSO | 120 MW | 1.5 h | –/12/52b |
| 23 | K3PO4 | DMSO | 120 MW | 1.5 h | –/–/62 |
| 24 | t-BuOK | DMSO | 120 MW | 1.5 h | –/–/65 |
| 25 | DBU | DMSO | 120 MW | 1.5 h | –/15/48b |
| 26 | TEA | DMSO | 120 MW | 1.5 h | –/20/45b |
aIsolated yields. bYields based on HPLC (C18 reversed-phase column, 150 × 4.8 mm, 5 µm). NR = no reaction.
However, a change in the nature of solvent from toluene to CH3CN, DMF or DMSO produced a dramatic effect on the outcome of the reaction, resulting in the formation of products comprising intermediates (4a and/or 5a) and/or indole-based polyheterocycle indolodiazepinotriazole 6a. Use of the polar solvent CH3CN at 90 °C for 15 h furnished a single product in 65% isolated yield, which was characterized as 2-[2-(4-methylphenyl)ethynyl]-1-(oxiran-2-ylmethyl)-1H-indole (4a, Table 1, entry 4). In contrast, use of the polar aprotic solvent DMF with high dielectric constant produced both intermediates 4a/5a as well as the annulated product 6a. Interestingly, a significant increase in the yield of the title compound 6a was observed by prolonging the reaction. Carrying out the reaction in DMF at rt also failed to promote annulation even after 15 h of prolonged stirring (Table 1, entry 5). Increasing the temperature to 120 °C furnished the intermediate 4a as a single product in 77% isolated yield within 1 h (Table 1, entry 6). Further stirring up to 4 h at 120 °C led to the partial conversion of 4a (by ring opening of the epoxide with NaN3) into yet another intermediate 1-azido-3-{2-[2-(4-methylphenyl)ethynyl]-1H-indol-1-yl}propan-2-ol (5a, Table 1, entry 7) in 30% isolated yield. Nonetheless, extending the reaction times up to 15 h, led to the complete disappearance of 4a and furnished a mixture of the intermediate 5a in 15% isolated yield and the title compound 6a characterized as 1-(4-methylphenyl)-6,7-dihydro-5H-[1,2,3]triazolo[5',1':3,4][1,4]diazepino[1,2-a]indol-6-ol in 50% isolated yield (Table 1, entry 8). The findings clearly suggest that the formation of indole-based annulated product 6a in the three-component domino format occurs via 4a and 5a intermediacy and requires higher temperature and prolonged stirring. This was again evident from the fact that a prolonged stirring up to 18 h led to the complete disappearance of the intermediates 4a and 5a and afforded 6a as a single product in 60% isolated yield (Table 1, entry 9). The role of intermediates 4a and 5a in the formation of 6a was further substantiated by treating 4a with NaN3 in DMF at 120 °C and by heating 5a in DMF at 120 °C. As envisaged, both reactions furnished 6a as a single product in 87% and 90% isolated yield, respectively (Scheme 2). Replacing DMF with yet another polar aprotic solvent, i.e., DMSO, produced similar results except for a marginal increase in the isolated yield of 6a to 64% in 15 h (Table 1, entries 10–13).
Scheme 2: Transformation of intermediates 4a and 5a to 6a.
Scheme 2: Transformation of intermediates 4a and 5a to 6a.
Next, in order to reduce the reaction times and to enhance the isolated yield of the annulated product 6a, we applied microwave conditions instead of conventional heating and monitored the progress of the reaction at different time intervals. A significant increase in the yield of 6a resulting from the increase in the reaction times under microwave conditions was observed. Initially, a 10 min irradiation of the reaction mixture furnished the intermediate 4a as the only product in 80% isolated yield (Table 1, entry 14), whereas a 30 min irradiation resulted in a mixture of 4a/5a/6a in 20/45/10% yields as evident from HPLC (Table 1, entry 15). Extending exposure to microwave conditions for 1 h produced a mixture of 5a and 6a (Table 1, entry 16); however, a further exposure up to 1.5 h furnished the desired compound 6a as the only product in 71% isolated yield (Table 1, entry 17). Thus, under microwave irradiation conditions, not only the isolated yield of 6a increased from 60% under conventional heating to 71%, but the duration of reaction was also reduced from 15 h to 1.5 h. Switching the solvent from DMSO to DMF under microwave conditions furnished 6a in slightly reduced yield (Table 1, entry 18) while the use of CH3CN and CH3OH failed to produce the desired product (Table 1, entry 19 and 20). Replacing Cs2CO3 with other bases such as K2CO3, Na2CO3, K3PO4, t-BuOK, DBU and TEA either produced a mixture of 5a/6a or furnished 6a in reduced yields (Table 1, entries 21–26). The observations clearly suggest that the formation of 6a in the three-component format involved intermolecular N-1 alkylation of the 2-alkynylindole 1a with epichlorohydrin to form 4a, ring opening of 4a with sodium azide to form 5a, and finally an intramolecular Huisgen azide–internal alkyne 1,3-dipolar cycloaddition reaction.
Once the reaction conditions for the three-component format had been optimized, several 2-alkynylindoles bearing different functional groups were treated with epichlorohydrin and sodium azide in order to establish the scope and limitation of the strategy. In total 22 compounds 6a–v (Scheme 3) were synthesized, with their isolated yields varying from 54–73%. The findings suggest that although the electronic properties of the substitution (R1) on the phenyl ring of the indole had no effect on the outcome of the isolated yield of the final products, the nature of R2 had a profound effect on the yields. When the aromatic group was used as R2, the final products 6a–c and 6e–q were obtained in isolated yields ranging from 66–73%, whereas substituting R2 with aliphatic/trimethylsilyl moieties furnished the cyclized products (6d and 6r–v) in diminished (54–65%) isolated yields.
Scheme 3: Three-component domino reaction for the synthesis of tetracyclic indolodiazepinotriazole compounds based on 6a. Reaction conditions: 1a (1.0 mmol), 2 (1.1 mmol), 3 (1.5 mmol) and Cs2CO3 (1.5 mmol) in DMSO (2.5 mL) at 120 °C, MW under N2 atmosphere.
Scheme 3: Three-component domino reaction for the synthesis of tetracyclic indolodiazepinotriazole compounds ...
Conclusion
In conclusion, we have developed a simple and efficient three-component domino reaction for the synthesis of highly substituted indolodiazepinotriazoles in good yields under microwave conditions. The domino sequence comprising N-1 alkylation, ring opening of the epoxide, and intramolecular Huisgen azide–internal alkyne 1,3-dipolar cycloaddition reaction, led to the generation of the diazepine and triazole rings annulated to the indole through the formation of four new sigma bonds in a single step.
Supporting Information
| Supporting Information File 1: Experimental section, copies of 1H, 13C NMR and HRMS spectra of starting and final compounds 1e, 1h, 1j–1l, 1n–1t, 1v, 4a, 5a and 6a–6v. | ||
| Format: PDF | Size: 8.0 MB | Download |
References
-
Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
Return to citation in text: [1] -
Prescher, J. A.; Bertozzi, C. R. Nat. Chem. Biol. 2005, 1, 13. doi:10.1038/nchembio0605-13
Return to citation in text: [1] -
Miaoa, T.; Wang, L. Synthesis 2008, 363. doi:10.1055/s-2008-1032037
Return to citation in text: [1] -
Wangler, C.; Schirrmacher, R.; Bartenstein, P.; Wangler, B. Curr. Med. Chem. 2010, 17, 1092. doi:10.2174/092986710790820615
Return to citation in text: [1] -
Walsh, J. C.; Kolb, H. C. Chimia 2010, 64, 29. doi:10.2533/chimia.2010.29
Return to citation in text: [1] -
Lal, S.; Díez-González, S. J. Org. Chem. 2011, 76, 2367. doi:10.1021/jo200085j
Return to citation in text: [1] -
Saha, B.; Sharma, S.; Sawant, D.; Kundu, B. Synlett 2007, 1591. doi:10.1055/s-2007-982543
Return to citation in text: [1] -
Li, P.; Wang, L. Lett. Org. Chem. 2007, 4, 23. doi:10.2174/157017807780037513
Return to citation in text: [1] -
Kumar, I.; Rode, C. V. Chem. Lett. 2007, 36, 592. doi:10.1246/cl.2007.592
Return to citation in text: [1] -
Liu, M.; Reiser, O. Org. Lett. 2011, 13, 1102. doi:10.1021/ol103134c
Return to citation in text: [1] -
Zhang, L.; Chen, X.; Xue, P.; Sun, H. H. Y.; Williams, I. D.; Sharpless, K. B.; Fokin, V. V.; Jia, G. J. Am. Chem. Soc. 2005, 127, 15998. doi:10.1021/ja054114s
Return to citation in text: [1] -
Yoo, E. J.; Ahlquist, M.; Bae, I.; Sharpless, K. B.; Fokin, V. V.; Chang, S. J. Org. Chem. 2008, 73, 5520. doi:10.1021/jo800733p
Return to citation in text: [1] -
Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952. doi:10.1021/cr0783479
Return to citation in text: [1] -
Sau, M.; Rodríguez-Escrich, C.; Pericàs, M. A. Org. Lett. 2011, 13, 5044. doi:10.1021/ol201869y
Return to citation in text: [1] -
Kwok, S. W.; Fotsing, J. R.; Fraser, R. J.; Rodionov, O. V.; Fokin, V. V. Org. Lett. 2010, 12, 4217. doi:10.1021/ol101568d
Return to citation in text: [1] -
Becer, C. R.; Hoogenboom, R.; Schubert, U. S. Angew. Chem., Int. Ed. 2009, 48, 4900. doi:10.1002/anie.200900755
Return to citation in text: [1] -
Agalave, S. G.; Maujan, S. R.; Pore, V. S. Chem.–Asian J. 2011, 6, 2696. doi:10.1002/asia.201100432
Return to citation in text: [1] -
Qin, A.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2010, 39, 2522. doi:10.1039/b909064a
Return to citation in text: [1] -
Mandadapu, A. K.; Sharma, S. K.; Gupta, S.; Krishna, D. G. V.; Kundu, B. Org. Lett. 2011, 13, 3162. doi:10.1021/ol201092k
Return to citation in text: [1] -
Aizpurua, J. M.; Azcune, I.; Fratila, R. M.; Balentova, E.; Sagartzazu-Aizpurua, M.; Miranda, J. I. Org. Lett. 2010, 12, 1584. doi:10.1021/ol1003127
Return to citation in text: [1] -
Fiandanese, V.; Bottalico, D.; Marchese, G.; Punzi, A.; Quarta, M. R.; Fittipaldi, M. Synthesis 2009, 3853. doi:10.1055/s-0029-1217017
Return to citation in text: [1] -
Arigela, R. K.; Mandadapu, A. K.; Sharma, S. K.; Kumar, B.; Kundu, B. Org. Lett. 2012, 14, 1804. doi:10.1021/ol300399y
Return to citation in text: [1] -
Guggenheim, K. G.; Toru, H.; Kurth, M. J. Org. Lett. 2012, 14, 3732. doi:10.1021/ol301592z
Return to citation in text: [1] -
Kunick, C. Liebigs Ann. Chem. 1993, 1141. doi:10.1002/jlac.1993199301182
Return to citation in text: [1] -
Akritopoulou-Zanze, I.; Gracias, V.; Djuric, S. W. Tetrahedron Lett. 2004, 45, 8439. doi:10.1016/j.tetlet.2004.09.117
Return to citation in text: [1] -
Oliva, A. I.; Christmann, U.; Font, D.; Cuevas, F.; Ballester, P.; Buschmann, H.; Torrens, A.; Yenes, S.; Pericàs, M. A. Org. Lett. 2008, 10, 1617. doi:10.1021/ol800291t
Return to citation in text: [1] -
Majumdar, K. C.; Ray, K.; Ganai, S. Synthesis 2010, 2101. doi:10.1055/s-0029-1218763
Return to citation in text: [1] -
Donald, J. R.; Martin, S. F. Org. Lett. 2011, 13, 852. doi:10.1021/ol1028404
Return to citation in text: [1] -
Conrad, W. E.; Rodriguez, K. X.; Nguyen, H. H.; Fettinger, J. C.; Haddadin, M. J.; Kurth, M. J. Org. Lett. 2012, 14, 3870. doi:10.1021/ol3015804
Return to citation in text: [1] -
Gracias, V.; Darczak, D.; Gasiecki, A. F.; Djuric, S. W. Tetrahedron Lett. 2005, 46, 9053. doi:10.1016/j.tetlet.2005.10.090
Return to citation in text: [1] -
Sharma, S. K.; Mandadapu, A. K.; Saifuddin, M.; Gupta, S.; Agarwal, P. K.; Mandwal, A. K.; Gauniyal, H. M.; Kundu, B. Tetrahedron Lett. 2010, 51, 6022. doi:10.1016/j.tetlet.2010.09.054
Return to citation in text: [1] -
Sharma, S. K.; Gupta, S.; Saifuddin, M.; Mandadapu, A. K.; Agarwal, P. K.; Gauniyal, H. M.; Kundu, B. Tetrahedron Lett. 2011, 52, 65. doi:10.1016/j.tetlet.2010.10.147
Return to citation in text: [1] -
Gupta, S.; Kumar, B.; Kundu, B. J. Org. Chem. 2011, 76, 10154. doi:10.1021/jo201994v
Return to citation in text: [1] -
Gupta, S.; Sharma, S. K.; Mandadapu, A. K.; Gauniyal, H. M.; Kundu, B. Tetrahedron Lett. 2011, 52, 4288. doi:10.1016/j.tetlet.2011.06.021
Return to citation in text: [1] -
Sharma, S. K.; Mandadapu, A. K.; Kumar, B.; Kundu, B. J. Org. Chem. 2011, 76, 6798. doi:10.1021/jo201228t
Return to citation in text: [1] -
Nagamochi, M.; Fang, Y.-Q.; Lautens, M. Org. Lett. 2007, 9, 2955. doi:10.1021/ol071370w
Return to citation in text: [1] -
Fiandanese, V.; Bottalico, D.; Marchese, G.; Punzi, A. Tetrahedron 2008, 64, 7301. doi:10.1016/j.tet.2008.05.059
Return to citation in text: [1]
| 1. | Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 |
| 2. | Prescher, J. A.; Bertozzi, C. R. Nat. Chem. Biol. 2005, 1, 13. doi:10.1038/nchembio0605-13 |
| 3. | Miaoa, T.; Wang, L. Synthesis 2008, 363. doi:10.1055/s-2008-1032037 |
| 4. | Wangler, C.; Schirrmacher, R.; Bartenstein, P.; Wangler, B. Curr. Med. Chem. 2010, 17, 1092. doi:10.2174/092986710790820615 |
| 5. | Walsh, J. C.; Kolb, H. C. Chimia 2010, 64, 29. doi:10.2533/chimia.2010.29 |
| 6. | Lal, S.; Díez-González, S. J. Org. Chem. 2011, 76, 2367. doi:10.1021/jo200085j |
| 17. | Agalave, S. G.; Maujan, S. R.; Pore, V. S. Chem.–Asian J. 2011, 6, 2696. doi:10.1002/asia.201100432 |
| 18. | Qin, A.; Lam, J. W. Y.; Tang, B. Z. Chem. Soc. Rev. 2010, 39, 2522. doi:10.1039/b909064a |
| 14. | Sau, M.; Rodríguez-Escrich, C.; Pericàs, M. A. Org. Lett. 2011, 13, 5044. doi:10.1021/ol201869y |
| 15. | Kwok, S. W.; Fotsing, J. R.; Fraser, R. J.; Rodionov, O. V.; Fokin, V. V. Org. Lett. 2010, 12, 4217. doi:10.1021/ol101568d |
| 16. | Becer, C. R.; Hoogenboom, R.; Schubert, U. S. Angew. Chem., Int. Ed. 2009, 48, 4900. doi:10.1002/anie.200900755 |
| 11. | Zhang, L.; Chen, X.; Xue, P.; Sun, H. H. Y.; Williams, I. D.; Sharpless, K. B.; Fokin, V. V.; Jia, G. J. Am. Chem. Soc. 2005, 127, 15998. doi:10.1021/ja054114s |
| 12. | Yoo, E. J.; Ahlquist, M.; Bae, I.; Sharpless, K. B.; Fokin, V. V.; Chang, S. J. Org. Chem. 2008, 73, 5520. doi:10.1021/jo800733p |
| 13. | Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952. doi:10.1021/cr0783479 |
| 7. | Saha, B.; Sharma, S.; Sawant, D.; Kundu, B. Synlett 2007, 1591. doi:10.1055/s-2007-982543 |
| 8. | Li, P.; Wang, L. Lett. Org. Chem. 2007, 4, 23. doi:10.2174/157017807780037513 |
| 9. | Kumar, I.; Rode, C. V. Chem. Lett. 2007, 36, 592. doi:10.1246/cl.2007.592 |
| 10. | Liu, M.; Reiser, O. Org. Lett. 2011, 13, 1102. doi:10.1021/ol103134c |
| 29. | Conrad, W. E.; Rodriguez, K. X.; Nguyen, H. H.; Fettinger, J. C.; Haddadin, M. J.; Kurth, M. J. Org. Lett. 2012, 14, 3870. doi:10.1021/ol3015804 |
| 30. | Gracias, V.; Darczak, D.; Gasiecki, A. F.; Djuric, S. W. Tetrahedron Lett. 2005, 46, 9053. doi:10.1016/j.tetlet.2005.10.090 |
| 36. | Nagamochi, M.; Fang, Y.-Q.; Lautens, M. Org. Lett. 2007, 9, 2955. doi:10.1021/ol071370w |
| 37. | Fiandanese, V.; Bottalico, D.; Marchese, G.; Punzi, A. Tetrahedron 2008, 64, 7301. doi:10.1016/j.tet.2008.05.059 |
| 25. | Akritopoulou-Zanze, I.; Gracias, V.; Djuric, S. W. Tetrahedron Lett. 2004, 45, 8439. doi:10.1016/j.tetlet.2004.09.117 |
| 26. | Oliva, A. I.; Christmann, U.; Font, D.; Cuevas, F.; Ballester, P.; Buschmann, H.; Torrens, A.; Yenes, S.; Pericàs, M. A. Org. Lett. 2008, 10, 1617. doi:10.1021/ol800291t |
| 27. | Majumdar, K. C.; Ray, K.; Ganai, S. Synthesis 2010, 2101. doi:10.1055/s-0029-1218763 |
| 28. | Donald, J. R.; Martin, S. F. Org. Lett. 2011, 13, 852. doi:10.1021/ol1028404 |
| 22. | Arigela, R. K.; Mandadapu, A. K.; Sharma, S. K.; Kumar, B.; Kundu, B. Org. Lett. 2012, 14, 1804. doi:10.1021/ol300399y |
| 23. | Guggenheim, K. G.; Toru, H.; Kurth, M. J. Org. Lett. 2012, 14, 3732. doi:10.1021/ol301592z |
| 24. | Kunick, C. Liebigs Ann. Chem. 1993, 1141. doi:10.1002/jlac.1993199301182 |
| 19. | Mandadapu, A. K.; Sharma, S. K.; Gupta, S.; Krishna, D. G. V.; Kundu, B. Org. Lett. 2011, 13, 3162. doi:10.1021/ol201092k |
| 20. | Aizpurua, J. M.; Azcune, I.; Fratila, R. M.; Balentova, E.; Sagartzazu-Aizpurua, M.; Miranda, J. I. Org. Lett. 2010, 12, 1584. doi:10.1021/ol1003127 |
| 21. | Fiandanese, V.; Bottalico, D.; Marchese, G.; Punzi, A.; Quarta, M. R.; Fittipaldi, M. Synthesis 2009, 3853. doi:10.1055/s-0029-1217017 |
| 31. | Sharma, S. K.; Mandadapu, A. K.; Saifuddin, M.; Gupta, S.; Agarwal, P. K.; Mandwal, A. K.; Gauniyal, H. M.; Kundu, B. Tetrahedron Lett. 2010, 51, 6022. doi:10.1016/j.tetlet.2010.09.054 |
| 32. | Sharma, S. K.; Gupta, S.; Saifuddin, M.; Mandadapu, A. K.; Agarwal, P. K.; Gauniyal, H. M.; Kundu, B. Tetrahedron Lett. 2011, 52, 65. doi:10.1016/j.tetlet.2010.10.147 |
| 33. | Gupta, S.; Kumar, B.; Kundu, B. J. Org. Chem. 2011, 76, 10154. doi:10.1021/jo201994v |
| 34. | Gupta, S.; Sharma, S. K.; Mandadapu, A. K.; Gauniyal, H. M.; Kundu, B. Tetrahedron Lett. 2011, 52, 4288. doi:10.1016/j.tetlet.2011.06.021 |
| 35. | Sharma, S. K.; Mandadapu, A. K.; Kumar, B.; Kundu, B. J. Org. Chem. 2011, 76, 6798. doi:10.1021/jo201228t |
© 2013 Arigela et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)